Correlation of Perfusion Metrics with Ki-67 Proliferation Index and Axillary Involvement as a Prognostic Marker in Breast Carcinoma Cases: A Dynamic Contrast-Enhanced Perfusion MRI Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPherson, K.; Steel, C.M.; Dixon, J.M. Breast Cancer—Epidemiology, Risk Factors, and Genetics. BMJ 2000, 321, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Vuong, D.; Simpson, P.T.; Green, B.; Cummings, M.C.; Lakhani, S.R. Molecular Classification of Breast Cancer. Virchows Arch. Int. J. Pathol. 2014, 465, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Panel members Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Cheang, M.C.U.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 Index, HER2 Status, and Prognosis of Patients with Luminal B Breast Cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-Enhanced MRI for Breast Cancer Screening. J. Magn. Reson. Imaging JMRI 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Bassingthwaighte, J.B. Blood Flow Distributions by Microsphere Deposition Methods. Cardiovasc. Res. 2000, 45, 13–21. [Google Scholar] [CrossRef]
- Tofts, P.S. T1-Weighted DCE Imaging Concepts: Modelling, Acquisition and Analysis. MAGNETOM Flash 2010, 45, 30–39. [Google Scholar]
- Leach, M.O.; Brindle, K.M.; Evelhoch, J.L.; Griffiths, J.R.; Horsman, M.R.; Jackson, A.; Jayson, G.C.; Judson, I.R.; Knopp, M.V.; Maxwell, R.J.; et al. The Assessment of Antiangiogenic and Antivascular Therapies in Early-Stage Clinical Trials Using Magnetic Resonance Imaging: Issues and Recommendations. Br. J. Cancer 2005, 92, 1599–1610. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Kang, B.J. Prognostic Factors of Disease Recurrence in Breast Cancer Using Quantitative and Qualitative Magnetic Resonance Imaging (MRI) Parameters. Sci. Rep. 2020, 10, 7598. [Google Scholar] [CrossRef]
- Meyer, H.J.; Wienke, A.; Surov, A. Correlation Between Ktrans and Microvessel Density in Different Tumors: A Meta-Analysis. Anticancer. Res. 2018, 38, 2945–2950. [Google Scholar] [PubMed]
- Gao, Y.; Feng, W.; Lu, X.-R.; Guo, Z.-Z.; Lei, J.-Q. Difference of DCE-MRI Parameters at Different Time Points and Their Predictive Value for Axillary Lymph Node Metastasis of Breast Cancer. Acad. Radiol. 2022, 29, S79–S86. [Google Scholar] [CrossRef]
- Lim, G.H.; Leong, L.C.H. Oncologic Outcomes in Breast Cancer Patients with Metastatic Nodes and Pathological Nodal Response Following Neoadjuvant Chemotherapy without Axillary Dissection: A Literature Review. Ann. Transl. Med. 2023, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, M.; Furukawa, M.; Yamamoto, S.; Nakamura, K.; Tanabe, M.; Okada, M.; Iida, E.; Ito, K. The Kinetic Analysis of Breast Cancer: An Investigation of the Optimal Temporal Resolution for Dynamic Contrast-Enhanced MR Imaging. Clin. Imaging 2020, 61, 4–10. [Google Scholar] [CrossRef]
- Nam, J.G.; Kang, K.M.; Choi, S.H.; Lim, W.H.; Yoo, R.-E.; Kim, J.-H.; Yun, T.J.; Sohn, C.-H. Comparison between the Prebolus T1 Measurement and the Fixed T1 Value in Dynamic Contrast-Enhanced MR Imaging for the Differentiation of True Progression from Pseudoprogression in Glioblastoma Treated with Concurrent Radiation Therapy and Temozolomide Chemotherapy. AJNR Am. J. Neuroradiol. 2017, 38, 2243–2250. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Connolly, J.L.; D’Orsi, C.J.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Giuliano, A. Breast. In AJCC Cancer Staging Manual; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 589–636. ISBN 978-3-319-40617-6. [Google Scholar]
- Ecanow, J.S.; Abe, H.; Newstead, G.M.; Ecanow, D.B.; Jeske, J.M. Axillary Staging of Breast Cancer: What the Radiologist Should Know. RadioGraphics 2013, 33, 1589–1612. [Google Scholar] [CrossRef]
- Peppercorn, J.; Perou, C.M.; Carey, L.A. Molecular Subtypes in Breast Cancer Evaluation and Management: Divide and Conquer. Cancer Investig. 2008, 26, 1–10. [Google Scholar] [CrossRef]
