Abstract
Aim: The purpose of this work was to investigate the prevalence of SIRT1 rs3818292, rs3758391, and rs7895833 single nucleotide polymorphisms and SIRT1 serum levels associated with multiple sclerosis (MS) in the Lithuanian population. Methods: A total of 250 MS patients and 250 healthy controls were included in the study. Genotyping was performed using the RT-PCR method. Statistical analysis was performed using “IBM SPSS version 29.0”. The serum SIRT1 level was determined by the ELISA method. Results: We found that rs3818292 was associated with increased odds of developing MS under the dominant (p = 0.007) and allelic genetic (p = 0.004) models. rs3758391 was associated with increased odds of developing under the co-dominant (p < 0.001), overdominant (p < 0.001), dominant (p < 0.001), and allelic (p = 0.002) genetic models. rs7895833 was associated with increased odds of developing MS under co-dominant (p < 0.001), overdominant (p < 0.001), dominant (p < 0.001), and allelic (p < 0.001) genetic models. Additional sex-differentiated analysis within females revealed that the rs3758391 was associated with an increased odds ratio for the occurrence of MS among the co-dominant (p = 0.006), dominant (p = 0.002), and allelic (p = 0.001). rs7895833 was associated with an increased odds ratio for the development of MS under the co-dominant (p < 0.001), overdominant (p < 0.001), dominant (p < 0.001), and allelic (p < 0.001) genetic models. Age-differentiated analysis showed that rs3758391 was associated with an increased odds ratio for the development of MS in younger patients under the codominant (p = 0.002), overdominant (p = 0.003), and dominant (p = 0.004) genetic models. rs7895833 was associated with an increased odds ratio for the occurrence of MS under the overdominant genetic model (p = 0.013). In elderly patients, rs3818292 was associated with an increased odds ratio for the occurrence of MS under the dominant (p = 0.008) and allelic (p = 0.009) genetic models. rs7895833 was associated with an increased odds ratio for the occurrence of MS under the codominant (p = 0.011 and p = 0.012), dominant (p = 0.001), and allelic (p < 0.001) genetic models. We also found that serum SIRT1 levels were statistically significantly different between MS patients and control group subjects (p < 0.001). In addition, comparison of SIRT1 levels between study groups and genotypes showed that rs3818292 AA (p = 0.001), rs3758391 CT (p < 0.001), and rs7895833 AA (p = 0.002) and AG (p = 0.004) had higher SIRT1 levels in the control group than in the MS group. All results were provided after strict Bonferroni correction. Conclusions: Genetic variations in SIRT1 rs3818292, rs3758391, and rs7895833 are associated with multiple sclerosis, with possible differences in gender and age, as well as lower serum SIRT1 levels.
1. Introduction
Multiple sclerosis (MS) is a chronic and unpredictable disease of the central nervous system characterized by the development of focal inflammatory lesions in the CNS that can cause a variety of neurological dysfunctions in early adulthood [1]. The exact cause of multiple sclerosis is unknown. It is thought to be a combination of genetic and environmental factors that trigger an autoimmune system. There is evidence that genetic factors may play a role in MS susceptibility [2]. Studies have shown that people with a family history of MS have a higher risk of developing the disease than people without this history. In addition, certain variations in genes involved in immune function have been associated with an increased risk of MS [3]. Regarding environmental factors, the incidence of MS varies greatly by region, race, age, and gender [4]. According to a systematic review, higher rates of MS have generally been reported in women and in populations living at higher latitudes, such as in Northern Europe, North America, and parts of Asia [5], as well as in Lithuania [6]. SIRT1 is a member of the sirtuin family of highly conserved III NAD class-dependent deacetylases involved in the regulation of cellular processes such as energy metabolism, DNA repair, aging, and inflammation [7]. Expression of SIRT1 has been detected in various mouse ocular tissues, including the cornea, lens, iris, ciliary body, inner nuclear layer, outer nuclear layer, and retinal ganglion cell layer [8]. In addition, SIRT1 is found in various neurons, including stem and progenitor cells, mature neurons, microglia, and astrocytes. SIRT1 is known to play a role in modulating immune response and reducing inflammation in various cell types, including immune cells in the central nervous system [9,10,11]. SIRT1 also controls neuronal development, axon growth, synaptic plasticity, and hormone secretion [12]. Both preclinical and clinical studies have shown that increasing the expression of SIRT1 can reduce autoimmunity as well as reduce the incidence of neurodegenerative diseases and neuroexcitation [13].
