A Severity Comparison between Italian and Israeli Rett Syndrome Cohorts
Abstract
1. Introduction
1.1. Comparing Israeli and Italian Patients with RTT
- Genetic and environmental factors—Comparing individuals from different geographical regions allows researchers to investigate potential variations in genetic backgrounds and environmental factors that may influence the presentation and progression of RTT. Differences in genetic mutations or environmental exposures could contribute to distinct clinical features or responses to treatments.
- Phenotypic variability—RTT is known for its phenotypic variability, meaning that individuals with the same genetic mutation can exhibit a wide range of symptoms and functional abilities. By comparing individuals from different populations, researchers may identify specific patterns of symptoms and variations in the syndrome’s clinical manifestations.
- Therapeutic approaches—Comparative studies can shed light on the effectiveness of the different therapeutic approaches used in Israel and Italy. Variations in medical practices, therapeutic interventions, and healthcare systems could influence outcomes and provide valuable insights into optimizing treatments for RTT.
- Data generalizability—Research findings based on a diverse sample of individuals from Israel and Italy can lead to more generalizable conclusions about RTT. Having data from multiple populations strengthens the external validity of research studies, allowing for a broader application of findings to other populations worldwide.
- Cross-cultural perspectives—Comparing RTT cases between countries enables a cross-cultural perspective on caregiving practices, societal support, and family dynamics. Such an approach can help identify cultural factors that may influence the quality of life and care received by individuals with RTT.
- Identifying best practices—If there are notable differences in treatment outcomes or management strategies between the two countries, a comparative analysis can help identify “best practices” that lead to improved quality of life and functional outcomes for individuals with RTT.
1.2. Models of Caring in Italy and Israel
1.3. Study Objectives
2. Materials and Methods
2.1. Participants
2.2. Rett Assessment Rating Scale
- Cognitive area—This area addresses the compromised cognitive abilities in girls with RTT. Due to the initial regression, their cognitive level typically remains severely delayed. Precise indicators related to cognitive development include attentional abilities, spatial and temporal orientation, memory, verbal communication skills, non-verbal communication through facial expressions, the ability to maintain eye contact and shared attention, and the presence of responsive smiling.
- Sensory area—Girls with RTT may experience visual issues characterized by peripheral gaze and hearing problems involving fluctuations in auditory sensitivity. Therefore, two items related to vision and hearing are included in the RARS.
- Motor area—Motor difficulties in girls with RTT primarily affect their walking ability and hand stereotypes. The diagnostic criteria for RTT include the appearance of hand stereotypies such as hand-washing, hand-clapping, and hand-wringing, as well as the emergence of ataxic and apraxic gait and trunk movements. The motor area of the RARS includes four items related to the body, hands, scoliosis, and feet.
- Emotional area—People interacting with girls with RTT find it easy to establish connections with them as they respond to social stimuli and smile with an intense gaze. Their emotional states are typically related to their well-being [42]. The items in the emotional area concern the basic emotions (assessing the ability to express and understand emotions, including the emotions of others), mood swings, and anxiety, which are common in individuals affected by the syndrome.
- Autonomy area—This area includes evaluating the control of sphincters, the ability to feed independently, and skills related to personal hygiene, such as washing and dressing.
- Typical characteristics of RTT—These can be categorized into disease-related and behavioral features. The items related to disease-related features investigate the presence and intensity of epilepsy, convulsions, dyspnea crises, and aerophagia. The items concerning the behavioral features explore the presence of hyperactivity, aggressiveness, bruxism, eating preferences, oculogyric crises, and muscle tension.
- The last item of the test (no. 31) refers to the overall impression that parents or therapists, who fill out the RARS, have regarding the severity of the disease in the child.
2.3. Data Analysis
3. Results
4. Discussion
4.1. National Groups Comparison
- Arrival at school—The day begins with the girl’s arrival and a meeting between the caregiver and the special education teacher to exchange information about the girl’s status.
- Morning roll call—During the morning roll call, the girl is addressed and responds to her name in the best way she can. This response may involve eye-tracking technology, raising a hand, or occasionally using her voice.
- Girls’ attendance and responses—This step marks the attendance and engagement of the girl in the class. For example, if the lesson regards food, the child must choose the food she prefers. The choice could be made adequately for the girl’s ability (e.g., using eye-tracking technology with two stimuli, raising a hand, or using her voice).
