Inner Retinal Thinning Comparison between Branch Retinal Artery Occlusion and Primary Open-Angle Glaucoma
Abstract
:1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Spectralis GMPE
2.4. Data Collection
2.5. Statistical Analysis
3. Results
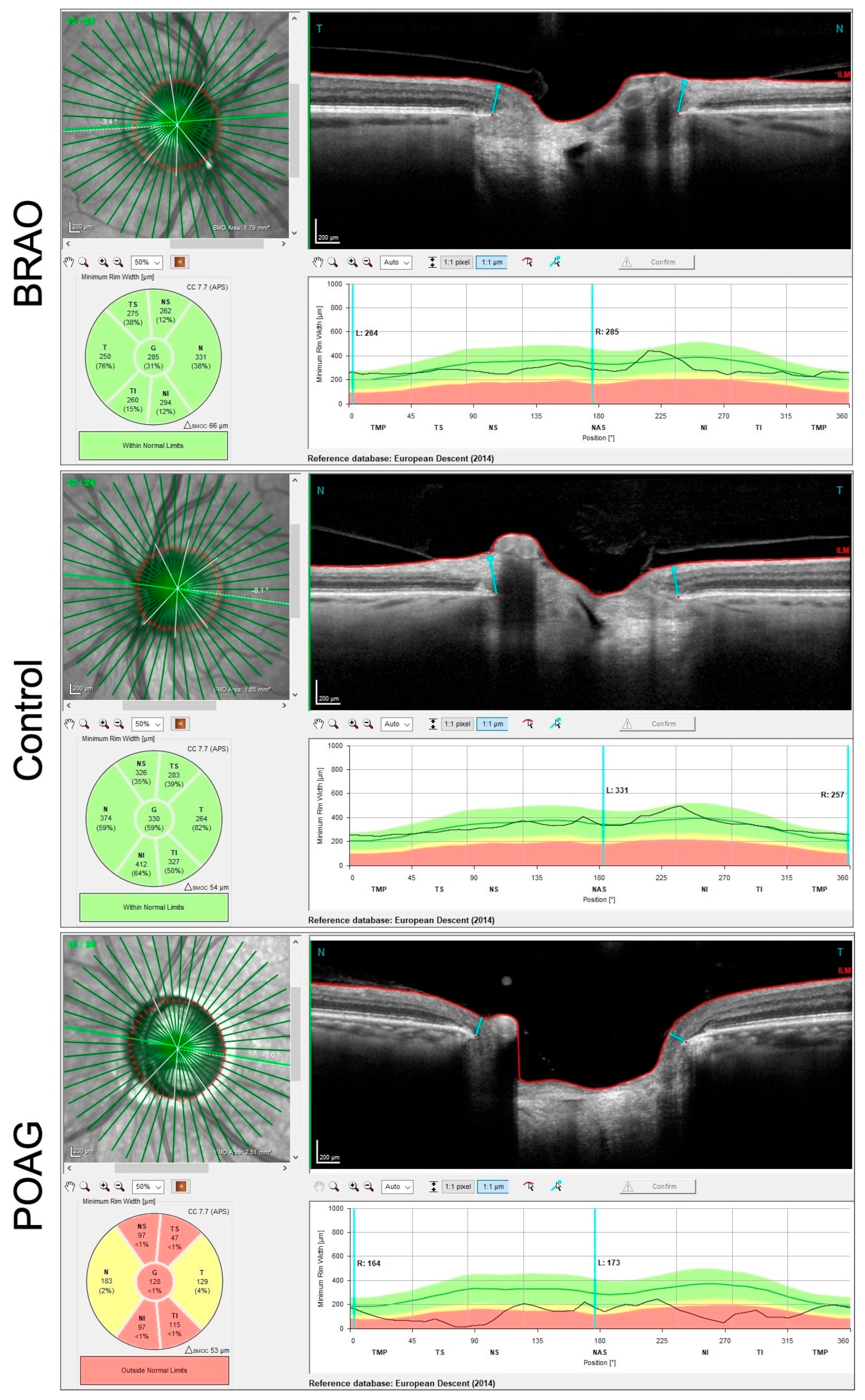
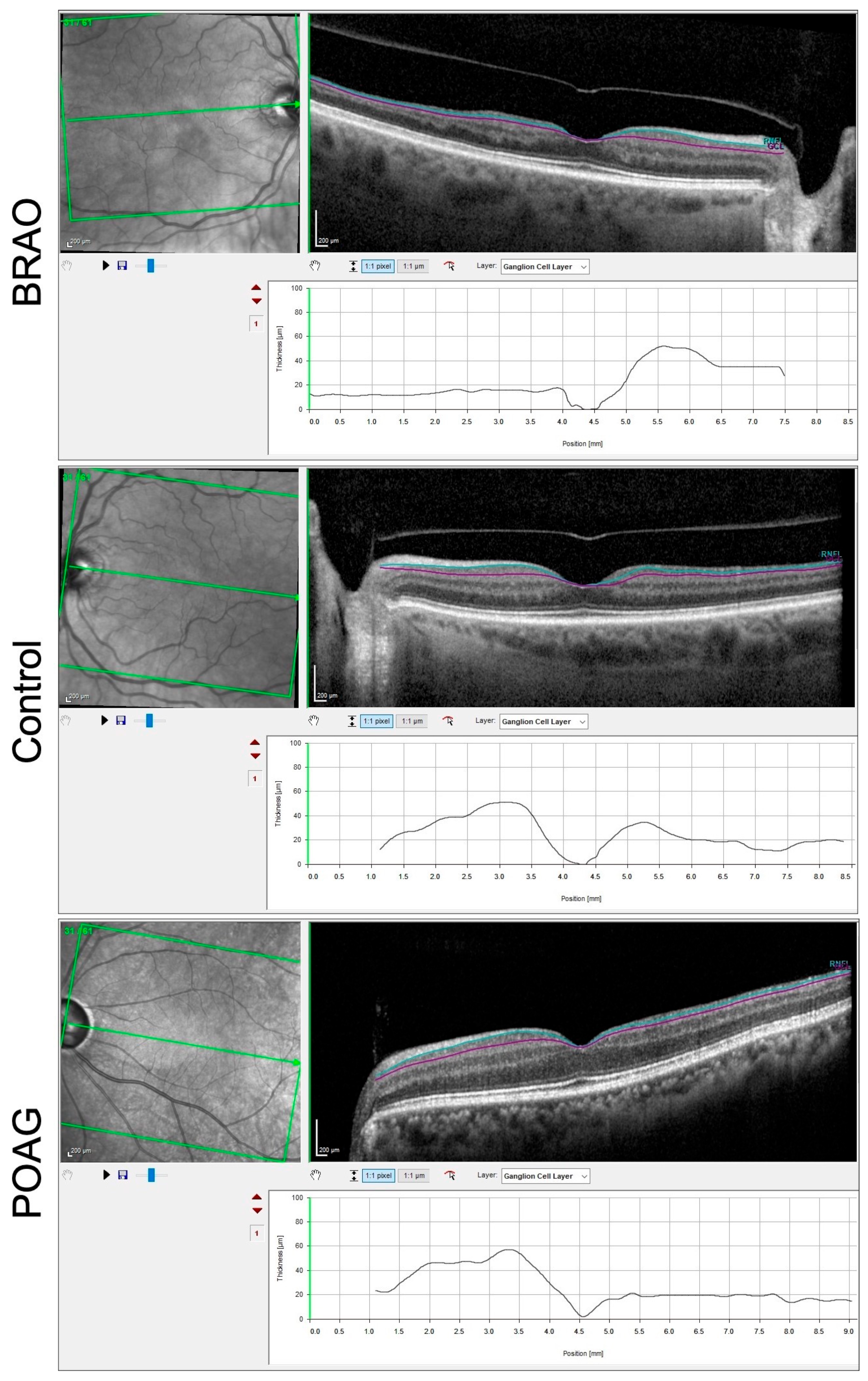
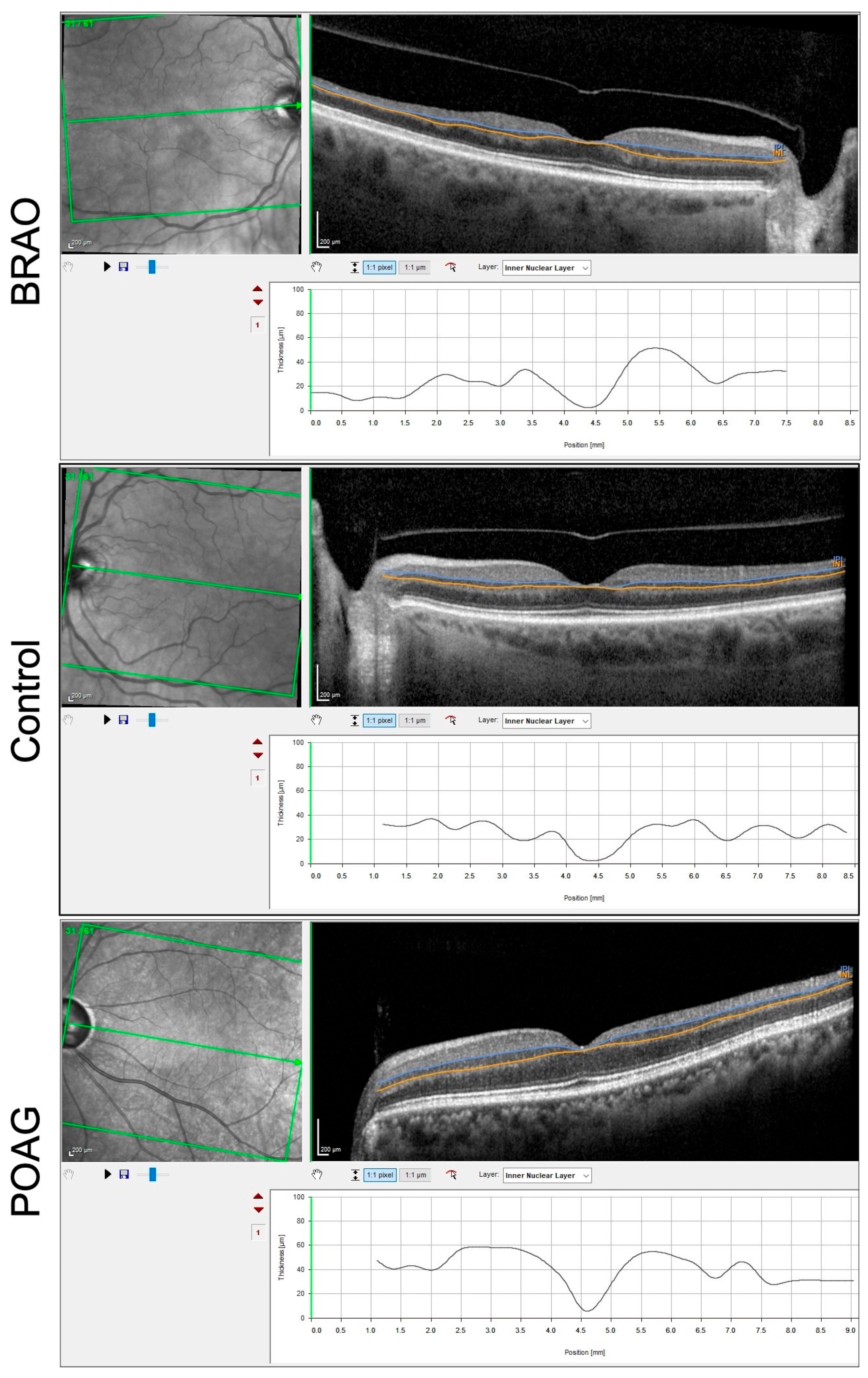
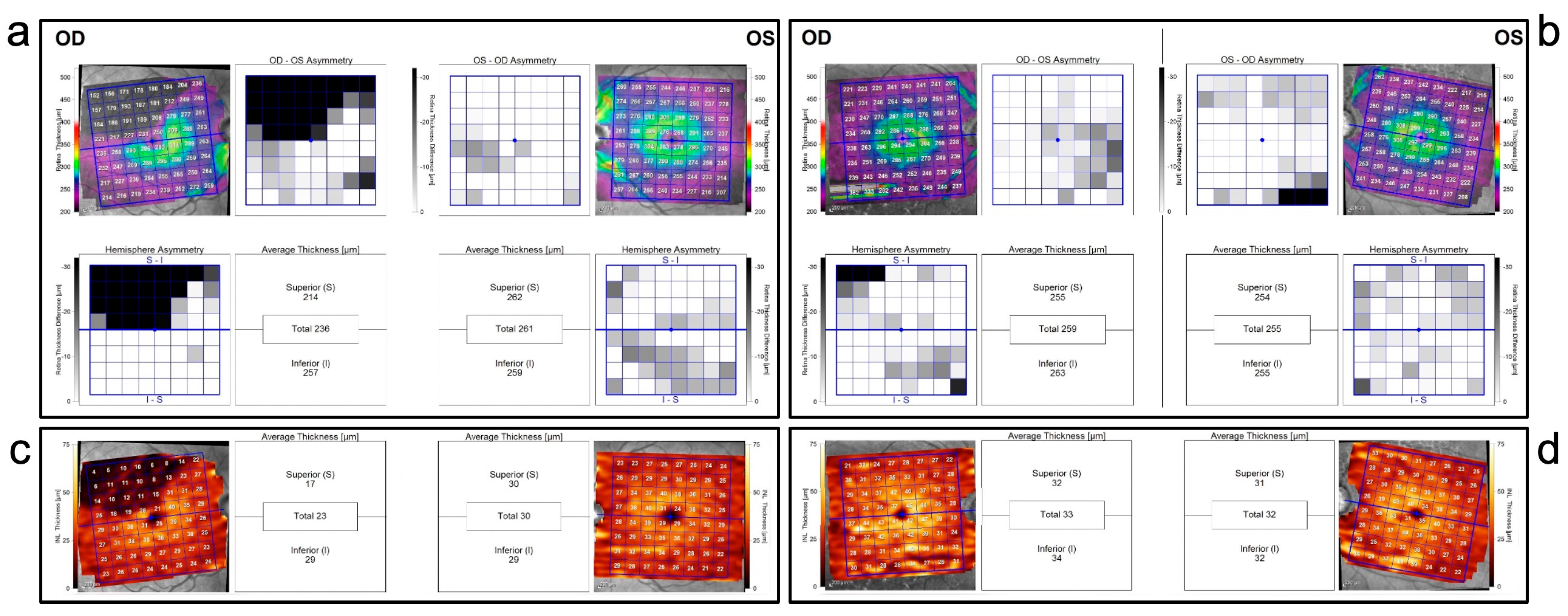
3.1. BRAO Cohort
3.2. POAG Cohort
3.3. Comparison between POAG and BRAO Cohort
4. Discussion
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayreh, S.; Zimmerman, M. Fundus changes in branch retinal arteriolar occlusion. Retina 2015, 35, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Feltgen, N.; Pielen, A. Retinaler arterienverschluss. Der Ophthalmol. 2017, 114, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Mee, M.; Amin, P.; Pensyl, D.; Katiyar, S. Differentiating occult branch retinal artery occlusion from primary open-angle glaucoma. Optom. Vis. Sci. 2018, 95, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Pang, C.; Gong, Y.; Freund, K.B.; Yannuzzi, L.A.; Rahimy, E.; Lujan, B.J.; Tabandeh, H.; Coonley, M.J.; Sarraf, D. The spectrum of superficial and deep capillary ischemia in retinal artery occlusion. Am. J. Ophthalmol. 2015, 159, 53–63.e2. [Google Scholar] [CrossRef]