Although the exact mechanisms of SIRT1 in the pathogenesis of MS are not fully understood and are controversial, it is hypothesized that SIRT1 dysregulation may have an impact on the development and progression of MS. The impact of SIRT1 on this disease would be through its effects on immune function as well as oxidative stress, mitochondrial function, and autophagy networks [14]. In addition, SIRT1 can regulate inflammation by modulating the activation of master regulators such as NFkB and influencing antigen presentation by dendritic cells. The effects of SIRT1 on inflammation can be both anti-inflammatory and pro-inflammatory, and overexpression of SIRT1 improves symptoms in animal models of MS [15]. SIRT1 may also cooperate with Nrf2, a transcription factor involved in antioxidant production, mitochondrial biogenesis, and oxidative phosphorylation. Nrf2 has been linked to neurodegeneration and the pathogenesis of MS [16]. Studies have revealed the crucial role of SIRTs, including SIRT1, in the interaction between neuroinflammation, neurodegeneration, and metabolic changes [9], and SIRT1 has been implicated in the pathogenesis of several neurological diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease [17,18]. As a result, SIRT1 has been identified as a potential therapeutic target for neurological diseases [19,20,21].
In this study, we investigated the potential association between SIRT1 gene polymorphisms (rs3818292, rs3758391, and rs7895833) and serum levels of SIRT1 in patients with multiple sclerosis in Lithuania. The intronic rs3818292 variant can affect the gene splicing processes and rs3758391, together with rs7895833, are functional variants located in the promoter region [22,23,24]. We believe that those variants could lead to the altered SIRT1 protein expression.
2. Materials and Methods
2.1. Subjects and Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki, and all participants gave informed consent. The study included 500 participants and was conducted in the Laboratory of Ophthalmology of the Neuroscience Institute of the Lithuanian University of Health Sciences. Participants were divided into two different groups:
2.2. Group I: Patients with Multiple Sclerosis (n = 250)
The selected 250 MS patients were treated in the clinics of LUHS (Lithuanian University of Health Sciences) in Kaunas between 1 January 2020 and 31 December 2023. The study included only patients with a confirmed MS diagnosis. MS diagnosis was made according to the widely accepted and revised McDonald criteria (2017) [25]. At the time of diagnosis, a lumbar puncture and CSF examination were performed. CSF samples were analyzed by isoelectric focusing and IgG-specific immunofixation to test for the presence of intrathecal specific OCBs. Demographic and clinical data and magnetic resonance imaging results were obtained from all patients. Disability was measured using the Kurtzke Expanded Disability Status Scale. Data were obtained from outpatient records, and retrospective analysis was performed. The following variables were considered in the selection process: patient age (at the time of diagnosis and first symptoms), gender, and disease progression [26].
Exclusion criteria for the study were systemic diseases such as diabetes mellitus, oncologic diseases, systemic tissue disorders, chronic infectious diseases, autoimmune diseases, and conditions after organ or tissue transplantation.
2.3. Group II: Control Group (n = 250)
The control group included subjects who matched the age and sex of the MS group, had no history of autoimmune or neurologic disease, and were in good general health.
2.4. Polymorphism Selection
In this study, we aimed to investigate the relationship between MS and three specific genetic variations in SIRT1: rs3818292, rs3758391, and rs7895833. Our literature search revealed that although these specific polymorphisms have not been directly associated with multiple sclerosis, they have been linked to other diseases associated with the development of MS, such as autoimmune diseases and neurodegenerative diseases. SIRT1 rs3818292 is known to be located in the intronic region, which has functional effects on gene expression and regulation [22,27]. SIRT1 rs3758391 and rs7895833 are located in the promoter region that can affect gene expression [23,24]. Therefore, we decided to evaluate these specific polymorphisms as potential genetic risk factors for MS in our study.
The study also examined the distribution of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in MS and the control groups across different sexes and ages. For this purpose, we divided participants into two age groups based on the average age of the study population: those who were 40 years old or younger and those who were over 40 years old.
2.5. DNA Extraction, SIRT1 Genotyping, and SIRT1 Serum-Level Determination
DNA extraction and analysis of SIRT1 rs3818292, rs3758391, and rs7895833 were performed in the Ophthalmology Laboratory of the Neuroscience Institute of the Lithuanian University of Health Sciences. DNA samples were obtained from venous blood using the DNA salting-out method. Briefly, venous blood samples (white blood cells) were collected and suspended in a buffer solution, followed by the addition of detergents to degrade cell membranes, proteinase K to hydrolyze proteins, and chloroform to deproteinize them. The DNA was then precipitated with ethanol.
TaqMan® genotyping assays (Thermo Scientific, Pleasanton, CA, USA) were used to determine all single nucleotide polymorphisms (SNPs). Genotyping of SIRT1 rs3818292, rs3758391, and rs7895833 was performed using a Step One Plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s recommendations. Real-time PCR mixtures were prepared according to the appropriate protocol for SNP determination.
