Nomogram Based on Dual-Layer Spectral Detector CTA Parameter for the Prediction of Infarct Core in Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Imaging Acquisition
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Cut-Off Values for Iodine Density and Effective Atomic Number
3.3. Univariate and Multivariate Logistic Analyses of Clinical and Spectral CT Parameters
3.4. Development and Performance of the Prognostic Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480,687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef]
- Wu, X.; Zou, S.; Zhu, B.; Shi, J. The hospital costs of stroke patients in Chinese island populations: An 11-year tendency analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2015, 24, 988–992. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, H.; Liu, F.; Chen, C.; Chen, C.; Bivard, A.; Parsons, M.W.; Li, G. Bridging Thrombolysis Before Endovascular Therapy in Stroke Patients With Faster Core Growth. Neurology 2023, 100, e2083–e2092. [Google Scholar] [CrossRef]
- Almekhlafi, M.A.; Kunz, W.G.; McTaggart, R.A.; Jayaraman, M.V.; Najm, M.; Ahn, S.H.; Fainardi, E.; Rubiera, M.; Khaw, A.V.; Zini, A.; et al. Imaging Triage of Patients with Late-Window (6–24 hours) Acute Ischemic Stroke: A Comparative Study Using Multiphase CT Angiography versus CT Perfusion. AJNR Am. J. Neuroradiol. 2020, 41, 129–133. [Google Scholar] [CrossRef]
- Demirler Simsir, B.; Danse, E.; Coche, E. Benefit of dual-layer spectral CT in emergency imaging of different organ systems. Clin. Radiol. 2020, 75, 886–902. [Google Scholar] [CrossRef]
- Conrad, J.; Ertl, M.; Oltmanns, M.H.; Zu Eulenburg, P. Prediction contribution of the cranial collateral circulation to the clinical and radiological outcome of ischemic stroke. J. Neurol. 2020, 267, 2013–2021. [Google Scholar] [CrossRef]
- Ermine, C.M.; Bivard, A.; Parsons, M.W.; Baron, J.C. The ischemic penumbra: From concept to reality. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 497–509. [Google Scholar] [CrossRef]
