Relationships between Heart Chamber Morphology or Function and Respiratory Parameters in Patients with HFrEF and Various Types of Sleep-Disordered Breathing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Echocardiographic Measurements
2.3. Polysomnographic Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javaheri, S. Sleep Disorders in Systolic Heart Failure: A Prospective Study of 100 Male Patients. The Final Report. Int. J. Cardiol. 2006, 106, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-Disordered Breathing in Patients with Symptomatic Heart Failure. A Contemporary Study of Prevalence in and Characteristics of 700 Patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yumino, D.; Wang, H.; Floras, J.S.; Newton, G.E.; Mak, S.; Ruttanaumpawan, P.; Parker, J.D.; Bradley, T.D. Prevalence and Physiological Predictors of Sleep Apnea in Patients With Heart Failure and Systolic Dysfunction. J. Card. Fail. 2009, 15, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Stat, D.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K. Prevalence and Predictors of Sleep-Disordered Breathing in Patients with Stable Chronic Heart Failure the SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Blau, A.; Börgel, J.; Duchna, H.W.; Fietze, I.; Koper, I.; Prenzel, R.; Schädlich, S.; Schmitt, J.; Tasci, S.; et al. Sleep Apnoea in Heart Failure. Eur. Respir. J. 2007, 29, 1201–1205. [Google Scholar] [CrossRef]
- Ayas, N.T. Risk Factors for Obstructive Sleep Apnea. Encycl. Sleep 2013, 291, 212–214. [Google Scholar] [CrossRef]
- Pływaczewski, R.; Bednarek, M.; Jonczak, L.; Zieliński, J. Sleep-Disordered Breathing in a Middle-Aged and Older Polish Urban Population. J. Sleep Res. 2008, 17, 73–81. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; Ten Have, T.; Leiby, B.E.; Vela-Bueno, A.; Kales, A. Association of Hypertension and Sleep-Disordered Breathing. Arch. Intern. Med. 2000, 160, 2289–2295. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. The Sympathetic Nervous System and Obstructive Sleep Apnea: Implications for Hypertension. J. Hypertens. 1997, 15, 1613–1619. [Google Scholar] [CrossRef]
- Patel, N.; Donahue, C.; Shenoy, A.; Patel, A.; El-Sherif, N. Obstructive Sleep Apnea and Arrhythmia: A Systemic Review. Int. J. Cardiol. 2017, 228, 967–970. [Google Scholar] [CrossRef]