- Individualized programs—Individualized programs are implemented throughout the day to cater to the girl’s specific needs and abilities. For example, if the girl can discriminate between the photo of the mother and father, another photo is presented to discriminate between other people. If she can bring food to the mouth with help, the teacher tries to fade the help.
- Meeting moments with classmates—The girl has scheduled moments for interaction with her classmates. These interactions are structured to promote social engagement.
- Adaptation of educational activities for participation—Educational activities are adapted and modified to ensure active participation by the girl, considering her unique requirements.
- Snack time—A designated time for snack breaks is provided during the school day together with their peers to ensure that the girls’ nutritional needs are met, taking advantage of the sociality of the meal.
- End-of-Day Greeting—The school day concludes with an end-of-day greeting, providing closure to the day’s activities.
- Resource allocation—It comprises the resources needed for dedicated personnel (special educational teacher, educator, support staff), structural interventions to overcome the architectural barriers (to make the scholar environment accessible), specific educational materials (including the time to construct it), and training courses (in general about inclusion strategies and specifically about the person’s disorder).
- Protocols—This refers to pre-established practices of the school as a system aimed at facilitating the inclusion process. It includes dedicated times for discussion (between the teachers, family members, and clinicians) and training courses, specific evaluation protocols of the inclusion process, semi-structured observation of the person with a disability, classroom and educational environment, and semi-structured processes of individualized educational intervention planning.
- Observation—the observation process is highly demanding for the teaching staff, but it significantly simplifies all subsequent activities if well-planned and conducted. It benefits from input from all involved with the student and proceeds from the general to the specific observation of spontaneous and facilitated activities through practical tests. Elements of interest during the observation of the students with disabilities include the skills possessed in various areas of development, what they can do consistently, occasionally, and with the teacher’s assistance, how the attitude and approach to interaction (of the student towards the context and vice versa), what the teachers can do to promote their functioning and interaction (what facilitates and what limits), which realistic progress can be most important for the student’s quality of life, and what motivational factors can be used.