- Chao, B.H.; Yan, F.; Hua, Y.; Liu, J.M.; Yang, Y.; Ji, X.M.; Peng, B.; Zhao, G.G.; Wang, Y.J.; Kang, D.Z.; et al. Stroke prevention and control system in China: CSPPC-Stroke Program. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 265–272. [Google Scholar] [CrossRef]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Mokin, M.; Levy, E.I.; Saver, J.L.; Siddiqui, A.H.; Goyal, M.; Bonafé, A.; Cognard, C.; Jahan, R.; Albers, G.W. Predictive Value of RAPID Assessed Perfusion Thresholds on Final Infarct Volume in SWIFT PRIME (Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke 2017, 48, 932–938. [Google Scholar] [CrossRef]
- Heiss, W.D. PET imaging in ischemic cerebrovascular disease: Current status and future directions. Neurosci. Bull. 2014, 30, 713–732. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Sobesky, J.; von Kummer, R.; Frackowiak, M.; Zaro Weber, O.; Lehnhardt, F.G.; Dohmen, C.; Neveling, M.; Möller-Hartmann, W.; Jacobs, A.H.; Heiss, W.D. Early ischemic edema on cerebral computed tomography: Its relation to diffusion changes and hypoperfusion within 6 h after human ischemic stroke. A comparison of CT, MRI and PET. Cerebrovasc. Dis. 2006, 21, 336–339. [Google Scholar] [CrossRef]
- Heiss, W.D.; Zaro Weber, O. Validation of MRI Determination of the Penumbra by PET Measurements in Ischemic Stroke. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 187–193. [Google Scholar] [CrossRef]
- Vagal, A.; Wintermark, M.; Nael, K.; Bivard, A.; Parsons, M.; Grossman, A.W.; Khatri, P. Automated CT perfusion imaging for acute ischemic stroke: Pearls and pitfalls for real-world use. Neurology 2019, 93, 888–898. [Google Scholar] [CrossRef]
- Rao, V.; Christensen, S.; Yennu, A.; Mlynash, M.; Zaharchuk, G.; Heit, J.; Marks, M.P.; Lansberg, M.G.; Albers, G.W. Ischemic Core and Hypoperfusion Volumes Correlate With Infarct Size 24 Hours After Randomization in DEFUSE 3. Stroke 2019, 50, 626–631. [Google Scholar] [CrossRef]
- Cereda, C.W.; Christensen, S.; Campbell, B.C.V.; Mishra, N.K.; Mlynash, M.; Levi, C.; Straka, M.; Wintermark, M.; Bammer, R.; Albers, G.W.; et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1780–1789. [Google Scholar] [CrossRef]