- Mooe, T.; Franklin, K.A.; Holmstrom, K.; Rabben, T.; Wiklund, U. Sleep-Disordered Breathing and Coronary Artery Disease: Long-Term Prognosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Mohsenin, V. Sleep-Related Breathing Disorders and Risk of Stroke. Stroke 2001, 32, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.; Liu, P.P.; Colloway, R.; Rutherford, R.; Xie, A.; Bradley, T.D. Obstructive Sleep Apnoea in Patients with Dilated Cardiomyopathy: Effects of Continuous Positive Airway Pressure. Lancet 1991, 338, 1480–1484. [Google Scholar]
- Dredla, B.K.; Castillo, P.R. Cardiovascular Consequences of Obstructive Sleep Apnea. Curr. Cardiol. Rep. 2019, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive Sleep Apnea and the Risk of Sudden Cardiac Death: A Longitudinal Study of 10,701 Adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef]
- Javaheri, S.; Blackwell, T.; Ancoli-Israel, S.; Ensrud, K.E.; Stone, K.L.; Redline, S. Sleep-Disordered Breathing and Incident Heart Failure in Older Men. Am. J. Respir. Crit. Care Med. 2016, 193, 561–568. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. The Present and Future State-of-the-Art Rreview Sleep Apnea Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Yumino, D.; Redolfi, S.; Ruttanaumpawan, P.; Su, M.C.; Smith, S.; Newton, G.E.; Mak, S.; Bradley, T.D. Nocturnal Rostral Fluid Shift: A Unifying Concept for the Pathogenesis of Obstructive and Central Sleep Apnea in Men with Heart Failure. Circulation 2010, 121, 1598–1605. [Google Scholar] [CrossRef]
- Perger, E.; Jutant, E.M.; Redolfi, S. Targeting Volume Overload and Overnight Rostral Fluid Shift: A New Perspective to Treat Sleep Apnea. Sleep Med. Rev. 2018, 42, 160–170. [Google Scholar] [CrossRef]
- White, L.H.; Bradley, T.D. Role of Nocturnal Rostral Fluid Shift in the Pathogenesis of Obstructive and Central Sleep Apnoea. J. Physiol. 2013, 591, 1179–1193. [Google Scholar] [CrossRef]
- Solin, P.; Bergin, P.; Richardson, M.; Kaye, D.M.; Walters, E.H.; Naughton, M.T. Influence of Pulmonary Capillary Wedge Pressure on Central Apnea in Heart Failure. Circulation 1999, 99, 1574–1579. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Sleep Apnea and Heart Failure—Part II: Central Sleep Apnea. Circulation 2003, 107, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Lyons, O.D.; Bradley, T.D. Heart Failure and Sleep Apnea. Can. J. Cardiol. 2015, 31, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Ammon, S. Managing Patients with Heart Failure. Am. J. Nurs. 2001, 101, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.; Javaheri, S. Cardiovascular Effects of Sleep-Related Breathing Disorders. In Principles and Prac-Tices of Sleep Medicine, 6th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1243–1252. [Google Scholar]