We added 1.5 μL of the samples’ DNA and 8.5 μL of the PCR reaction mixture to each of the 96 wells of the plate, along with the negative control. Real-time PCR was performed using the Allelic Discrimination program, and the assay was performed according to the manufacturer’s instructions. The program analyzed each genotype based on the fluorescence intensity of the different detectors (VIC and FAM).
Serum SIRT1 levels were measured in both control subjects and patients using a human SIRT1 enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, UK). Serum SIRT1 levels were measured in duplicate in 41 control subjects and 20 patients with MS.
The ELISA assay was performed according to the manufacturer’s instructions. Optical density at a wavelength of 450 nm was measured using a microplate reader (Multiskan FC microplate photometer, Thermo Scientific, Waltham, MA, USA). SIRT1 concentrations were calculated using the standard curve with a sensitivity range of 0.63–40 ng/mL and 132 pg/mL.
2.6. Statistical Analysis
Statistical analysis was performed with SPSS/W 29.0 software (IBM Corp, Armonk, NY, USA). Sex distribution was presented in absolute numbers and percentages and compared with the chi-square test. Continuous data (age and serum SIRT1 level) were expressed as median with interquartile range (IQR). Data that were not normally distributed between the 2 groups or subgroups were compared with the Mann–Whitney U test.
We performed Hardy–Weinberg analysis with the χ2 test to analyze the observed and expected frequencies of SIRT1 rs3818292, rs3758391, and rs7895833 in the control group. The analysis showed that all three SNPs met the HWE criteria (p > 0.05), indicating that the genotype and allele frequencies in the study were consistent with HWE expectations. We used the χ2 test to analyze the differences in the distribution of SIRT1 rs3818292, rs3758391, and rs7895833 between the groups with MS and the control group. We also performed binary logistic regression analysis to evaluate the effects of genotypes on the development of multiple sclerosis, reporting odds ratios (OR) and 95% confidence intervals (CI). The best genetic model was selected based on the Akaike information criterion (AIC). According to the Akaike Information Criterion (AIC), the model with the lowest value is the most appropriate inheritance model. We considered statistically significant differences as those with p < 0.05 and adjusted our significance threshold for multiple comparisons to alpha = 0.017 (0.05/3, because we examined three SNPs in the SIRT1 gene). Continuous data (age and serum SIRT1 level) were expressed as median with interquartile range (IQR) and compared between two groups or subgroups using the Mann–Whitney U test. Sex distribution was presented as absolute numbers with percentages and compared with the χ2 test.
3. Results
The study included a total of 500 subjects divided into two groups: 250 patients with MS and 250 control subjects. The control group was selected based on gender and age distribution to match the MS group. Females made up 65.5% (n = 164) of the MS group and 65.5% (n = 250) of the control group, while males made up 34.3% (n = 86) of the MS group and 34.3% (n = 86) of the control group (Table 1).

Table 1.
Demographic characteristics of the study groups.
In the MS group, the rs3818292 AA genotype and A allele were less common, whereas the AG genotype was more common compared with the control group (87.2% vs. 78.0%, p = 0.007; 93.0% vs. 87.0%, p = 0.002; and 11.6% vs. 18.0, p < 0.001, respectively). Similarly, the rs3758391 CC genotype and C allele were less common, whereas the CT genotype was more common in the MS group than in the control group (58.4% vs. 42.0%, p < 0.001; 75.8% vs. 67.2%, p = 0.003; and 34.8% vs. 50.4%, p < 0.001, respectively). Finally, the rs7895833 AA genotype and A allele were less common, whereas the AG genotype was more common in the MS group than in the control group (75.2% vs. 59.2%, p = <0.001; 21.2% vs. 34.8%, p = 0.001; and 85.8% vs. 76.6%, p < 0.001, respectively) (Table 2).

Table 2.
Distribution of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in the patients with multiple sclerosis and control groups.
Our analysis revealed that individuals with the rs3818292 AG+GG genotype and each G allele had a 1.9-fold and 1.8-fold increased odds of developing MS under the dominant and allelic genetic models, respectively (OR = 1.921; CI: 1.193–3.095; p = 0.007 and OR = 1.806; CI: 1.203–2.711; p = 0.004, respectively). Similarly, the rs3758391 CT, CT+TT genotypes, and each T allele were associated with a 2-fold, 1.9-fold, 1.9-fold, and 1.6-fold increased odds of developing MS under the co-dominant, overdominant, dominant, and allelic genetic models, respectively (OR = 2.014; CI: 1.390–2.918; p < 0.001; OR = 1.904; CI: 1.329–2.727; p < 0.001; OR = 1.939; CI: 1.359–2.766; p < 0.001; and OR = 1.567; CI: 1.175–2.089; p = 0.002, respectively). Finally, individuals with the SIRT1 rs7895833 AG, AG+GG genotypes, and each G allele had a 2.1-fold, 2-fold, 2.1-fold, and 1.8-fold increased odds of developing MS under the co-dominant, overdominant, dominant, and allelic genetic models, respectively (OR = 2.085; CI: 1.392–3.122; p < 0.001; OR = 1.984; CI: 1.330–2.959; p < 0.001; OR = 2.090; CI: 1.426–3.062; p < 0.001 and OR = 1.775; CI: 1.290–2.443; p < 0.001, respectively) (Table 3).