- Bouslama, M.; Ravindran, K.; Rodrigues, G.M.; Pisani, L.; Haussen, D.C.; Frankel, M.R.; Nogueira, R.G. Falsely normal CT perfusion ischemic core readings are common and often associated with deep infarcts. J. Neurointerventional Surg. 2023, 15, 183–187. [Google Scholar] [CrossRef]
- Hoang, J.K.; Wang, C.; Frush, D.P.; Enterline, D.S.; Samei, E.; Toncheva, G.; Lowry, C.; Yoshizumi, T.T. Estimation of radiation exposure for brain perfusion CT: Standard protocol compared with deviations in protocol. Am. J. Roentgenol. 2013, 201, W730–W734. [Google Scholar] [CrossRef]
- Sellerer, T.; Noël, P.B.; Patino, M.; Parakh, A.; Ehn, S.; Zeiter, S.; Holz, J.A.; Hammel, J.; Fingerle, A.A.; Pfeiffer, F.; et al. Dual-energy CT: A phantom comparison of different platforms for abdominal imaging. Eur. Radiol. 2018, 28, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yassi, N.; Sharma, G.; Yan, B.; Desmond, P.M.; Davis, S.M.; Campbell, B.C. Diagnosing acute lacunar infarction using CT perfusion. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2016, 29, 70–72. [Google Scholar] [CrossRef]
- Campbell, B.C.; Weir, L.; Desmond, P.M.; Tu, H.T.; Hand, P.J.; Yan, B.; Donnan, G.A.; Parsons, M.W.; Davis, S.M. CT perfusion improves diagnostic accuracy and confidence in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2013, 84, 613–618. [Google Scholar] [CrossRef]
- Benson, J.C.; Payabvash, S.; Mortazavi, S.; Zhang, L.; Salazar, P.; Hoffman, B.; Oswood, M.; McKinney, A.M. CT Perfusion in Acute Lacunar Stroke: Detection Capabilities Based on Infarct Location. AJNR Am. J. Neuroradiol. 2016, 37, 2239–2244. [Google Scholar] [CrossRef]
- Wu, D.; Yin, L.; Zhang, Y.; Lin, Y.; Deng, W.; Zheng, C.; Liu, H.; Jiang, F.; Lan, S.; Wu, Q.; et al. Evaluation of microcirculation in asymptomatic cerebral infarction with multi-parameter imaging of spectral CT. Brain Res. Bull. 2023, 203, 110775. [Google Scholar] [CrossRef]