- Planning—It refers to identifying the specific educational objectives and strategies to be implemented. Accurate planning cannot be done without comprehensive observation and allows the class staff to know what to do, how, and when. The educational objectives identification should follow the SMART principles (Specific, Measurable, Achievable, Realistic, and Time-bound) [63]. The educational strategies should be selected to pursue the objectives and adapted based on identified facilitators, barriers, and motivational factors. Moreover, the planning process also benefits from discussing with family members and clinicians to align objectives and strategies to pursue a shared goal. Finally, the evaluation time points and the strategies to assess the attainment of the objectives within and at the end of the school year should be planned a priori.
4.2. Age Groups Comparison
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petriti, U.; Dudman, D.C.; Scosyrev, E.; Lopez-Leon, S. Global Prevalence of Rett Syndrome: Systematic Review and Meta-Analysis. Syst. Rev. 2023, 12, 5. [Google Scholar] [CrossRef]
- Laurvick, C.L.; de Klerk, N.; Bower, C.; Christodoulou, J.; Ravine, D.; Ellaway, C.; Williamson, S.; Leonard, H. Rett Syndrome in Australia: A Review of the Epidemiology. J. Pediatr. 2006, 148, 347–352. [Google Scholar] [CrossRef]
- Baptista, P.M.; Mercadante, M.T.; Macedo, E.C.; Schwartzman, J.S. Cognitive Performance in Rett Syndrome Girls: A Pilot Study Using Eyetracking Technology. J. Intellect. Disabil. Res. 2006, 50, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, J.; Haas, R.H. Cognitive Profile of Rett Syndrome. J. Child Neurol. 1988, 3, 20–24. [Google Scholar] [CrossRef]
- Demeter, K. Assessing the Developmental Level in Rett Syndrome: An Alternative Approach? Eur. Child Adolesc. Psychiatry 2000, 9, 227–233. [Google Scholar] [CrossRef]
- Byiers, B.; Symons, F. The Need for Unbiased Cognitive Assessment in Rett Syndrome: Is Eye Tracking the Answer? Dev. Med. Child. Neurol. 2013, 55, 301–302. [Google Scholar] [CrossRef]
- Byiers, B.J.; Symons, F.J. Issues in Estimating Developmental Level and Cognitive Function in Rett Syndrome. Int. Rev. Res. Dev. Disabil. 2012, 43, 147–185. [Google Scholar] [CrossRef]
- Hetzroni, O.; Rubin, C.; Konkol, O. The Use of Assistive Technology for Symbol Identification by Children with Rett Syndrome. J. Intellect. Dev. Disabil. 2002, 27, 57–71. [Google Scholar] [CrossRef]
- Djukic, A.; Valicenti McDermott, M. Social Preferences in Rett Syndrome. Pediatr. Neurol. 2012, 46, 240–242. [Google Scholar] [CrossRef]
- Bartolotta, T.E.; Zipp, G.P.; Simpkins, S.D.; Glazewski, B. Communication Skills in Girls with Rett Syndrome. Focus Autism Other Dev. Disabl. 2011, 26, 15–24. [Google Scholar] [CrossRef]
- Didden, R.; Korzilius, H.; Smeets, E.; Green, V.A.; Lang, R.; Lancioni, G.E.; Curfs, L.M. Communication in Individuals with Rett Syndrome: An Assessment of Forms and Functions. J. Dev. Phys. Disabil. 2010, 22, 105–118. [Google Scholar] [CrossRef]
- Urbanowicz, A.; Downs, J.; Girdler, S.; Ciccone, N.; Leonard, H. Aspects of Speech-Language Abilities Are Influenced by MECP2 Mutation Type in Girls with Rett Syndrome. Am. J. Med. Genet. A 2015, 167A, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, A.; Leonard, H.; Girdler, S.; Ciccone, N.; Downs, J. Parental Perspectives on the Communication Abilities of Their Daughters with Rett Syndrome. Dev. Neurorehabil. 2016, 19, 17–25. [Google Scholar] [CrossRef]