Table 3.
Binary logistic regression analysis of patients with multiple sclerosis and control groups.
The findings of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in MS and control groups between different gender distributions suggest that, in women, the SIRT1 rs3758391 CC genotype and each C allele were less frequent in those with MS compared with the control group (62.8% vs. 45.7%, p = 0.002; and 79.9% vs. 69.2% p = 0.002, respectively). The rs7895833 AA genotype and A allele were less common, whereas the AG genotype was more common in the MS group than in the control group (78.0% vs. 59.1%, p < 0.001; 87.8% vs. 77.7%, p = 0.001; and 19.5% vs. 37.2%, p < 0.001; respectively). Regarding men, the results showed that the rs3758391 CT genotype was more common in those with MS than in the control group (36.0% vs. 57.0%, p = 0.006) (Table 4).

Table 4.
Distribution of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in MS and control groups between different genders.
A binary logistic regression analysis within different genders indicated that the SIRT1 gene rs3758391 CT and CT+TT genotypes, as well as each T allele, were significantly associated with an increased odds ratio of MS occurrence under the co-dominant, dominant, and allelic genetic models. Specifically, the odds ratios were 1.9-fold, 2-fold, and 1.9-fold, respectively (OR = 1.888; CI: 1.198–2.976; p = 0.006; OR = 2.004; CI: 1.289–3.115; p = 0.002 and OR = 1.859; CI: 1.273–2.716; p = 0.001).
Similarly, the rs7895833 AG and AG+GG genotypes, as well as each G allele, were also significantly associated with an increased odds ratio of developing MS under the co-dominant, overdominant, dominant, and allelic genetic models. The odds ratios were 2.5-fold, 2.4-fold, 2.5-fold, and 2.1-fold, respectively (OR = 2.515; CI: 1.522–4.158; p < 0.001; OR = 2.443; CI: 1.483–4.025; p < 0.001; OR = 2.456; CI: 1.515–3.982; p < 0.001, and OR = 2.079; CI: 1.352–3.195; p < 0.001) (Table 5). However, there was no significant difference observed among men.

Table 5.
Binary logistic regression analysis of patients with multiple sclerosis and control groups between different genders.
The results of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in MS and control groups between different ages (40 or younger, and over 40 years old) indicated that the rs3758391 CC genotype was less frequent, whereas that of the CT genotype was higher in the MS group than in the control group in the younger participants (56.7% vs. 38.6%, p = 0.004; and 35.0% vs. 53.8%, p = 0.003, respectively). The frequency of the rs7895833 AG genotype was also higher in the MS group than in the control group (23.3% vs. 37.9%; p = 0.012) (Table 6).

Table 6.
Distribution of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in the patients with multiple sclerosis and control groups between younger participants.
However, in the older participants, the frequency of the rs3818292 AA genotype and A allele was lower than in the control group (89.2% vs. 76.3% and 93.8% vs. 85.6%; p = 0.007 and p = 0.002, respectively), and the frequency of the rs7895833 AA genotype and A allele was also lower than in the control group (78.5% vs. 59.3%, p = 0.001; and 88.1% vs. 75.0%, p < 0.001; respectively) (Table 7).

Table 7.
Distribution of genotypes and alleles of SIRT1 rs3818292, rs3758391, and rs7895833 in patients with multiple sclerosis and control groups between older participants.
Binary logistic regression analysis in younger patients revealed that SIRT1 rs3758391 CT and CT+TT genotypes were associated with a 2.3-fold, 2.2-fold, and 2.1-fold increased odds of MS occurrence under the co-dominant, overdominant, and dominant genetic models (OR = 2.254; CI: 1.331–3.443; p = 0.002; OR = 2.162; CI: 1.301–3.592; p = 0.003 and OR = 2.077; CI: 1.256–3.435; p = 0.004, respectively). rs7895833 AG genotype was associated with a 2–fold increased odds of MS occurrence under the overdominant genetic model (OR = 2.003; CI: 1.156–3.473; p = 0.013) (Table 8).

Table 8.
Binary logistic regression analysis of multiple sclerosis and control groups’ younger participants (<40 years).