- Shetty, R.; Bolling, J.; Stewart, M.; Heckman, M. Differences in optical coherence tomography of the macula in advanced glaucoma and after a retinal artery occlusion. Ophthalmic Surg. Lasers Imaging Retin. 2007, 38, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.; Richards, C.; Ghazi, N. Comparison of optical coherence tomography findings in a patient with central retinal artery occlusion in one eye and end-stage glaucoma in the fellow eye. Middle East Afr. J. Ophthalmol. 2012, 19, 247. [Google Scholar]
- Reis, A.; O’Leary, N.; Yang, H.; Sharpe, G.P.; Nicolela, M.T.; Burgoyne, C.F.; Chauhan, B.C. Influence of Clinically Invisible, but Optical Coherence Tomography Detected, Optic Disc Margin Anatomy on Neuroretinal Rim Evaluation. Investig. Opthalmol. Vis. Sci. 2012, 53, 1852. [Google Scholar] [CrossRef]
- SPECTRALIS Glaucoma Module Premium Edition|Heidelberg Engineering. Business-Lounge.heidelbergengineering.com. 2019. Available online: https://business-lounge.heidelbergengineering.com/us/en/products/spectralis/glaucoma-module/ (accessed on 15 September 2020).
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1998, 240, 1285–1293. [Google Scholar] [CrossRef]
- Jampol, L.M. Arteriolar occlusive diseases of the macula. Ophthalmology 1983, 90, 534–539. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Oltramari, L.; Sousa, N.A.; Makarczyk, L.S.Q.; Abe, R.Y. Differentiating Branch Retinal Artery Occlusion From Normal Tension Glaucoma With Optical Coherence Tomography Angiography. J. Glaucoma 2023, 32, e19–e23. [Google Scholar] [CrossRef]