- Hagberg, B. Clinical Manifestations and Stages of Rett Syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 61–65. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.M.; Bird, A.; Coenraads, M.; Gray, S.J.; Menon, D.U.; Philpot, B.D.; Tarquinio, D.C. Rett Syndrome: Crossing the Threshold to Clinical Translation. Trends Neurosci. 2016, 39, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.B.; Lee, H.S.; Smith, L.W.; Cheng, P.; Percy, A.K.; Glaze, D.G.; Neul, J.L.; Motil, K.J.; Barrish, J.O.; Skinner, S.A.; et al. Clinical Severity and Quality of Life in Children and Adolescents with Rett Syndrome. Neurology 2011, 77, 1812–1818. [Google Scholar] [CrossRef]
- Lee, J.Y.L.; Leonard, H.; Piek, J.P.; Downs, J. Early Development and Regression in Rett Syndrome. Clin. Genet. 2013, 84, 572–576. [Google Scholar] [CrossRef]
- de Monteiro, C.B.M.; Savelsbergh, G.J.; Smorenburg, A.R.; Graciani, Z.; Torriani-Pasin, C.; de Abreu, L.C.; Valenti, V.E.; Kok, F.; Monteiro, C.B.; Savelsbergh, G.J.; et al. Quantification of Functional Abilities in Rett Syndrome: A Comparison between Stages III and IV. Neuropsychiatr. Dis. Treat. 2014, 10, 1213. [Google Scholar] [CrossRef]
- Humphreys, P.; Barrowman, N. The Incidence and Evolution of Parkinsonian Rigidity in Rett Syndrome: A Pilot Study. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2016, 43, 567–573. [Google Scholar] [CrossRef]
- Smeets, E.E.; Schrander-Stumpel, C.T.R.M. Rett Syndrome. In Management of Genetic Syndromes, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA; Department of Clinical Genetics, Academic Hospital Maastricht: Maastricht, The Netherlands, 2010; pp. 677–691. ISBN 9780470191415. [Google Scholar]
- Sansom, D.; Krishnan, V.H.R.; Corbett, J.; Kerr, A. Emotional And Behavioural Aspects of Rett Syndrome. Dev. Med. Child Neurol. 2008, 35, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Mount, R.H.; Hastings, R.P.; Reilly, S.; Cass, H.; Charman, T. Behaviour Problems in Adult Women with Rett Syndrome. J. Intellect. Disabil. Res. 2002, 46, 619–624. [Google Scholar] [CrossRef]
- Mount, R.H.; Hastings, R.P.; Reilly, S.; Cass, H.; Charman, T. Towards a Behavioral Phenotype for Rett Syndrome. Am. J. Ment. Retard. 2003, 108, 1–12. [Google Scholar] [CrossRef]
- Lotan, M.; Zwilling, M.; Romano, A. Psychometric Values of a New Scale: The Rett Syndrome Fear of Movement Scale (RSFMS). Diagnostics 2023, 13, 2148. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B. Rett Syndrome: Clinical Peculiarities and Biological Mysteries. Acta Paediatr. 2008, 84, 971–976. [Google Scholar] [CrossRef]
- Hagberg, B.; Anvret, M.; Wahlstrom, J.; Wahlström, J. Rett Syndrome-Clinical and Biological Aspects: Studies on 130 Swedish Females; Cambridge University Press: London, UK, 1993; ISBN 0521412838. [Google Scholar]