Moreover, in older patients, SIRT1 rs3818292 AG+GG genotypes and each G allele were associated with a 2.6-fold and 2.2-fold increased odds of MS occurrence under the dominant and allelic genetic models (OR = 2.578; CI: 1.282–5.181; p = 0.008; OR = 2.177; CI: 1.219–3.890; p = 0.009, respectively). rs7895833 AG and GG genotypes were associated with a 2.2-fold and 5.3-fold increased odds of MS occurrence under the co-dominant genetic model (OR = 2.157; CI: 1.194–3.897; p = 0.011 and OR = 5.343; CI: 1.438–19.848; p = 0.012, respectively). Also, AG+GG genotypes and each G allele were associated with a 2.5-fold and 2.2-fold increased odds of MS occurrence under the dominant and allelic genetic models (OR = 2.498; CI: 1.432–4.358; p = 0.001 and OR = 2.225; CI: 1.403–3.528; p < 0.001, respectively) (Table 9).

Table 9.
Binary logistic regression analysis of multiple sclerosis and control groups’ older participants.
SIRT1 Serum Levels in Early and Multiple Sclerosis and Controls
Serum SIRT1 levels were measured in groups of patients with MS (n = 20) and healthy subjects (n = 41). We found that SIRT1 serum levels statistically significantly differ between MS patients and control group subjects (1.833 (2.488) ng/mL vs. 0.094 (0.038) ng/mL, p < 0.001) (Figure 1).
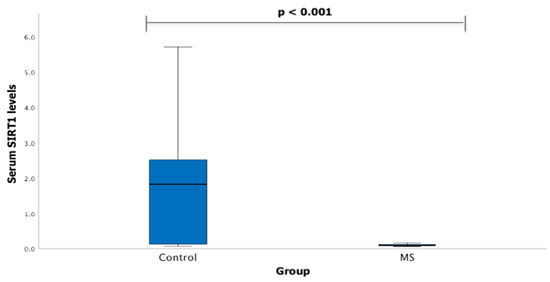
Figure 1.
SIRT1 serum levels in patients with multiple sclerosis and control group subjects. Mann–Whitney U test was used to assess serum SIRT1 levels differences between patients with multiple sclerosis and control groups; p < 0.001.
Serum SIRT1 levels were measured in groups of patients with MS (n = 13) and healthy subjects (n = 23). We found that females with MS had decreased SIRT1 serum levels compared to control group females (0.090 (0.047) ng/mL vs. 1.963 (2.614) ng/mL, p < 0.001) (Figure 2).
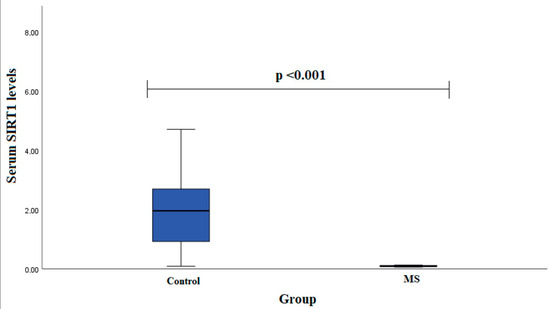
Figure 2.
SIRT1 serum levels in patients with multiple sclerosis and control group subjects in female group. Mann–Whitney U test was used to assess serum SIRT1 levels differences between patients with multiple sclerosis and control groups; p < 0.001.
Serum SIRT1 levels were measured in groups of patients with MS (n = 7) and healthy subjects (n = 19). We found that SIRT1 serum levels did not statistically significantly differ between MS patients and control group subjects in males (0.102 (0.028) ng/mL vs. 2.291 (4.095) ng/mL, p < 0.001) (Figure 3).
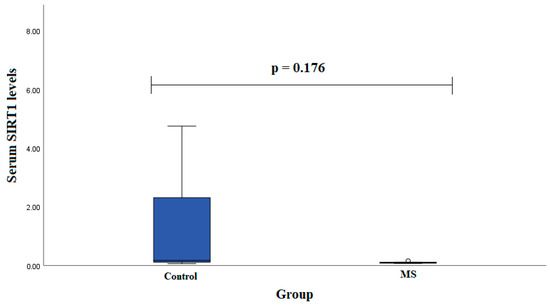
Figure 3.
SIRT1 serum levels in patients with multiple sclerosis and control group subjects in male group. Student test was used to assess serum SIRT1 levels differences between patients with multiple sclerosis and control groups; p =0.176.