- Choi, J.H.; Yang, H.K.; Lee, J.E. Incidental branch retinal artery occlusion on optical coherence tomography angiography presenting as segmental optic atrophy in a child: A case report. BMC Ophthalmol. 2017, 17, 256. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.M.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Longitudinal Changes of Retinal Thicknesses in Branch Retinal Artery Occlusion: Spectral-Domain Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.K.; Yang, J.Y.; Kim, S.S. Optical Coherence Tomography Measurement and Visual Outcome in Acute Central Retinal Artery Occlusion. Korean J. Ophthalmol. 2018, 32, 303–311. [Google Scholar] [CrossRef]
- Mirza, E.; Mirza, G.D.; Oltulu, R.; Belviranli, S.; Kerimoglu, H. Subclinical inner retinal layer thickness changes in the fellow eyes of patients with unilateral central retinal artery occlusion: A pilot study. Int. Ophthalmol. 2020, 40, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.G.; Tilton, E.P.; Patel, B.; Knape, R.M.; Newman, S.A. Comparison of macular optical coherence tomography findings between post-acute retinal artery occlusion and non-acute optic neuropathy. Retina 2010, 30, 578–585. [Google Scholar] [CrossRef]
- Dahrling, B.E., II. The histopathology of early central retinal artery occlusion. Arch. Ophthalmol. 1965, 73, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Zina, S.; Ksiaa, I.; Abdelhedi, C.; Ben Amor, H.; Attia, S.; Khochtali, S.; Khairallah, M. Multimodal imaging in IRVAN syndrome presenting with Branch Retinal Artery Occlusion. Eur. J. Ophthalmol. 2022, 32, NP28–NP32. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Budenz, D.L. Optical coherence tomography platforms and parameters for glaucoma diagnosis and progression. Curr. Opin. Ophthalmol. 2016, 27, 102–110. [Google Scholar] [CrossRef]