- Pelgrim, G.J.; van Hamersvelt, R.W.; Willemink, M.J.; Schmidt, B.T.; Flohr, T.; Schilham, A.; Milles, J.; Oudkerk, M.; Leiner, T.; Vliegenthart, R. Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur. Radiol. 2017, 27, 3904–3912. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.P.; Kopp, F.K.; Münzel, D.; Dangelmaier, J.; Renz, M.; Renger, B.; Braren, R.; Fingerle, A.A.; Rummeny, E.J.; Noël, P.B. Accuracy of iodine quantification in dual-layer spectral CT: Influence of iterative reconstruction, patient habitus and tube parameters. Eur. J. Radiol. 2018, 102, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Delgado Sanchez-Gracian, C.; Oca Pernas, R.; Trinidad Lopez, C.; Santos Armentia, E.; Vaamonde Liste, A.; Vazquez Caamano, M.; Tardaguila de la Fuente, G. Quantitative myocardial perfusion with stress dual-energy CT: Iodine concentration differences between normal and ischemic or necrotic myocardium. Initial experience. Eur. Radiol. 2016, 26, 3199–3207. [Google Scholar] [CrossRef]
- Gordic, S.; Puippe, G.D.; Krauss, B.; Klotz, E.; Desbiolles, L.; Lesurtel, M.; Müllhaupt, B.; Pfammatter, T.; Alkadhi, H. Correlation between Dual-Energy and Perfusion CT in Patients with Hepatocellular Carcinoma. Radiology 2016, 280, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mileto, A.; Marin, D.; Alfaro-Cordoba, M.; Ramirez-Giraldo, J.C.; Eusemann, C.D.; Scribano, E.; Blandino, A.; Mazziotti, S.; Ascenti, G. Iodine quantification to distinguish clear cell from papillary renal cell carcinoma at dual-energy multidetector CT: A multireader diagnostic performance study. Radiology 2014, 273, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, W.M.; Haberland, U.; Kaufmann, S.; Spira, D.; Thomas, C.; Nikolaou, K.; Horger, M.; Sauter, A.W. Iodine concentration as a perfusion surrogate marker in oncology: Further elucidation of the underlying mechanisms using Volume Perfusion CT with 80 kVp. Eur. Radiol. 2016, 26, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Fransson, V.; Mellander, H.; Wasselius, J.; Ydström, K. Detection of Perfusion Deficits in Multiphase Computed Tomography Angiography-A Stroke Imaging Technique Based on Iodine Mapping on Spectral Computed Tomography: Initial Findings. J. Comput. Assist. Tomogr. 2021, 45, 618–624. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Wang, X.; Hao, L.; Zhang, J.; Zhang, X.; Sheng, Z.; Liu, K. The diagnostic value of quantitative parameters on dual-layer detector-based spectral CT in identifying ischaemic stroke. Front. Neurol. 2023, 14, 1056941. [Google Scholar] [CrossRef]