A serum SIRT1 level comparison between study groups and genotypes was performed and did show statistically significantly differences between two groups. We found that rs3818292 AA, rs3758391 CT, and rs7895833 AA and AG carriers had higher SIRT1 levels in the control group than the MS group (0.239 (2.377) vs. 1.245 (0.045); p = 0.001, 0.304 (2.770) vs. 0.089 (0.037); p < 0.001, 1.813 (2.812) vs. 0.094 (0.054); p = 0.002 and 1.872 (2.763) vs.0.089 (0.028); p = 0.004, respectively). A Mann–Whitney U test was used to compare SIRT1 levels between the two groups. The bars represent the median with the interquartile range (Table 10).

Table 10.
Genotype distribution and serum SIRT1 levels between patients with multiple sclerosis and control group.
4. Discussion
In this study, we investigated the possible association between SIRT1 gene polymorphisms and SIRT1 serum levels in patients with multiple sclerosis in Lithuania. We performed genotyping analysis of three specific single nucleotide polymorphisms (SNPs) within the SIRT1 gene (rs3818292, rs3758391, and rs7895833). The analysis was performed on two groups consisting of 250 patients with MS and 250 control subjects. The results indicated an association between the three SNPs and a higher probability of developing MS.
As previously mentioned, gender is considered one of the risk factors for MS [4]. Our research results suggest that the variants of SIRT1 gene, rs3758391 and rs7895833, are significantly associated with increased probability of developing MS. However, no significant difference was found in the results between males. Previous studies have shown that there are differences in the occurrence and clinical presentation of MS between genders [28]. Females tend to have an earlier onset of the disease MS and have a higher likelihood of relapse, while males tend to have a more aggressive form of the disease with faster progression of disability [29,30]. Hormonal factors may play a role in these sex differences, but the exact mechanisms are not fully understood [31].
Our research shows that the variants of the SIRT1 gene, rs3758391 and rs7895833, are significantly associated with an increased likelihood of developing MS in younger patients, while the variants rs3818292 and rs7895833 are significantly associated with an increased likelihood of developing MS in older patients. It is known that age may also be a factor determining the prognosis of many neurodegenerative diseases, including MS [32]. The transition from the relapsing phase of MS, which is primarily inflammatory, to the secondary progressive phase of the disease, which is thought to be primarily neurodegenerative, is strongly associated with age and is considered the strongest predictor of this transition [33]. MS affects people of all ages but is most commonly diagnosed between 20 and 40 years of age [34]. The age of onset and clinical course of MS can vary widely, but patients with early-onset multiple sclerosis typically have relapsing-remitting disease, whereas patients with later-onset disease may experience more rapid progression to permanent disability [35].
Our study focused on specific genetic variations of SIRT1 based on their location in the gene. The intronic variant rs3818292 may affect the splicing processes of the gene, while rs3758391 and rs7895833 are functional variants located in the promoter region [22,23,24,27]. These variants likely result in altered expression of the SIRT1 protein, as indicated by differences in serum levels between different groups and between carriers of different genotypes. We found that serum SIRT1 levels were higher in the control group than in the multiple sclerosis group. These results confirm previous conclusions that increasing SIRT1 expression can decrease autoimmunity and reduce the incidence of neurodegenerative disorders and neuroexcitation. To prevent neurological complications, it is critical to understand SIRT1 signaling and identify immune-mediated damage to neurons for potential therapeutic intervention [13].
The location of these SIRT1 polymorphisms may play an important role in regulating gene expression and contribute to various disease susceptibilities.
Studies have shown that rs3818292 has a weak association with the risk of developing Parkinson’s disease (PD) [36]. Both diseases, PD and MS, affect the human nervous system [37]. In addition, mutation in the rs3818292 locus may be associated with a lower risk of developing diabetic kidney disease (DKD) [38]. The pathogenesis of kidney disease in patients with MS may be related to lower urinary tract dysfunction, recurrent urinary tract infections, and treatment with immunomodulatory agents such as interferons [39].
In addition, rs3818292 has been associated with visceral body fat in men with obesity [40]. Recent research has consistently shown that there is an association between obesity and an increased risk of developing multiple sclerosis [41].
Another SIRT1 genetic variant, rs3758391, is a polymorphism that has been associated with various diseases such as type 2 diabetes, breast cancer, autoimmune thyroid disease, lupus erythematosus, and others [42].
Studies conducted with SIRT1 rs7895833 are closely related to multiple sclerosis pathogenesis. There is an association between SIRT1 expression in the elderly and the rs7895833 variant in the SIRT1 gene [43]. Another study showed that 42% of elderly patients in Brazil had variant allele G of the SIRT1 gene polymorphism, which was associated with dyslipidemia [44]. It is well known that multiple sclerosis and dyslipidemia are linked through the association between inflammation and alterations in lipid metabolism [45]. In addition, an association between this polymorphism and increased risk of hypertension, higher body fat percentage, higher body mass index, and higher diastolic blood pressure has been demonstrated [46].