- Oddone, F.; Lucenteforte, E.; Michelessi, M.; Rizzo, S.; Donati, S.; Parravano, M.; Virgili, G. Macular versus Retinal Nerve Fiber Layer Parameters for Diagnosing Manifest Glaucoma: A Systematic Review of Diagnostic Accuracy Studies. Ophthalmology 2016, 123, 939–949. [Google Scholar] [CrossRef]
- Zangalli, C.S.; Ahmed, O.M.; Waisbourd, M.; HAli, M.; Cvintal, V.; Affel, E.; Gupta, L.; Katz, L.J.; Sergott, R.C. Segmental Analysis of Macular Layers in Patients with Unilateral Primary Open-Angle Glaucoma. J. Glaucoma 2016, 25, e401–e407. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Toschi, N.; Cesareo, M.; Giannini, C.; Pocobelli, G.; Garaci, F.; Mancino, R.; Nucci, C. Spectral domain optical coherence tomography assessment of macular and optic nerve alterations in patients with glaucoma and correlation with visual field index. J. Ophthalmol. 2018, 2018, 6581846. [Google Scholar] [CrossRef] [PubMed]
- Yuksel Elgin, C.; Chen, D.; Al-Aswad, L.A. Ophthalmic imaging for the diagnosis and monitoring of glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 183–197. [Google Scholar] [CrossRef]