- Lattimore, J.D.; Celermajer, D.S.; Wilcox, I. Obstructive Sleep Apnea and Cardiovascular Disease. J. Am. Coll. Cardiol. 2003, 41, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Damy, T.; Margarit, L.; Noroc, A.; Bodez, D.; Guendouz, S.; Boyer, L.; Drouot, X.; Stoica, M.; Paulino, A.; Adnot, S.; et al. Prognostic Impact of Sleep-Disordered Breathing and Its Treatment with Nocturnal Ventilation for Chronic Heart Failure. Eur. J. Heart Fail. 2012, 14, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Young, T.; Finn, L.; Skatrud, J.B.; Ryan, C.M.; Newton, G.E.; Mak, S.; Parker, J.D.; Floras, J.S.; Bradley, T.D. Sleepiness and Sleep in Patients with Both Systolic Heart Failure and Obstructive Sleep Apnea. Arch. Intern. Med. 2006, 166, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Ahbab, S.; Ataoǧlu, H.E.; Tuna, M.; Karasulu, L.; Çetin, F.; Temiz, L.Ü.; Yenigün, M. Neck Circumference, Metabolic Syndrome and Obstructive Sleep Apnea Syndrome; Evaluation of Possible Linkage. Med. Sci. Monit. 2013, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67, Erratum in Circulation 2022, 145, e775. [Google Scholar] [CrossRef]
- Hastings, P.C.; Vazir, A.; O’Driscoll, D.M.; Morrell, M.J.; Simonds, A.K. Symptom Burden of Sleep-Disordered Breathing in Mild-to-Moderate Congestive Heart Failure Patients. Eur. Respir. J. 2006, 27, 748–755. [Google Scholar] [CrossRef]
- Javaheri, S.; Parker, T.J.; Liming, J.D.; Corbett, W.S.; Nishiyama, H.; Wexler, L.; Roselle, G.A. Sleep Apnea in 81 Ambulatory Male Patients with Stable Heart Failure: Types and Their Prevalences, Consequences, and Presentations. Circulation 1998, 97, 2154–2159. [Google Scholar] [CrossRef]
- Pepperell, J.C.T.; Maskell, N.A.; Jones, D.R.; Langford-Wiley, B.A.; Crosthwaite, N.; Stradling, J.R.; Davies, R.J.O. A Randomized Controlled Trial of Adaptive Ventilation for Cheyne-Stokes Breathing in Heart Failure. Am. J. Respir. Crit. Care Med. 2003, 168, 1109–1114. [Google Scholar] [CrossRef]
- Pak, V.M.; Strouss, L.; Yaggi, H.K.; Redeker, N.S.; Mohsenin, V.; Riegel, B. Mechanisms of Reduced Sleepiness Symptoms in Heart Failure and Obstructive Sleep Apnea. J. Sleep Res. 2019, 28, e12778. [Google Scholar] [CrossRef]