- Cai, X.; Li, N. Association between Use of Spironolactone and Risk of Stroke in Hypertensive Patients: A Cohort Study. Pharmaceuticals 2022, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Liebeskind, D.S.; Chan, S.L. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 2129–2149. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Chan, S.L. Impact of Acute and Chronic Hypertension on Changes in Pial Collateral Tone In Vivo During Transient Ischemia. Hypertension 2020, 76, 1019–1026. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Sweet, J.G.; Chan, S.L. Effect of hypertension and peroxynitrite decomposition with FeTMPyP on CBF and stroke outcome. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 1276–1285. [Google Scholar] [CrossRef]
- Pikija, S.; Trkulja, V.; Juvan, L.; Ivanec, M.; Dukši, D. Higher on-admission serum triglycerides predict less severe disability and lower all-cause mortality after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2013, 22, e15–e24. [Google Scholar] [CrossRef]
- Pikija, S.; Milevcić, D.; Trkulja, V.; Kidemet-Piskac, S.; Pavlicek, I.; Sokol, N. Higher serum triglyceride level in patients with acute ischemic stroke is associated with lower infarct volume on CT brain scans. Eur. Neurol. 2006, 55, 89–92. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. United States Am. 2003, 100, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Serhat Tokgoz, O.; Guney, F.; Kaya, A.; Bugrul, A.; Eruyar, E.; Buyukgol, H.; Seyithanoglu, A.; Sinan Iyisoy, M. Acute-Phase Stroke Outcome and Lipids. Sisli Etfal Hastan. Tip Bul. 2021, 55, 538–544. [Google Scholar] [CrossRef] [PubMed]
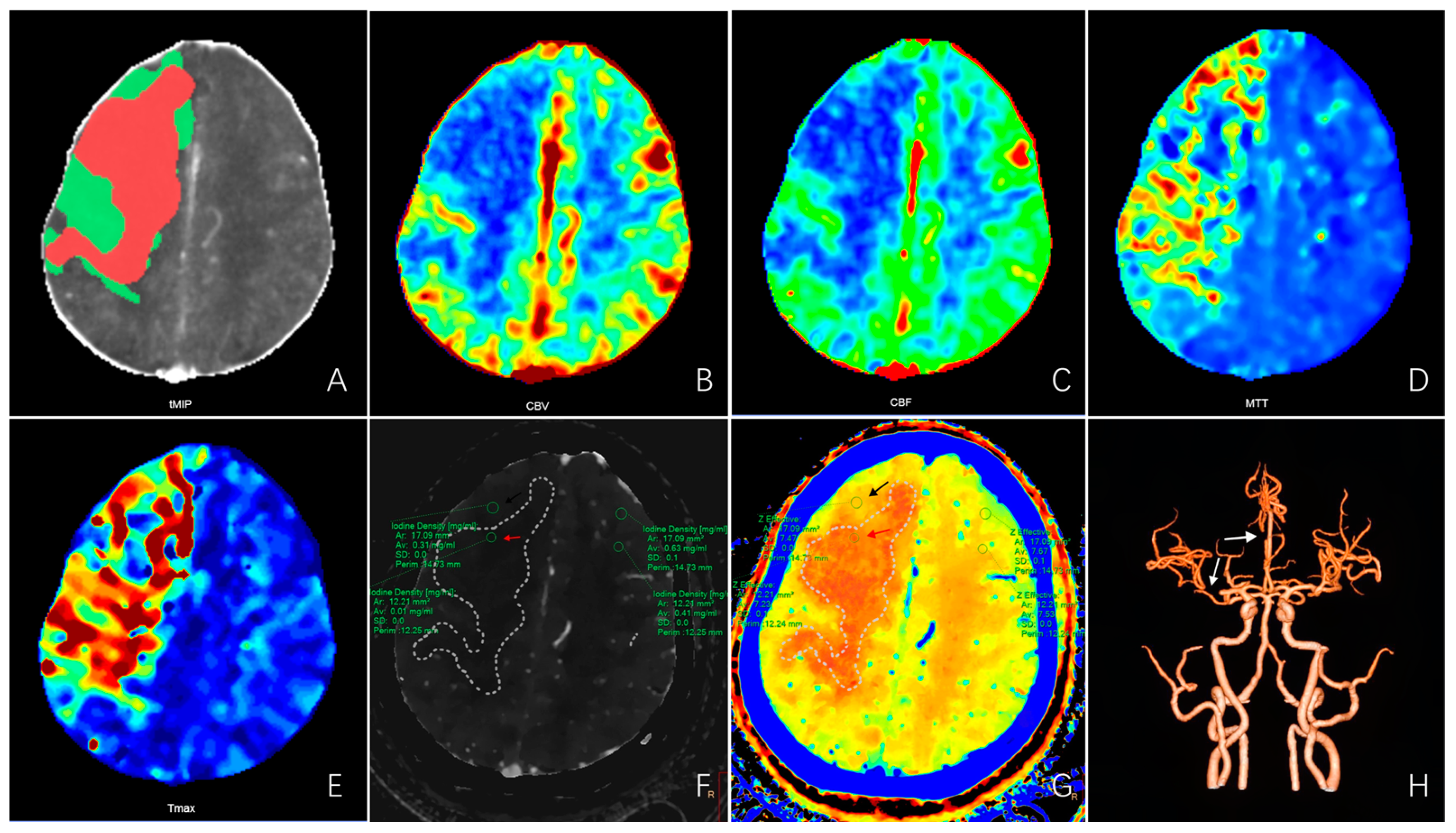

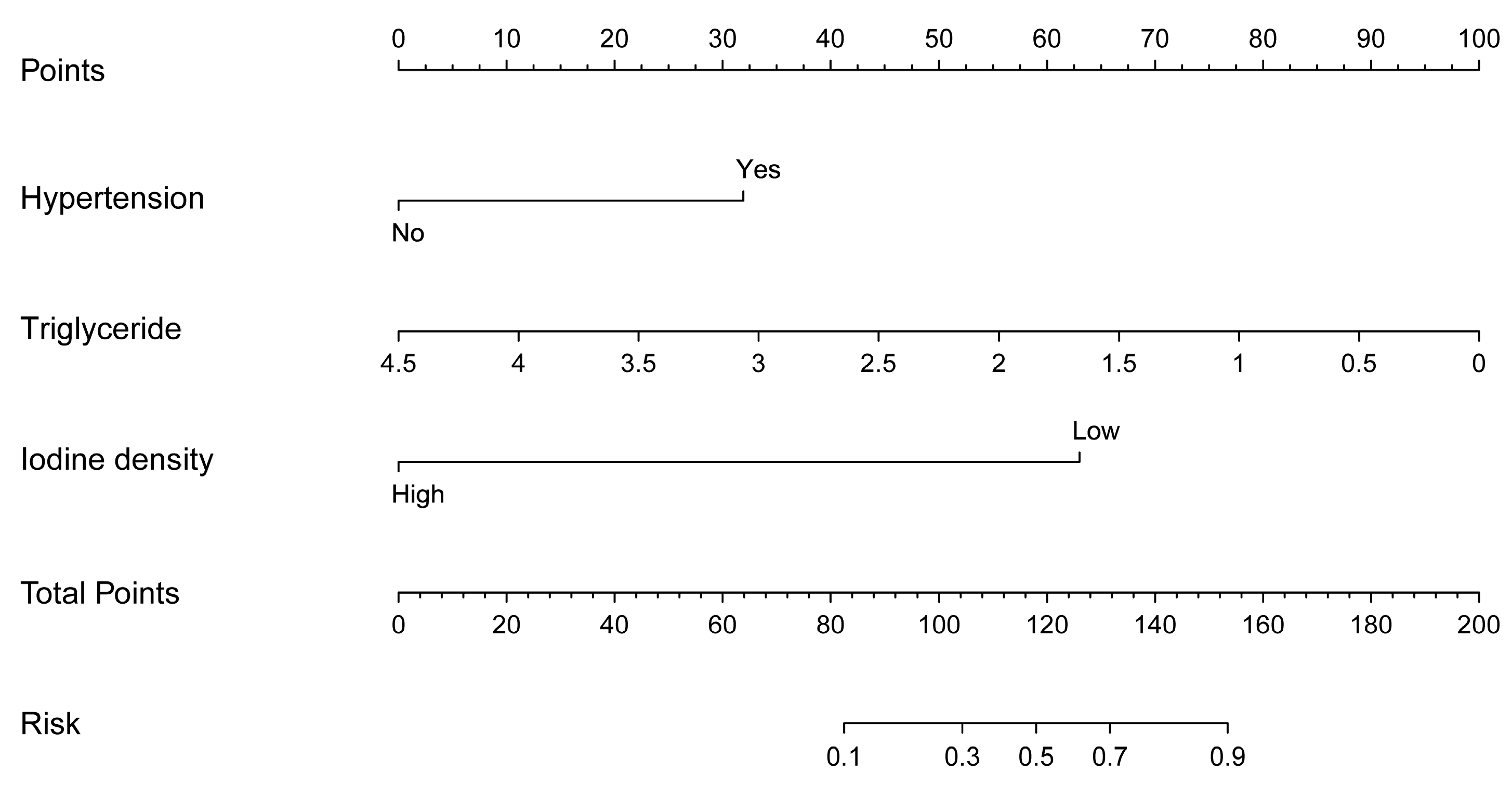

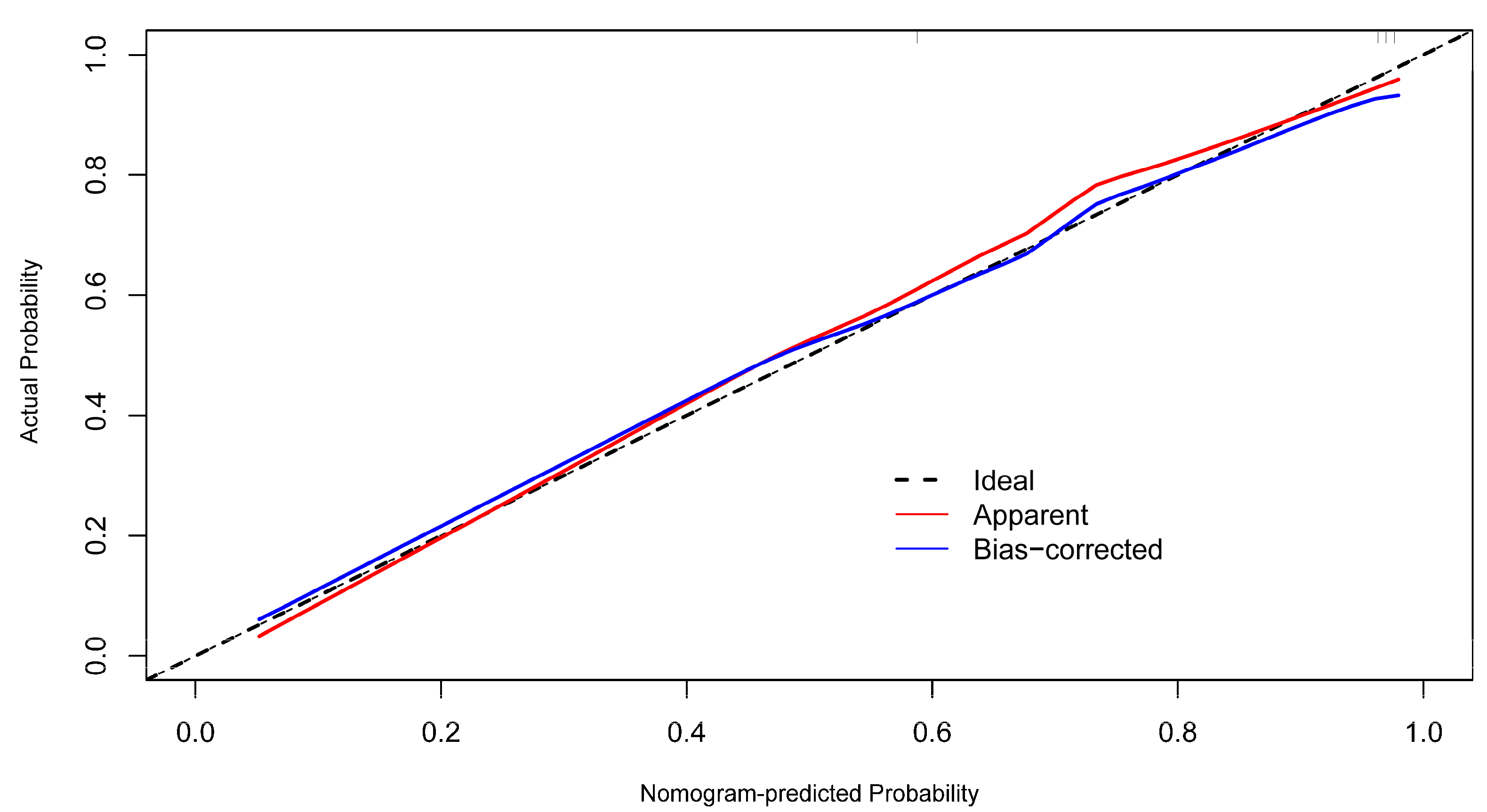

| Variables | Infarct Core Group (n = 67) | Non-Infarct Core Group (n = 35) | Test Statistic | p-Value |
|---|---|---|---|---|
| Iodine density (mg/mL) | 0.14 (0.11, 0.20) a | 0.24 ± (0.20, 0.42) c | 5.406 | <0.001 * |