- Deshpande, G.A.; Gupta, R.; Bawankule, P.; Raje, D.; Chakraborty, M. Evaluation of ganglion cell-inner plexiform layer thickness in the diagnosis of preperimetric glaucoma and comparison to retinal nerve fiber layer. Indian J. Ophthalmol. 2021, 69, 1113–1119. [Google Scholar] [CrossRef]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical Coherence Tomography Angiography in Glaucoma. J. Glaucoma 2020, 29, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zangwill, L.M.; Bowd, C. Retinal nerve fiber layer analysis in the diagnosis of glaucoma. Curr. Opin. Ophthalmol. 2006, 17, 120–131. [Google Scholar]
- Nakatani, Y.; Sugiyama, K. Comparison of the Structure-Function Relationship in Glaucoma Using Optical Microangiography in the Peripapillary Retinal Nerve Fiber Layer. J. Glaucoma 2022, 31, 160–169. [Google Scholar] [CrossRef]
- Leung, C.K.S.; Guo, P.Y.; Lam, A.K.N. Retinal Nerve Fiber Layer Optical Texture Analysis: Involvement of the Papillomacular Bundle and Papillofoveal Bundle in Early Glaucoma. Ophthalmology 2022, 129, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, T.W.; Weinreb, R.N.; Park, K.H.; Kim, S.H.; Kim, D.M. Detection of localized retinal nerve fiber layer defects with posterior pole asymmetry analysis of spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4347–4353. [Google Scholar] [CrossRef]
| Mean Values ± SD | p Value | |||||
|---|---|---|---|---|---|---|
| BRAO | Control | POAG | BRAO vs. Control | POAG vs. Control | POAG vs. BRAO | |
| All layers total | 265 ± 20 | 285 ± 25 | 273 ± 16 | 0.0016 | 0.1703 | 0.2024 |
| mRNFL total | 32 ± 6.0 | 39 ± 6.3 | 29 ± 7.9 | 0.0005 | 0.0008 | 0.0927 |