- Taranto Montemurro, L.; Floras, J.S.; Millar, P.J.; Kasai, T.; Gabriel, J.M.; Spaak, J.; Coelho, F.M.S.; Bradley, T.D. Inverse Relationship of Subjective Daytime Sleepiness to Sympathetic Activity in Patients with Heart Failure and Obstructive Sleep Apnea. Chest 2012, 142, 1222–1228. [Google Scholar] [CrossRef]
- Sin, D.D.; Fitzgerald, F.; Parker, J.D.; Newton, G.; Floras, J.S.; Bradley, T.D. Risk Factors for Central and Obstructive Sleep Apnea in 450 Men and Women with Congestive Heart Failure. Am. J. Respir. Crit. Care Med. 1999, 160, 1101–1106. [Google Scholar] [CrossRef]
- Szymanski, F.M.; Filipiak, K.J.; Platek, A.E.; Hrynkiewicz-Szymanska, A.; Karpinski, G.; Opolski, G. OSACS Score-a New Simple Tool for Identifying High Risk for Obstructive Sleep Apnea Syndrome Based on Clinical Parameters. Anadolu Kardiyol. Derg. 2015, 15, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Alonderis, A.; Raskauskiene, N.; Gelziniene, V.; Mickuviene, N.; Brozaitiene, J. The Association of Sleep Disordered Breathing with Left Ventricular Remodeling in CAD Patients: A Cross-Sectional Study. BMC Cardiovasc. Disord. 2017, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.; Xie, A.; Rutherford, R.; Ando, S.; Floras, J.S.; Bradley, T.D. Cycle Length of Periodic Breathing in Patients with and without Heart Failure. Am. J. Respir. Crit. Care Med. 1996, 154 Pt 1, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wedewardt, J.; Bitter, T.; Prinz, C.; Faber, L.; Horstkotte, D.; Oldenburg, O. Cheyne-Stokes Respiration in Heart Failure: Cycle Length Is Dependent on Left Ventricular Ejection Fraction. Sleep Med. 2010, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Spieker, M.; Klose, S.; Keymel, S.; Boentert, M.; Krüger, S.; Horn, P.; Kelm, M.; Westenfeld, R. Reduction of Sleep-Disordered Breathing Following Effective Percutaneous Mitral Valve Repair with the MitraClip System. Sleep Breath. 2019, 23, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Maripov, A.; Mamazhakypov, A.; Sartmyrzaeva, M.; Akunov, A.; Muratali Uulu, K.; Duishobaev, M.; Cholponbaeva, M.; Sydykov, A.; Sarybaev, A. Right Ventricular Remodeling and Dysfunction in Obstructive Sleep Apnea: A Systematic Review of the Literature and Meta-Analysis. Can. Respir. J. 2017, 2017, 1587865. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Terzano, C.; Saponara, M.; Gaudio, C.; Mangieri, E.; Capotosto, L.; Pergolini, M.; D’Orazio, S.; Continanza, G.; Cimino, E. Assessment of Right Ventricular Function in Obstructive Sleep Apnea Syndrome and Effects of Continuous Positive Airway Pressure Therapy: A Pilot Study. Can. J. Cardiol. 2015, 31, 823–831. [Google Scholar] [CrossRef]