- Fabio, R.A.; Colombo, B.; Russo, S.; Cogliati, F.; Masciadri, M.; Foglia, S.; Antonietti, A.; Tavian, D. Recent Insights into Genotype-Phenotype Relationships in Patients with Rett Syndrome Using a Fine Grain Scale. Res. Dev. Disabil. 2014, 35, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, A.; Anderson, A.; Ravine, D.; Fyfe, S.; Pineda, M.; de Klerk, N.; Ben-Zeev, B.; Yatawara, N.; Percy, A.; Kaufmann, W.E. Investigating Genotype–Phenotype Relationships in Rett Syndrome Using an International Data Set. Neurology 2008, 70, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Halbach, N.S.J.; Smeets, E.E.J.; van den Braak, N.; van Roozendaal, K.E.P.; Blok, R.M.J.; Schrander-Stumpel, C.T.R.M.; Frijns, J.P.; Maaskant, M.A.; Curfs, L.M.G. Genotype-Phenotype Relationships as Prognosticators in Rett Syndrome Should Be Handled with Care in Clinical Practice. Am. J. Med. Genet. A 2012, 158A, 340–350. [Google Scholar] [CrossRef]
- Fabio, R.A.; Giannatiempo, S.; Semino, M.; Caprì, T. Longitudinal Cognitive Rehabilitation Applied with Eye-Tracker for Patients with Rett Syndrome. Res. Dev. Disabil. 2021, 111, 103891. [Google Scholar] [CrossRef]
- Migliorelli, C.; Medina-Rivera, I.; Bachiller, A.; Tost, A.; Alonso, J.F.; López-Sala, A.; Armstrong, J.; O’Callahan, M.d.M.; Pineda, M.; Mañanas, M.A.; et al. Cognitive stimulation has potential for brain activation in individuals with Rett syndrome. J. Intellect. Disabil. Res. 2022, 66, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Byiers, B.J.; Dimian, A.; Symons, F.J. Functional Communication Training in Rett Syndrome: A Preliminary Study. Am. J. Intellect. Dev. Disabil. 2014, 119, 340–350. [Google Scholar] [CrossRef]
- Fabio, R.A.; Castelli, I.; Marchetti, A.; Antonietti, A. Training Communication Abilities in Rett Syndrome through Reading and Writing. Front. Psychol. 2013, 4, 911. [Google Scholar] [CrossRef]
- Romano, A.; Ippolito, E.; Risoli, C.; Malerba, E.; Favetta, M.; Sancesario, A.; Lotan, M.; Moran, D.S. Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome. J. Clin. Med. 2022, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, M. Health-Enhancing Participation in Girls and Women with Rett Syndrome—A Balancing Act; Lund University: Lund, Sweden, 2018; ISBN 978-91-7619-646-5. [Google Scholar]
- Stahlhut, M.; Downs, J.; Wong, K.; Bisgaard, A.M.; Nordmark, E. Feasibility and Effectiveness of an Individualized 12-Week “Uptime” Participation (U-PART) Intervention in Girls and Women With Rett Syndrome. Phys. Ther. 2020, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Cianfaglione, R.; Clarke, A.; Kerr, M.; Hastings, R.P.; Oliver, C.; Felce, D. Ageing in Rett Syndrome. J. Intellect. Disabil. Res. 2016, 60, 182–190. [Google Scholar] [CrossRef]
- Good, K.V.; Vincent, J.B.; Ausió, J. MeCP2: The Genetic Driver of Rett Syndrome Epigenetics. Front. Genet. 2021, 12, 620895. [Google Scholar] [CrossRef]
- Rodocanachi Roidi, M.L.; Isaias, I.U.; Cozzi, F.; Grange, F.; Scotti, F.M.; Gestra, V.F.; Gandini, A.; Ripamonti, E. Motor Function in Rett Syndrome: Comparing Clinical and Parental Assessments. Dev. Med. Child Neurol. 2019, 61, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Lotan, M.; Ippolito, E.; Favetta, M.; Romano, A. Skype Supervised, Individualized, Home-Based Rehabilitation Programs for Individuals With Rett Syndrome and Their Families—Parental Satisfaction and Point of View. Front. Psychol. 2021, 12, 3995. [Google Scholar] [CrossRef]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Development and Standardization of the “Rars”(Rett Assessment Rating Scale). Life Span. Disabil. 2005, 8, 257–281. [Google Scholar]