| GCL total | 24 ± 3.9 | 30 ± 5.0 | 27 ± 5.8 | 0.0006 | 0.0478 | 0.1234 |
| IPL total | 23 ± 3.3 | 26 ± 4.9 | 25 ± 4.7 | 0.0027 | 0.2349 | 0.0067 |
| INL total | 25 ± 3.0 | 31 ± 3.9 | 32 ± 3.2 | 0.0039 | 0.0424 | <0.0001 |
| Mean Values ± SD | p Value | |||||
|---|---|---|---|---|---|---|
| BRAO | Control | POAG | BRAO vs. Control | POAG vs. Control | POAG vs. BRAO | |
| All layers-Hemisphere asymmetry | 38 ± 18 | 5.6 ± 5.5 | 7.4 ± 6/0 | <0.0001 | 0.4817 | <0.0001 |
| mRNFL-Hemisphere asymmetry | 15 ± 8.1 | 5.7 ± 3.2 | 5.3 ± 4.4 | 0.0005 | 0.4904 | <0.0001 |
| GCL-Hemisphere asymmetry | 10 ± 4.1 | 1.8 ± 2.8 | 2.1 ± 1.6 | <0.0001 | 0.1177 | <0.0001 |
| IPL-Hemisphere asymmetry | 5.3 ± 2.7 | 1.2 ± 0.91 | 1.3 ± 1.2 | 0.0004 | 0.9434 | <0.0001 |
| INL-Hemisphere asymmetry | 9.9 ± 3.9 | 1.1 ± 1.8 | 0.75 ± 0.85 | <0.0001 | 0.8303 | <0.0001 |
| Parameter | AUC | Test Accuracy | p Value |
|---|---|---|---|
| All layers Hemisphere difference | 0.9563 | a | <0.0001 |
| mRNFL Hemisphere difference | 0.8844 | b | <0.0001 |
| GCL Hemisphere difference | 0.9720 | a | <0.0001 |
| IPL total | 0.7594 | b | 0.0082 |
| IPL Hemisphere difference | 0.8953 | b | <0.0001 |
| INL total | 0.9672 | a | <0.0001 |
| INL Hemisphere difference | 0.9781 | a | <0.0001 |
| NRR Thinning (G) | 0.9000 | a | <0.0001 |
| RNFL Thinning at 3.50 mm (G) | 0.8188 | b | 0.0012 |
| Mean Values ± SD | p Value | |||||
|---|---|---|---|---|---|---|
| BRAO | Control | POAG | BRAO vs. Control | POAG vs. Control | POAG vs. BRAO | |