- Karamanzanis, G.; Panou, F.; Lazaros, G.; Oikonomou, E.; Nikolopoulos, I.; Mihaelidou, M.; Ntounis, G.; Lekakis, J. Impact of Continuous Positive Airway Pressure Treatment on Myocardial Performance in Patients with Obstructive Sleep Apnea. A Conventional and Tissue Doppler Echocardiographic Study. Sleep Breath. 2015, 19, 343–350. [Google Scholar] [CrossRef]
- Tugcu, A.; Guzel, D.; Yildirimturk, O.; Aytekin, S. Evaluation of Right Ventricular Systolic and Diastolic Function in Patients with Newly Diagnosed Obstructive Sleep Apnea Syndrome without Hypertension. Cardiology 2009, 113, 184–192. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D. Obstructive Sleep Apnea and Heart Failure: Pathophysiologic and Therapeutic Implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef]
- Bradley, T.D.; Hall, M.J.; Ando, S.I.; Floras, J.S. Hemodynamic Effects of Simulated Obstructive Apneas in Humans with and without Heart Failure. Chest 2001, 119, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Stansbury, R.; Hackett, B.; Fox, H. Sleep Apnea and Pulmonary Hypertension: A Riddle Waiting to Be Solved. Pharmacol. Ther. 2021, 227, 107935. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Stone, K.L.; Varosy, P.D.; Hoffman, A.R.; Marcus, G.M.; Blackwell, T.; Ibrahim, O.A.; Salem, R.; Redline, S. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009, 169, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.R.; Lavie, C.J.; Di Nicolantonio, J.J.; O’Keefe, J.; Morin, D.P.; Khatib, S.; Milani, R.V. Atrial fibrillation in the 21st century: A current understanding of risk factors and primary prevention strategies. Mayo Clin. Proc. 2013, 88, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Cadby, G.; McArdle, N.; Briffa, T.; Hillman, D.R.; Simpson, L.; Knuiman, M.; Hung, J. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest 2015, 148, 945–952. [Google Scholar] [CrossRef]
- Tung, P.; Levitzky, Y.S.; Wang, R.; Weng, J.; Quan, S.F.; Gottlieb, D.J.; Rueschman, M.; Punjabi, N.M.; Mehra, R.; Bertisch, S.; et al. Obstructive and Central Sleep Apnea and the Risk of Incident Atrial Fibrillation in a Community Cohort of Men and Women. J. Am. Heart Assoc. 2017, 6, e004500. [Google Scholar] [CrossRef]