| Zeff a | 7.34 (7.31, 7.38) | 7.41 (7.34, 7.43) | 2.394 | 0.021 * |
| Variables | Infarct Core Group (n = 67) | Non-Infarct Core Group (n = 35) | Test Statistic | p-Value |
|---|---|---|---|---|
| Gender b | 0.989 | 0.320 | ||
| Male | 52 (68.4) | 24 (31.6) | ||
| Female | 15 (57.7) | 11 (42.3) | ||
| Age (years) b | 0.080 | 0.778 | ||
| <70 | 44 (66.7) | 22 (33.3) | ||
| ≥70 | 23 (63.9) | 13 (36.1) | ||
| Smoking b | 34 (63) | 20 (37) | 0.378 | 0.539 |
| Drinking b | 25 (65.8) | 13 (34.2) | 0 | 0.987 |
| GCS c | 13.09 ± 2.57 | 12.94 ± 2.71 | 0.269 | 0.789 |
| Pre-stroke mRS b | 0.124 | 0.725 | ||
| 0–2 | 32 (64) | 18 (36) | ||
| 3–6 | 35 (67.3) | 17 (32.7) | ||
| Baseline NIHSS b | 2.195 | 0.138 | ||
| <15 | 42 (60.9) | 27 (39.1) | ||
| ≥15 | 25 (75.8) | 8 (24.2) | ||
| Lesion location b | 0.835 | 0.361 | ||
| Anterior | 57 (64) | 32 (36) | ||
| Post | 10 (76.9) | 3 (23.1) | ||
| TOAST classification b | 0.203 | 0.903 | ||
| LAA | 48 (66.7) | 24 (33.3) | ||
| CE | 8 (66.7) | 4 (33.3) | ||
| Others | 11 (61.1) | 7 (38.9) | ||
| Risk factors b | ||||
| History of stroke | 46 (67.6) | 22 (32.4) | 0.348 | 0.555 |
| Hypertension | 52 (71.2) | 21 (28.8) | 3.505 | 0.061 |
| Diabetes | 23 (65.7) | 12 (34.3) | 0 | 0.997 |
| Cancer | 4 (66.7) | 2 (33.3) | 0.030 | 0.958 |
| Paralysis | 15 (65.2) | 8 (34.8) | 0.003 | 0.957 |
| Atrial fibrillation | 9 (64.3) | 5 (35.7) | 0.014 | 0.905 |
| Coronary heart disease | 9 (81.8) | 2 (18.2) | 1.424 | 0.233 |
| Chronic heart failure | 12 (60) | 8 (40) | 0.357 | 0.550 |
| Pulmonary infection | 42 (71.2) | 17 (28.8) | 1.878 | 0.171 |
| Laboratory date | ||||
| Leukocyte (×109/L) c | 8.96 ± 3.18 | 8.15 ± 3.21 | 1.220 | 0.228 |
| Neutrophil (×109/L) a | 6.0 (4.30, 9.10) | 4.7 (3.40, 8.30) | 2.268 | 0.026 * |
| Lymphocyte (×109/L) a | 1.40 (1.20, 2.00) | 1.40 (1.00, 1.90) | 1.612 | 0.113 |
| Monocyte (×109/L) c | 0.52 ± 0.23 | 0.54 ± 0.20 | 0.444 | 0.658 |
| Platelets (×109/L) c | 226.60 ± 92.27 | 214.00 ± 76.32 | 0.690 | 0.492 |
| CRP (mg/L) a | 6.00 (4.80, 18.10) | 5.70 (5.20 ± 11.3) | 1.458 | 0.148 |
| Cholesterol (mmol/L) c | 4.03 ± 1.05 | 4.13 ± 0.99 | 0.442 | 0.660 |