| BMO area | 1.9 ± 0.44 | 1.9 ± 0.48 | 2 ± 0.43 | 0.99 | 0.9686 | 0.8689 |
| NRR Thinning (G) | 257 ± 45 | 281 ± 48 | 167 ± 52 | 0.006 | <0.0001 | <0.0001 |
| RNFL Thinning at 3.50 mm (G) | 87 ± 14 | 95 ± 11 | 67 ± 17 | 0.0245 | <0.0001 | 0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Salvo, G.; Oshallah, M.; Sepetis, A.E.; Borbara, R.; Oliverio, G.W.; Meduri, A.; Frisina, R.; Jacob, A. Inner Retinal Thinning Comparison between Branch Retinal Artery Occlusion and Primary Open-Angle Glaucoma. Diagnostics 2023, 13, 3428. https://doi.org/10.3390/diagnostics13223428
De Salvo G, Oshallah M, Sepetis AE, Borbara R, Oliverio GW, Meduri A, Frisina R, Jacob A. Inner Retinal Thinning Comparison between Branch Retinal Artery Occlusion and Primary Open-Angle Glaucoma. Diagnostics. 2023; 13(22):3428. https://doi.org/10.3390/diagnostics13223428
Chicago/Turabian StyleDe Salvo, Gabriella, Mohamed Oshallah, Anastasios E. Sepetis, Ramez Borbara, Giovanni William Oliverio, Alessandro Meduri, Rino Frisina, and Aby Jacob. 2023. "Inner Retinal Thinning Comparison between Branch Retinal Artery Occlusion and Primary Open-Angle Glaucoma" Diagnostics 13, no. 22: 3428. https://doi.org/10.3390/diagnostics13223428
APA StyleDe Salvo, G., Oshallah, M., Sepetis, A. E., Borbara, R., Oliverio, G. W., Meduri, A., Frisina, R., & Jacob, A. (2023). Inner Retinal Thinning Comparison between Branch Retinal Artery Occlusion and Primary Open-Angle Glaucoma. Diagnostics, 13(22), 3428. https://doi.org/10.3390/diagnostics13223428