- May, A.M.; Blackwell, T.; Stone, P.H.; Stone, K.L.; Cawthon, P.M.; Sauer, W.H.; Varosy, P.D.; Redline, S.; Mehra, R.; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central Sleep-disordered Breathing Predicts Incident Atrial Fibrillation in Older Men. Am. J. Respir. Crit. Care Med. 2016, 193, 783–791. [Google Scholar] [CrossRef]
- Anzai, T.; Grandinetti, A.; Katz, A.R.; Hurwitz, E.L.; Wu, Y.Y.; Masaki, K. Association between central sleep apnea and atrial fibrillation/flutter in Japanese-American men: The Kuakini Honolulu Heart Program (HHP) and Honolulu-Asia Aging Study (HAAS). J. Electrocardiol. 2020, 61, 10–17. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Chen, L.Y.; Chung, M.K.; Cornwell, W.K.; Furie, K.L.; Lakkireddy, D.R.; Marrouche, N.F.; Natale, A.; Olshansky, B.; Joglar, J.A.; et al. Managing Atrial Fibrillation in Patients with Heart Failure and Reduced Ejection Fraction: A Scientific Statement From the American Heart Association. Circ Arrhythm Electrophysiol. 2021, 14, HAE0000000000000078. [Google Scholar] [CrossRef]
| All Cases | CSA Group | OSA Group | p-Value | |
|---|---|---|---|---|
| Anthropometric parameters | ||||
| No (%) | 76 | 64 (76,2%) | 12 (14,3%) | |
| Sex (male), no (%) | 64 | 54 (84.4%) | 10 (83.3%) | 0.93 |
| Sex (female), no (%) | 12 | 10 (15.6%) | 2 (16.7%) | 0.93 |
| Age (years), X ± SD | 68.0 ± 8.7 | 67.9 ± 8.8 | 68.3 ± 9 | 0.91 |
| BMI (kg/m2), X ± SD | 30.7 ± 6.4 | 30.1 ± 5.3 | 34.3 ± 10.3 | 0.001 |
| Neck circumference (cm), X ± SD | 41.9 ± 3.8 | 41.7 ± 3.8 | 42.9 ± 3.7 | 0.98 |
| Comorbidities | ||||
| CAD with a history of MI, no (%) | 53 (69.7%) | 45 (70.3%) | 8 (66.7%) | 0.06 |
| Hypertension, no (%) | 60 (78.9%) | 50 (78.1%) | 10 (83.3%) | 0.90 |
| Diabetes, no (%) | 40 (52.6%) | 33 (51.6%) | 7 (58.3%) | 0.09 |
| Atrial fibrillation, no (%) | 27 (35.5%) | 26 (40.6%) | 1 (8.3%) | 0.03 |
| CKD, no (%) | 16 (21.1%) | 13 (20.3%) | 3 (25.0%) | 0.30 |
| PAD, no (%) | 8 (9.5%) | 4 (6.2%) | 3 (25.0%) | 0.10 |
| Hypothyroidism, no (%) | 6 (7.9%) | 6 (9.4%) | 0 (0.0%) | 0.50 |
| Stroke, no (%) | 4 (5.3%) | 4 (6.2%) | 0 (0.0%) | 0.50 |
| CAS, no (%) | 5 (6.6%) | 5 (7.8%) | 0 (0.0%) | 0.50 |
| Pharmacological treatment | ||||
| ACE-I, no (%) | 60 (78.9%) | 49 (76.6%) | 11 (91.7%) | 0.24 |
| ARB, no (%) | 2 (2.6%) | 2 (3.1%) | 0 (0.0%) | 0.54 |