This study has shown significant associations between the genetic variations of SIRT1 rs3818292, rs3758391, and rs7895833 and the development of multiple sclerosis, with possible differences in gender and age. In addition, these genetic variations were found to be associated with lower serum SIRT1 levels. Also, females with MS had decreased SIRT1 serum levels compared to control group females. These results suggest that genetic SIRT1 variations may be potential prognostic factors for multiple sclerosis and may contribute to the identification of new therapeutic targets. However, further studies are needed to explore the precise mechanisms underlying the associations between genetic SIRT1 variations and multiple sclerosis and to determine the generalizability of these findings to other populations.
This study is significant because it analyzes SIRT1 rs3818292, rs3758391, and rs7895833, and serum SIRT1 levels in individuals with multiple sclerosis in the Lithuanian population and compares these results with those of healthy control subjects without other diseases, such as optic neuritis. Our study has some limitations to acknowledge. The relatively small sample size indicates the need for additional research with a larger cohort to draw stronger conclusions. Additionally, our analysis did not account for other potential risk factors like smoking, vitamin D levels, infection agents, and dietary preferences. Lastly, as our study exclusively focused on the Lithuanian population, its generalizability to other populations is limited. Despite these limitations, our findings offer valuable insights, serving as a foundation for future research and potential clinical applications. However, future studies should consider these limitations for a more comprehensive understanding.
5. Conclusions
Genetic variations in SIRT1 rs3818292, rs3758391, and rs7895833 are associated with multiple sclerosis, with possible differences in sex and age, and lower serum SIRT1 levels.
Author Contributions
Conceptualization, R.L. and R.B.; methodology, K.K., G.G., R.B. and R.L.; formal analysis, K.K., G.G., R.B. and R.L.; investigation, K.K., G.G. and R.B.; resources, R.B. and R.L.; data curation, R.B. and R.L.; writing—original draft preparation, K.K.; writing—review and editing, K.K., G.G., R.B., I.U. and R.L.; supervision, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee for Biomedical Research, Lithuanian University of Health Sciences (no. BE-2-/102).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data relevant to the study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cotsapas, C.; Mitrovic, M.; Hafler, D. Multiple sclerosis. Handb. Clin. Neurol. 2018, 148, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Patsopoulos, N.A. Genetics and functional genomics of multiple sclerosis. Semin. Immunopathol. 2022, 44, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 2013, 13 (Suppl. S12), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Blizzard, L., Jr.; Otahal, P.; Van der Mei, I.; Taylor, B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Valadkeviciene, D.; Kavaliunas, A.; Kizlaitiene, R.; Jocys, M.; Jatuzis, D. Incidence rate and sex ratio in multiple sclerosis in Lithuania. Brain Behav. 2019, 9, e01150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Jaliffa, C.; Ameqrane, I.; Dansault, A.; Leemput, J.; Vieira, V.; Lacassagne, E.; Provost, A.; Bigot, K.; Masson, C.; Menasche, M.; et al. Sirt1 involvement in rd10 mouse retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3562–3572. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Donmez, G.; Arun, A.; Chung, C.Y.; McLean, P.J.; Lindquist, S.; Guarente, L. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 124–132. [Google Scholar] [CrossRef]
- LeBrasseur, N.K. Physical Resilience: Opportunities and Challenges in Translation. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front. Neurosci. 2018, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Shandilya, A.; Kumar, N.; Mehan, S. Dysregulation of SIRT-1 Signaling in Multiple Sclerosis and Neuroimmune Disorders: A Systematic Review of SIRTUIN Activators as Potential Immunomodulators and their Influences on other Dysfunctions. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1845–1868. [Google Scholar] [CrossRef] [PubMed]
- Foolad, F.; Khodagholi, F.; Javan, M. Sirtuins in Multiple Sclerosis: The crossroad of neurodegeneration, autoimmunity and metabolism. Mult. Scler. Relat. Disord. 2019, 34, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Piacente, F.; Bottero, M.; Benzi, A.; Vigo, T.; Uccelli, A.; Bruzzone, S.; Ferrara, G. Neuroprotective Potential of Dendritic Cells and Sirtuins in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 4352. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free. Radic. Biol. Med. 2015, 88 Pt B, 179–188. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. Alzheimer’s Disease Cooperative Study A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Ntetsika, T.; Papathoma, P.E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Q.; Zhang, X.; Zhen, Y.; He, X.Y.; Zhao, S.; Li, X.F.; Yang, B.; Gao, F.; Guo, F.Y.; Fu, L.; et al. A novel small-molecule activator of Sirtuin-1 induces autophagic cell death/mitophagy as a potential therapeutic strategy in glioblastoma. Cell Death Dis. 2018, 9, 767. [Google Scholar] [CrossRef]
- Rafalski, V.A.; Ho, P.P.; Brett, J.O.; Ucar, D.; Dugas, J.C.; Pollina, E.A.; Chow, L.M.; Ibrahim, A.; Baker, S.J.; Barres, B.A.; et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat. Cell Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef]
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. SIRT1: A Potential Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2021, 12, 779177. [Google Scholar] [CrossRef] [PubMed]
- RS3818292 Refsnp Report—dbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs3818292 (accessed on 22 March 2023).