- Martínez De Paz, A.; Khajavi, L.; Martin, H.; Claveria-Gimeno, R.; Tom Dieck, S.; Cheema, M.S.; Sanchez-Mut, J.V.; Moksa, M.M.; Carles, A.; Brodie, N.I.; et al. MeCP2-E1 Isoform Is a Dynamically Expressed, Weakly DNA-Bound Protein with Different Protein and DNA Interactions Compared to MeCP2-E2. Epigenet. Chromatin 2019, 12, 63. [Google Scholar] [CrossRef]
- Sheikh, T.I.; de Paz, A.M.; Akhtar, S.; Ausió, J.; Vincent, J.B. MeCP2_E1 N-Terminal Modifications Affect Its Degradation Rate and Are Disrupted by the Ala2Val Rett Mutation. Hum. Mol. Genet. 2017, 26, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Yang, Y.; Uvarov, O.; Cao, W.; Alexov, E. Impact of Rett Syndrome Mutations on MeCP2 MBD Stability. Biochemistry 2015, 54, 6357–6368. [Google Scholar] [CrossRef]
- Yang, Y.; Kucukkal, T.G.; Li, J.; Alexov, E.; Cao, W. Binding Analysis of Methyl-CpG Binding Domain of MeCP2 and Rett Syndrome Mutations. ACS Chem. Biol. 2016, 11, 2706–2715. [Google Scholar] [CrossRef]
- Moncla, A.; Kpebe, A.; Missirian, C.; Mancini, J.; Villard, L. Polymorphisms in the C-Terminal Domain of MECP2 in Mentally Handicapped Boys: Implications for Genetic Counselling. Eur. J. Hum. Genet. 2002, 10, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, A.; Percy, A.; Christodoulou, J.; Ravine, D.; Ho, G.; Jacoby, P.; Anderson, A.; Pineda, M.; Ben Zeev, B.; Bahi-Buisson, N.; et al. Updating the Profile of C-Terminal MECP2 Deletions in Rett Syndrome. J. Med. Genet. 2010, 47, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lyst, M.J.; Ekiert, R.; Ebert, D.H.; Merusi, C.; Nowak, J.; Selfridge, J.; Guy, J.; Kastan, N.R.; Robinson, N.D.; de Lima Alves, F.; et al. Rett Syndrome Mutations Abolish the Interaction of MeCP2 with the NCoR/SMRT Co-Repressor. Nat. Neurosci. 2013, 16, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, T.; Ghosh, R.P.; Horowitz-Scherer, R.A.; Hansen, J.C.; Grigoryev, S.A.; Woodcock, C.L. MeCP2-Chromatin Interactions Include the Formation of Chromatosome-like Structures and Are Altered in Mutations Causing Rett Syndrome. J. Biol. Chem. 2007, 282, 28237–28245. [Google Scholar] [CrossRef]
- Kerr, A.; Engerström, I.W. Rett Disorder and the Developing Brain; Oxford Medical Publications; Oxford University Press: Oxford, UK, 2001; ISBN 9780192630834. [Google Scholar]
- Downs, J.; Rodger, J.; Li, C.; Tan, X.; Hu, N.; Wong, K.; De Klerk, N.; Leonard, H. Environmental Enrichment Intervention for Rett Syndrome: An Individually Randomised Stepped Wedge Trial. Orphanet J. Rare Dis. 2018, 13, 3. [Google Scholar] [CrossRef]
- Leonard, H.; Ravikumara, M.; Baikie, G.; Naseem, N.; Ellaway, C.; Percy, A.; Abraham, S.; Geerts, S.; Lane, J.; Jones, M.; et al. Assessment and management of nutrition and growth in Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 451. [Google Scholar] [CrossRef]