| MRA, no (%) | 67 (88.2%) | 56 (87.5%) | 11 (91.7%) | 0.68 |
| Loop diuretics, no (%) | 66 (86.8%) | 55 (85.9%) | 11 (91.7%) | 0.59 |
| Beta-blockers, no (%) | 75 (98.7%) | 63 (98.4%) | 12 (100.0%) | 0,67 |
| Digitalis, no (%) | 6 (7.9%) | 6 (9.4%) | 0 (0.0%) | 0.27 |
| Ivabradine, no (%) | 15 (19.7%) | 14 (21.9%) | 1 (8.3%) | 0.28 |
| All Cases | CSA Group | OSA Group | p-Value | ||
|---|---|---|---|---|---|
| Cardiorespiratory fitness parameters | |||||
| No (%) | 76 | 64 (76.2%) | 12 (14.3%) | ||
| HF etiology (ICM), no (%) | 54 (71.1%) | 46 (71.9%) | 8 (66.7%) | 0.71 | |
| HF duration [months], X ± SD | 74.8 ± 82.4 | 81.5 ± 86,8 | 39.2 ± 37.8 | 0.10 | |
| NYHA class, X ± SD | 2.4 ± 0.5 | 2.4 ± 0.56 | 2.5 ± 0.52 | 0.66 | |
| 6 MWT [m], X ± SD | 282.2 ± 105 | 282.1 ± 110.3 | 281.7 ± 76.9 | 0.99 | |
| Echocardiographic parameters | |||||
| LVEDD [mm], X ± SD | 67.80 ± 7.91 | 67.92 ± 8.39 | 67.17 | 0.048 | |
| LVESD [mm], X ± SD | 57.22 ± 9.31 | 57.75 ± 9.68 | 54.42 ± 6.64 | 0.17 | |
| IVS [mm], X ± SD | 10.6 ± 2.8 | 10.4 ± 2.4 | 12.0 ± 4.3 | 0.06 | |
| PW [mm], X ± SD | 9.59 ± 1.72 | 9.55 ± 1.66 | 9.75 ± 2.09 | 0.25 | |
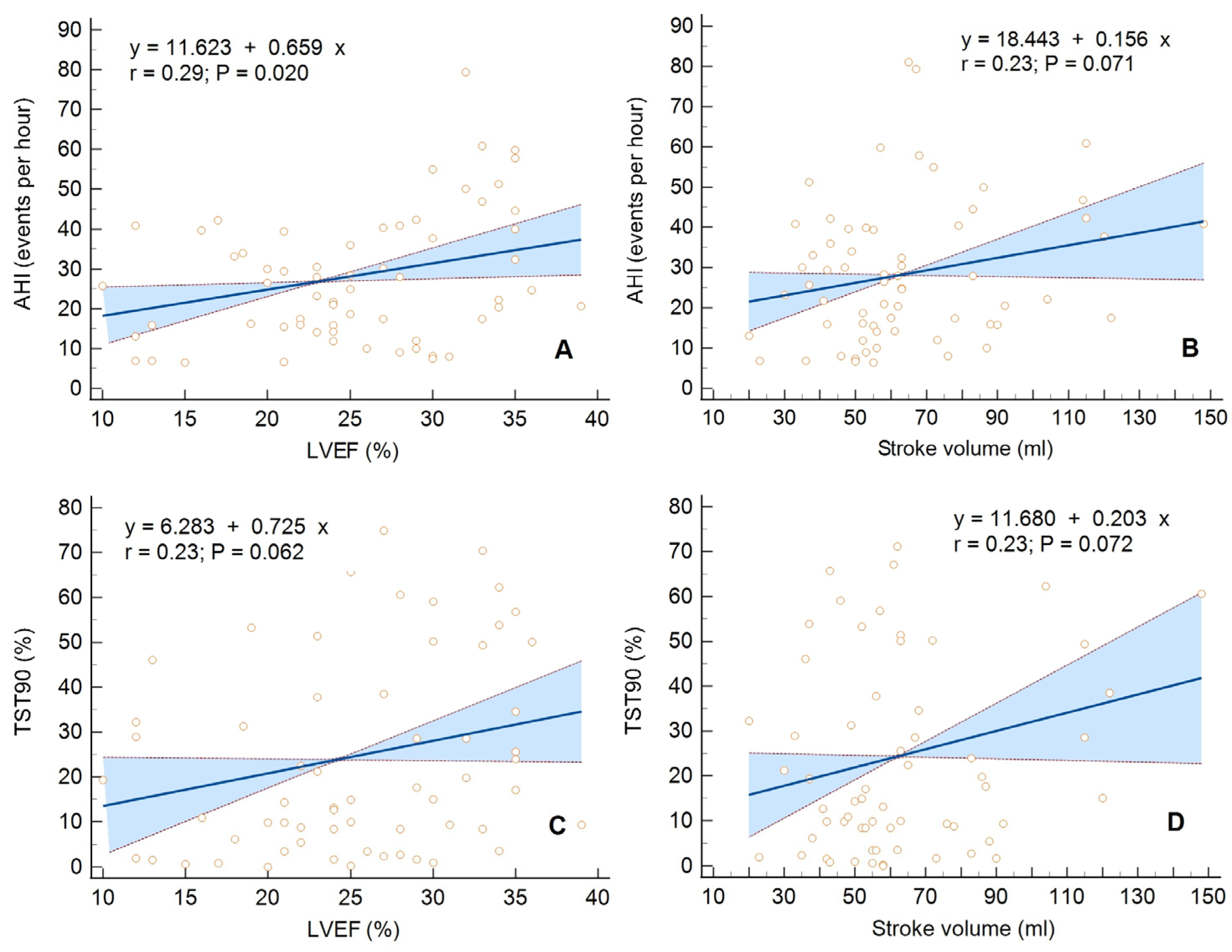
| LVEF [%], X ± SD | 25.4 ± 6.8 | 25.2 ± 7.2 | 25.9 ± 6 | 0.76 | |
| SV [mL], X ± SD | 64.5 ± 24.4 | 63.8 ± 25.8 | 68.7 ± 14.7 | 0.53 | |
| LVEDVI [mL/m2], X ± SD | 129.1 ± 39.9 | 129.5 ± 40.6 | 128.3 ± 39.2 | 0.93 | |
| LVESVI [mL/m2], X ± SD | 96.6 ± 34.5 | 97.2 ± 34.9 | 95.3 ± 36.4 | 0.87 | |
| LAA [cm2], X ± SD | 27.64 ± 6.60 | 27.92 ± 6.68 | 26.17 ± 6.24 | 0.86 | |
| MR | trace/mild, no (%) | 39 (51.3%) | 32 (50.0%) | 7 (58.3%) | 0.60 |
| moderate/severe, no (%) | 37 (48.7%) | 32 (50.0%) | 5 (41.7%) | ||
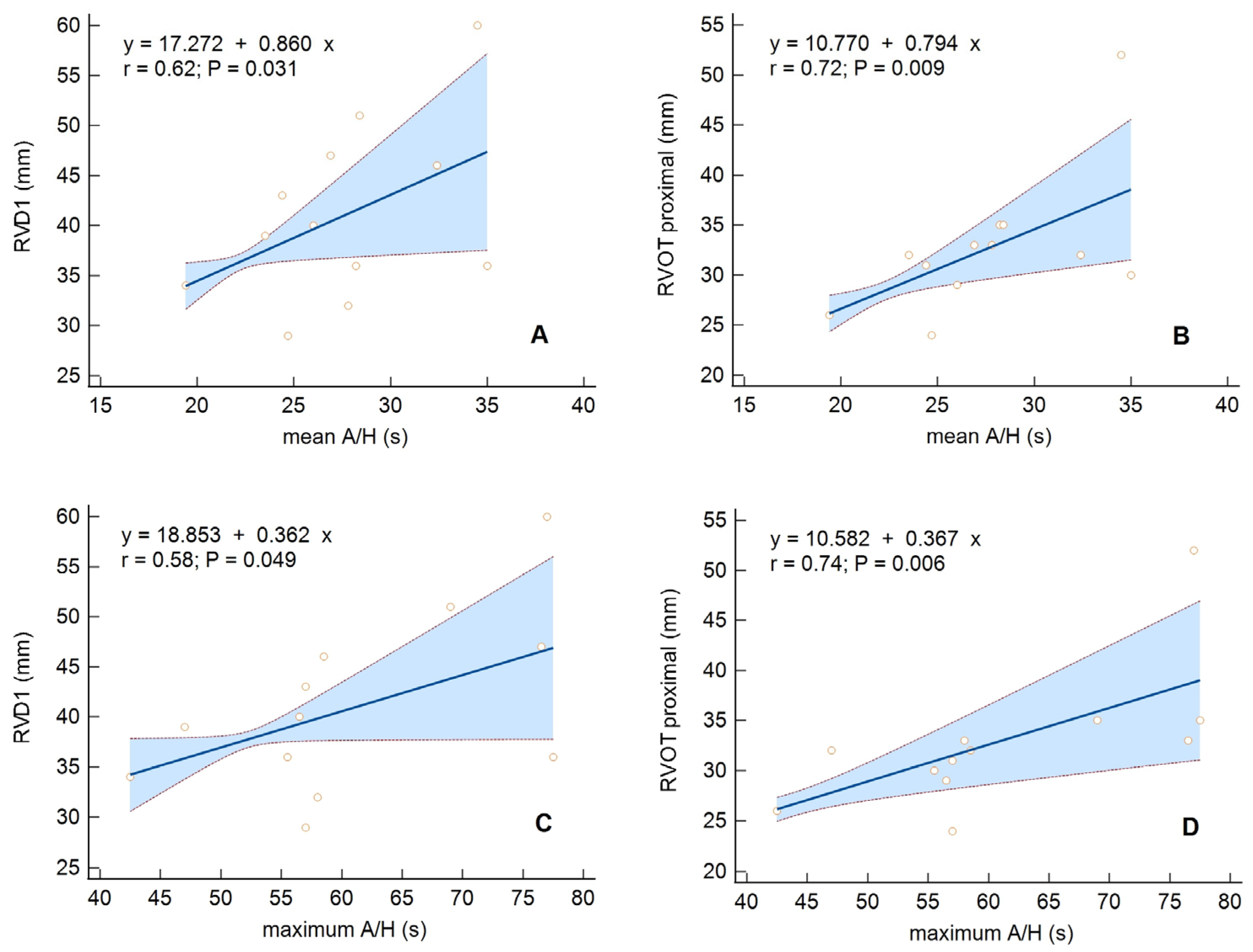
| RVOTproximal [mm], X ± SD | 34.2 ± 5.8 | 34.5 ± 5.6 | 32.7 ± 6.9 | 0.33 | |
| RVD1 [mm], X ± SD | 38.8 ± 6.9 | 38.5 ± 6.5 | 41.1 ± 8.8 | 0.24 | |
| TAPSE [mm], X ± SD | 18.3 ± 4.7 | 17.9 ± 4.3 | 20.6 ± 6.2 | 0.07 | |
| RAA [cm2], X ± SD | 20.13 ± 6.10 | 20.38 ± 6.34 | 18.83 ± 4.61 | 0.25 | |
| TR | trace/mild, no (%) | 53 (71.6%) | 45 (71.4%) | 8 (72.7%) | 0.93 |
| moderate/severe, no (%) | 21 (28.4%) | 18 (28.6%) | 3 (27.3%) | ||
| Peak TR velocity [m/s], X ± SD | 2.77 ± 0.80 | 2.76 ± 0.82 | 2.85 ± 0.72 | 0.88 | |
| TRPG [mm Hg], X ± SD | 31.21 ± 15.28 | 30.76 ± 15.30 | 34.56 ± 16.40 | 0.70 | |
| RVSP [mm Hg], X ± SD | 40.4 ± 17.7 | 40.1 ± 17.8 | 42.8 ± 19.6 | 0.76 | |