| Triglyceride (mmol/L) a | 1.23 (0.78, 1.53) | 1.51 (1.03, 2.40) | 2.556 | 0.014 * |
| HDL (mmol/L) a | 1.04 (0.92, 1.33) | 1.10 (0.85, 1.29) | 0.016 | 0.988 |
| LDL (mmol/L) c | 2.50 ± 1.16 | 2.55 ± 1.16 | 0.211 | 0.833 |
| HbA1c (≥6.5%) b | 19 (61.3) | 12 (38.7) | 0.510 | 0.475 |
| Variables | Univariable Models | Full Multivariable Model | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Hypertension | 2.311 (0.952~5.613) | 0.064 | 7.179 (1.766~29.186) | 0.006 ** |
| Baseline NIHSS (≥15) | 2.009 (0.791~5.099) | 0.142 | 1.872 (0.500~7.016) | 0.352 |
| Pulmonary infection | 1.779 (0.778~4.069) | 0.172 | 1.087 (0.318~3.721) | 0.894 |
| Triglyceride | 0.447 (0.246~0.813) | 0.008 ** | 0.255 (0.109~0.594) | 0.002 ** |
| Iodine density | 0.028 (0.007~0.106) | <0.001 ** | 0.022 (0.003~0.170) | <0.001 ** |
| Zeff | 0.093 (0.032~0.271) | <0.001 ** | 0.869 (0.122~6.177) | 0.889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Shi, D.; Shen, H.; Wang, Y.; Xu, D.; Xiao, A.; Jin, D.; Lu, K.; Cai, W.; Xu, L. Nomogram Based on Dual-Layer Spectral Detector CTA Parameter for the Prediction of Infarct Core in Patients with Acute Ischemic Stroke. Diagnostics 2023, 13, 3434. https://doi.org/10.3390/diagnostics13223434
Gu Y, Shi D, Shen H, Wang Y, Xu D, Xiao A, Jin D, Lu K, Cai W, Xu L. Nomogram Based on Dual-Layer Spectral Detector CTA Parameter for the Prediction of Infarct Core in Patients with Acute Ischemic Stroke. Diagnostics. 2023; 13(22):3434. https://doi.org/10.3390/diagnostics13223434
Chicago/Turabian StyleGu, Yan, Dai Shi, Hao Shen, Yeqing Wang, Dandan Xu, Aoqi Xiao, Dan Jin, Kuan Lu, Wu Cai, and Liang Xu. 2023. "Nomogram Based on Dual-Layer Spectral Detector CTA Parameter for the Prediction of Infarct Core in Patients with Acute Ischemic Stroke" Diagnostics 13, no. 22: 3434. https://doi.org/10.3390/diagnostics13223434
APA StyleGu, Y., Shi, D., Shen, H., Wang, Y., Xu, D., Xiao, A., Jin, D., Lu, K., Cai, W., & Xu, L. (2023). Nomogram Based on Dual-Layer Spectral Detector CTA Parameter for the Prediction of Infarct Core in Patients with Acute Ischemic Stroke. Diagnostics, 13(22), 3434. https://doi.org/10.3390/diagnostics13223434