- Jefferson, A.; Leonard, H.; Siafarikas, A.; Woodhead, H.; Fyfe, S.; Ward, L.M.; Munns, C.; Motil, K.; Tarquinio, D.; Shapiro, J.R.; et al. Clinical Guidelines for Management of Bone Health in Rett Syndrome Based on Expert Consensus and Available Evidence. PLoS ONE 2016, 11, e0146824. [Google Scholar] [CrossRef]
- Sernheim, Å.S.; Hemmingsson, H.; Witt Engerström, I.; Liedberg, G. Activities That Girls and Women with Rett Syndrome Liked or Did Not like to Do. Scand. J. Occup. Ther. 2018, 25, 267–277. [Google Scholar] [CrossRef]
- Fonzo, M.; Sirico, F.; Corrado, B. Evidence-based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review. Brain Sci. 2020, 10, 410. [Google Scholar] [CrossRef]
- Kerr, A.M. Reflections on the Constraints and Opportunities in Therapy in Rett Syndrome. Sci. World J. 2006, 6, 992–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lotan, M.; Stahlhut, M.; Romano, A.; Downs, J.; Elefant, C. Family-Centered Telehealth Supporting Motor Skills and Activity in Individuals With Rett Syndrome. In Assistive Technologies for Assessment and Recovery of Neurological Impairments; Stasolla, F., Ed.; IGI Global: Hershey, PA, USA, 2022; pp. 147–171. [Google Scholar]
- Stahlhut, M.; Esbensen, B.A.; Larsen, J.L.; Bisgaard, A.M.; Downs, J.; Nordmark, E. Facilitators and Barriers of Participation in “Uptime” Activities in Girls and Women With Rett Syndrome: Perspectives From Parents and Professionals. Qual. Health Res. 2019, 29, 609–619. [Google Scholar] [CrossRef]
- Fryxell, D.; Kennedy, C.H. Placement along the Continuum of Services and Its Impact on Students’ Social Relationships. J. Assoc. Pers. Sev. Handicap. 1995, 20, 259–269. [Google Scholar] [CrossRef]
- Evans, I.M.; Salisbury, C.L.; Palombaro, M.M.; Berryman, J.; Hollowood, T.M. Peer Interactions and Social Acceptance of Elementary-Age Children with Severe Disabilities in an Inclusive School. J. Assoc. Pers. Sev. Handicap. 1992, 17, 205–212. [Google Scholar] [CrossRef]
- Evans, M.I.; Meyer, L.H. Having Friends and Rett Syndrome: How Social Relationships Create Meaningful Contexts for Limited Skills. Disabil. Rehabil. 2001, 23, 167–176. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.H.; Botell, R.E.; Wade, D.T. Writing SMART Rehabilitation Goals and Achieving Goal Attainment Scaling: A Practical Guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Halbach, N.S.J.; Smeets, E.E.J.; Steinbusch, C.; Maaskant, M.A.; van Waardenburg, D.; Curfs, L.M.G. Aging in Rett Syndrome: A Longitudinal Study. Clin. Genet. 2013, 84, 223–229. [Google Scholar] [CrossRef]
- Halbach, N.S.J.; Smeets, E.E.J.; Schrander-Stumpel, C.T.R.M.; van Schrojenstein Lantman de Valk, H.H.J.; Maaskant, M.A.; Curfs, L.M.G. Aging in People with Specific Genetic Syndromes: Rett Syndrome. Am. J. Med. Genet. A 2008, 146A, 1925–1932. [Google Scholar] [CrossRef]
- Killian, J.T.; Lane, J.B.; Lee, H.S.; Skinner, S.A.; Kaufmann, W.E.; Glaze, D.G.; Neul, J.L.; Percy, A.K. Scoliosis in Rett Syndrome: Progression, Comorbidities, and Predictors. Pediatr. Neurol. 2017, 70, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Bergman, A.; Carter, P.; Anderson, A.; Palmer, G.M.; Roye, D.; van Bosse, H.; Bebbington, A.; Larsson, E.L.; Smith, B.G.; et al. Guidelines for Management of Scoliosis in Rett Syndrome Patients Based on Expert Consensus and Clinical Evidence. Spine 2009, 34, E607–E617. [Google Scholar] [CrossRef]