| All Cases | CSA Group | OSA Group | p-Value | |
|---|---|---|---|---|
| Polysomnographic Parameters | ||||
| No (%) | 76 | 64 (76.2%) | 12 (14.3%) | |
| ESS (points), X ± SD | 8.0 ± 4.5 | 7.6 ± 4.4 | 10.1 ± 5.0 | 0.08 |
| Participants with ESS score >10 pts, no (%) | 20 (26.3%) | 16 (25.0%) | 4 (33.3%) | 0.55 |
| Percentage of central apneas, M (IQR) | 52.41 (20.72–78.17) | 57.33 (29.29–84.09) | 20.20 (1.49–42.92) | 0.003 |
| Percentage of obstructive apneas, M (IQR) | 46.60 (20.81–76.59) | 40.21 (13.83–69.79) | 79.80 (57.09–98.52) | 0.002 |
| AHI [events/h], X ± SD | 27.9 ± 17.0 | 28.4 ± 17.3 | 25.8 ± 15.8 | 0.77 |
| mean A/H [s], X ± SD | 28.3 ± 4.5 | 28.7 ± 4.4 | 27.6 ± 4.6 | 0.44 |
| max A/H [s], X ± SD | 59.2 ± 10.6 | 60.3 ± 9.1 | 61.0 ± 11.6 | 0.83 |
| mean SpO2 [%], X ± SD | 91.1 ± 2.0 | 91.1 ± 2.1 | 91.1 ± 2.1 | 0.99 |
| min SpO2 [%], X ± SD | 78.6 ± 7.9 | 78.6 ± 7.8 | 77.1 ± 8.1 | 0.54 |
| TST 90 [%], X ± SD | 23.5 ± 21.4 | 24.7 ± 22.5 | 21.2 ± 18.0 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, K.; Łoboda, D.; Adamek, M.; Wilczek, J.; Gibiński, M.; Gardas, R.; Biernat, J.; Gołba, K.S. Relationships between Heart Chamber Morphology or Function and Respiratory Parameters in Patients with HFrEF and Various Types of Sleep-Disordered Breathing. Diagnostics 2023, 13, 3309. https://doi.org/10.3390/diagnostics13213309
Simionescu K, Łoboda D, Adamek M, Wilczek J, Gibiński M, Gardas R, Biernat J, Gołba KS. Relationships between Heart Chamber Morphology or Function and Respiratory Parameters in Patients with HFrEF and Various Types of Sleep-Disordered Breathing. Diagnostics. 2023; 13(21):3309. https://doi.org/10.3390/diagnostics13213309
Chicago/Turabian StyleSimionescu, Karolina, Danuta Łoboda, Mariusz Adamek, Jacek Wilczek, Michał Gibiński, Rafał Gardas, Jolanta Biernat, and Krzysztof S. Gołba. 2023. "Relationships between Heart Chamber Morphology or Function and Respiratory Parameters in Patients with HFrEF and Various Types of Sleep-Disordered Breathing" Diagnostics 13, no. 21: 3309. https://doi.org/10.3390/diagnostics13213309
APA StyleSimionescu, K., Łoboda, D., Adamek, M., Wilczek, J., Gibiński, M., Gardas, R., Biernat, J., & Gołba, K. S. (2023). Relationships between Heart Chamber Morphology or Function and Respiratory Parameters in Patients with HFrEF and Various Types of Sleep-Disordered Breathing. Diagnostics, 13(21), 3309. https://doi.org/10.3390/diagnostics13213309