- RS3758391 RefSNP Report—dbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs3758391#frequency_tab (accessed on 22 March 2023).
- RS7895833 Refsnp Report—dbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs7895833 (accessed on 22 March 2023).
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet. Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MAGNIMS Study Group MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet. Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Hargreaves, K.A.; Li, R.; Reiter, J.L.; Wang, Y.; Mort, M.; Cooper, D.N.; Zhou, Y.; Zhang, C.; Eadon, M.T.; et al. RegSNPs-intron: A computational framework for predicting pathogenic impact of intronic single nucleotide variants. Genome Biol. 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Airas, L. Hormonal and gender-related immune changes in multiple sclerosis. Acta Neurol. Scand. 2015, 132, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S.; Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain A J. Neurol. 2003, 126 Pt 4, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, R. Prognostic factors in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Kritsilis, M.; V Rizou, S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Daumer, M.; Ebers, G.C.; Muraro, P.A. Age and disability accumulation in multiple sclerosis. Neurology 2011, 77, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.S.; Krysko, K.M.; Hua, L.H.; Absinta, M.; Franklin, R.J.M.; Segal, B.M. Ageing and multiple sclerosis. Lancet. Neurol. 2023, 22, 66–77. [Google Scholar] [CrossRef]
- Maszlag-Török, R.; Boros, F.A.; Vécsei, L.; Klivényi, P. Gene variants and expression changes of SIRT1 and SIRT6 in peripheral blood are associated with Parkinson’s disease. Sci. Rep. 2021, 11, 10677. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Pasternak, B.; Stenager, E.; Koch-Henriksen, N.; Frisch, M. Multiple sclerosis and risk of Parkinson’s disease: A Danish nationwide cohort study. Eur. J. Neurol. 2014, 21, 107–111. [Google Scholar] [CrossRef]
- Yue, X.G.; Yang, Z.G.; Zhang, Y.; Qin, G.J.; Liu, F. Correlations between SIRT1 gene polymorphisms and diabetic kidney disease. R. Soc. Open Sci. 2018, 5, 171871. [Google Scholar] [CrossRef] [PubMed]
- Alugba, G.; Urhi, A.; Olateju, I.V.; Onyemarin, H.; Uzzi, C.; Oshiba-Fowode, T.; Obomanu, E.; Popoola, H.A.; Okoronkwo, E.J.; Ukenenye, E.; et al. Renal diseases associated with multiple sclerosis: A narrative review. Medicine 2022, 101, e31959. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.V.; Beckers, S.; Verrijken, A.; Mertens, I.; Roevens, P.; Peeters, P.J.; Van Hul, W.; Van Gaal, L.F. Association of SIRT1 gene variation with visceral obesity. Hum. Genet. 2008, 124, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Barcellos, L.F. Obesity and Multiple Sclerosis Susceptibility: A Review. J. Neurol. Neuromed. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Ramírez, Á.; Hernández, M.; Suárez-Sánchez, R.; Ortega, C.; Peralta, J.; Gómez, J.; Valladares, A.; Cruz, M.; Vázquez-Moreno, M.A.; Suárez-Sánchez, F. Type 2 diabetes-associated polymorphisms correlate with SIRT1 and TGF-β1 gene expression. Ann. Hum. Genet. 2020, 84, 185–194. [Google Scholar] [CrossRef]
- Kilic, U.; Gok, O.; Erenberk, U.; Dundaroz, M.R.; Torun, E.; Kucukardali, Y.; Elibol-Can, B.; Uysal, O.; Dundar, T. A remarkable age-related increase in SIRT1 protein expression against oxidative stress in elderly: SIRT1 gene variants and longevity in human. PLoS ONE 2015, 10, e0117954. [Google Scholar] [CrossRef]
- Casarotto, A.A.F.; Galera, B.B.; Sumiyoshi, L.M.; Floôr, T.M. Polymorphism rs7895833 in the SIRT1 gene and its association with dyslipidaemia in the elderly. Rev. Española Geriatría Gerontol. 2019, 54, 214–219. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Suzuki, K.; Hamajima, N.; Niwa, T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl. Res. J. Lab. Clin. Med. 2011, 157, 339–347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).