| MeCP2 Mutation | MeCP2 Domain | Number of Subjects (%) | |
|---|---|---|---|
| Israeli | Italian | ||
| R168X | Others | 5 (14%) | 4 (6%) |
| R255X | Others | 3 (9%) | 7 (10%) |
| R270X | Others | 4 (11%) | 8 (11%) |
| R133C | MBD | 1 (3%) | 3 (4%) |
| T158M | MBD | 4 (11%) | 13 (18%) |
| R306C | Others | 0 (0%) | 7 (10%) |
| R106W | Others | 3 (9%) | 1 (1%) |
| R294X | Others | 0 (0%) | 4 (6%) |
| C-terminal deletions | Others | 5 (14%) | 9 (13%) |
| Others | Various * | 10 (29%) | 15 (21%) |
| Total | 35 (100%) | 71 (100%) | |
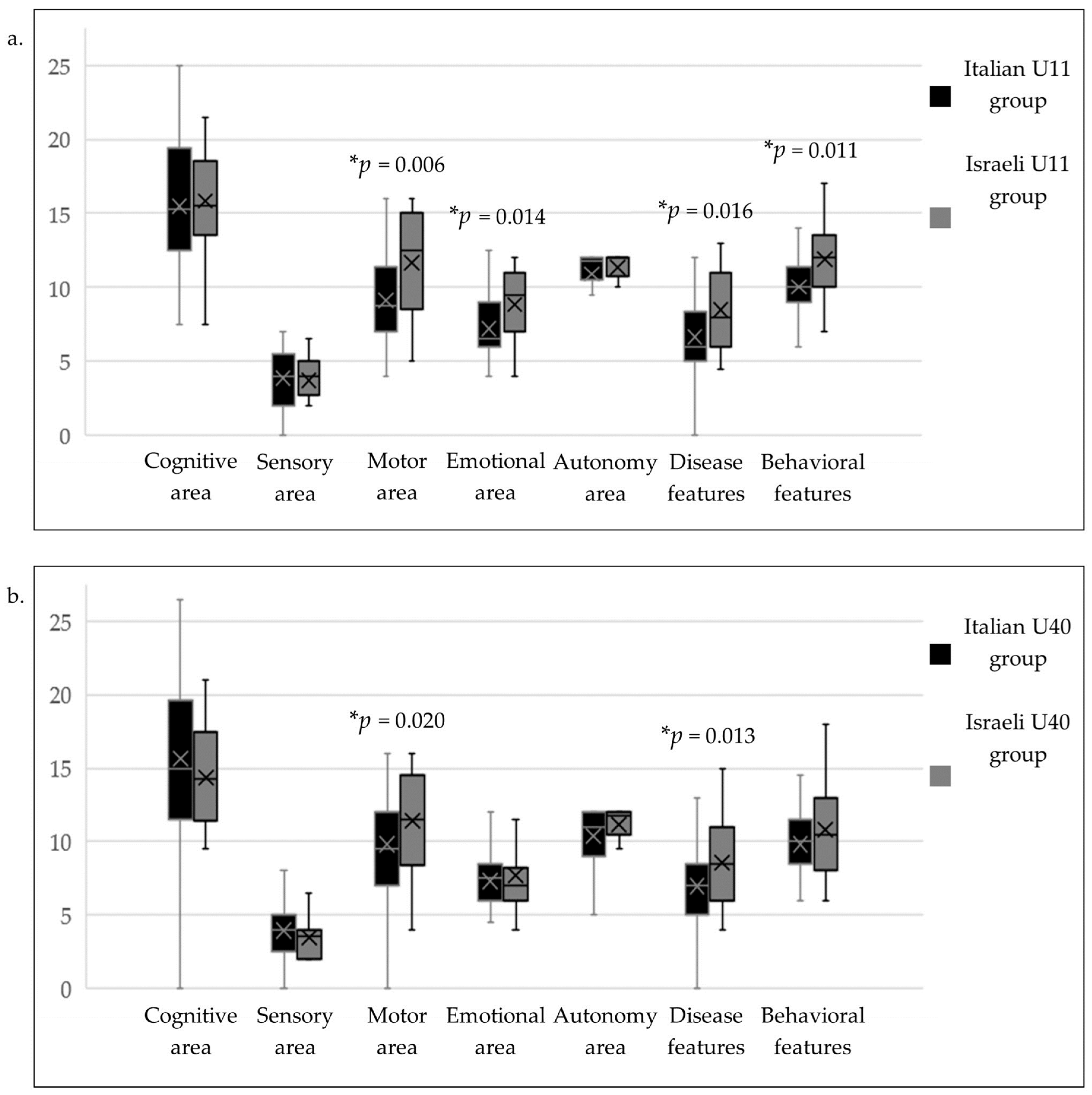
| U11 | U40 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Italian | Israeli | p-Value Ita vs. Is | Italian | Israeli | p-Value Ita vs. Is | |||||
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | |||
| Cognitive area | 15.7 (4.8) | 15.5 | 15.8 (4.3) | 15.5 | 0.944 | 15.8 (5.0) | 15.0 | 14.3 (3.4) | 14.3 | 0.190 |
| (25.0–7.0) | (27.5–7.5) | (26.5–7.5) | (21.0–9.5) | |||||||
| Sensory area | 4.0 (1.7) | 4.0 | 3.7 (1.2) | 4.0 | 0.720 | 4.0 (1.6) | 4.0 | 3.4 (1.4) | 3.5 | 0.101 |
| (7.0–2.0) | (6.5–2.0) | (8.0–2.0) | (6.5–2.0) | |||||||
| Motor area | 9.3 (2.8) | 9.0 | 11.6 (3.5) | 12.5 | 0.006 * | 9.9 (3.1) | 9.5 | 11.4 (3.3) | 11.5 | 0.020 * |
| (16.0–4.0) | (16.0–5.0) | (16.0–5.0) | (16.0–4.0) | |||||||
| Emotional area | 7.3 (2.2) | 6.5 | 8.9 (2.4) | 9.5 | 0.014 * | 7.4 (1.8) | 7.5 | 7.7 (2.4) | 7.0 | 0.991 |
| (12.5–4.0) | (12.0–4.0) | (12.0–4.5) | (13.0–4.0) | |||||||
| Autonomy area | 11.1 (1.3) | 12.0 | 11.3 (1.2) | 12.0 | 0.406 | 10.5 (1.8) | 11.0 | 11.2 (1.3) | 11.8 | 0.082 |
| (12.0–6.0) | (12.0–7.5) | (12.0–5.0) | (12.0–6.0) | |||||||
| Disease features | 6.8 (2.1) | 6.0 | 8.5 (2.8) | 8.0 | 0.016 * | 7.1 (2.3) | 7.0 | 8.5 (3.1) | 8.5 | 0.013 * |
| (12.0–4.0) | (13.0–4.5) | (14.0–4.0) | (15.0–4.0) | |||||||
| Behavioral features | 10.2 (2.1) | 10.0 | 11.9 (2.6) | 12.0 | 0.011 * | 9.9 (2.1) | 10.0 | 10.8 (3.0) | 10.5 | 0.236 |
| (15.0–6.0) | (17.0–7.0) | (14.5–6.0) | (18.0–6.0) | |||||||
| Total | 67.4 (13.0) | 66.5 | 74.9 (11.7) | 75.5 | 0.039 * | 67.6 (12.8) | 65.5 | 70.7 (12.8) | 68.0 | 0.210 |
| (95.5–37.0) | (95.0–54.0) | (97.0–41.0) | (99.5–46.0) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Lotan, M.; Fabio, R.A. A Severity Comparison between Italian and Israeli Rett Syndrome Cohorts. Diagnostics 2023, 13, 3390. https://doi.org/10.3390/diagnostics13213390
Romano A, Lotan M, Fabio RA. A Severity Comparison between Italian and Israeli Rett Syndrome Cohorts. Diagnostics. 2023; 13(21):3390. https://doi.org/10.3390/diagnostics13213390
Chicago/Turabian StyleRomano, Alberto, Meir Lotan, and Rosa Angela Fabio. 2023. "A Severity Comparison between Italian and Israeli Rett Syndrome Cohorts" Diagnostics 13, no. 21: 3390. https://doi.org/10.3390/diagnostics13213390
APA StyleRomano, A., Lotan, M., & Fabio, R. A. (2023). A Severity Comparison between Italian and Israeli Rett Syndrome Cohorts. Diagnostics, 13(21), 3390. https://doi.org/10.3390/diagnostics13213390