- Rosen, E.L.; Blackwell, K.L.; Baker, J.A.; Soo, M.S.; Bentley, R.C.; Yu, D.; Samulski, T.V.; Dewhirst, M.W. Accuracy of MRI in the Detection of Residual Breast Cancer after Neoadjuvant Chemotherapy. AJR Am. J. Roentgenol. 2003, 181, 1275–1282. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Robertson, S.; Rönnlund, C.; de Boniface, J.; Hartman, J. Re-Testing of Predictive Biomarkers on Surgical Breast Cancer Specimens Is Clinically Relevant. Breast Cancer Res. Treat. 2019, 174, 795–805. [Google Scholar] [CrossRef]
- Sutton, E.J.; Dashevsky, B.Z.; Oh, J.H.; Veeraraghavan, H.; Apte, A.P.; Thakur, S.B.; Morris, E.A.; Deasy, J.O. Breast Cancer Molecular Subtype Classifier That Incorporates MRI Features. J. Magn. Reson. Imaging JMRI 2016, 44, 122–129. [Google Scholar] [CrossRef]
- Wu, J.; Cao, G.; Sun, X.; Lee, J.; Rubin, D.L.; Napel, S.; Kurian, A.W.; Daniel, B.L.; Li, R. Intratumoral Spatial Heterogeneity at Perfusion MR Imaging Predicts Recurrence-Free Survival in Locally Advanced Breast Cancer Treated with Neoadjuvant Chemotherapy. Radiology 2018, 288, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.P.; Tessier, J.J.; Ashton, S.E.; Waterton, J.C.; Wilson, Z.; Worthington, P.L.; Ryan, A.J. Correlation of MRI Biomarkers with Tumor Necrosis in Hras5 Tumor Xenograft in Athymic Rats. Neoplasia 2007, 9, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.S.; Thng, C.H.; Hartono, S.; Dominguez, L.T.M.; Lim, T.K.H.; Huynh, H.; Martarello, L.; Ng, Q.S. Assessment of Tumor Necrotic Fraction by Dynamic Contrast-Enhanced MRI: A Preclinical Study of Human Tumor Xenografts with Histopathologic Correlation. NMR Biomed. 2014, 27, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.H.; Musall, B.C.; Adrada, B.E.; Hess, K.; Son, J.B.; Hwang, K.-P.; Candelaria, R.P.; Santiago, L.; Whitman, G.J.; Le-Petross, H.T.; et al. Tumor Necrosis by Pretreatment Breast MRI: Association with Neoadjuvant Systemic Therapy (NAST) Response in Triple-Negative Breast Cancer (TNBC). Breast Cancer Res. Treat. 2021, 185, 1–12. [Google Scholar] [CrossRef]
- Shin, J.K.; Kim, J.Y. Dynamic Contrast-Enhanced and Diffusion-Weighted MRI of Estrogen Receptor-Positive Invasive Breast Cancers: Associations between Quantitative MR Parameters and Ki-67 Proliferation Status. J. Magn. Reson. Imaging 2017, 45, 94–102. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Kang, B.J.; Song, B.J.; Kim, H.-B.; Lee, H.; Jin, M.-S.; Lee, A. Dynamic Contrast-Enhanced MRI Perfusion Parameters as Imaging Biomarkers of Angiogenesis. PLoS ONE 2016, 11, e0168632. [Google Scholar] [CrossRef]
- Nagasaka, K.; Satake, H.; Ishigaki, S.; Kawai, H.; Naganawa, S. Histogram Analysis of Quantitative Pharmacokinetic Parameters on DCE-MRI: Correlations with Prognostic Factors and Molecular Subtypes in Breast Cancer. Breast Cancer Tokyo Jpn. 2019, 26, 113–124. [Google Scholar] [CrossRef]
- Kang, S.R.; Kim, H.W.; Kim, H.S. Evaluating the Relationship Between Dynamic Contrast-Enhanced MRI (DCE-MRI) Parameters and Pathological Characteristics in Breast Cancer. J. Magn. Reson. Imaging 2020, 52, 1360–1373. [Google Scholar] [CrossRef]
- Thakran, S.; Gupta, P.K.; Kabra, V.; Saha, I.; Jain, P.; Gupta, R.K.; Singh, A. Characterization of Breast Lesion Using T1-Perfusion Magnetic Resonance Imaging: Qualitative vs. Quantitative Analysis. Diagn. Interv. Imaging 2018, 99, 633–642. [Google Scholar] [CrossRef]
- Loiselle, C.; Eby, P.R.; Kim, J.N.; Calhoun, K.E.; Allison, K.H.; Gadi, V.K.; Peacock, S.; Storer, B.E.; Mankoff, D.A.; Partridge, S.C.; et al. Preoperative MRI Improves Prediction of Extensive Occult Axillary Lymph Node Metastases in Breast Cancer Patients with a Positive Sentinel Lymph Node Biopsy. Acad. Radiol. 2014, 21, 92–98. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, J.Y.; Hwangbo, L.; Suh, H.B.; Son, Y.; Nickel, M.D.; Grimm, R. Ultrafast Dynamic Contrast-Enhanced MRI Using Compressed Sensing: Associations of Early Kinetic Parameters with Prognostic Factors of Breast Cancer. Am. J. Roentgenol. 2021, 217, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Nakazono, T.; Egashira, R.; Fukui, S.; Baba, K.; Hamamoto, T.; Irie, H. Maximum Slope of Ultrafast Dynamic Contrast-Enhanced MRI of the Breast: Comparisons with Prognostic Factors of Breast Cancer. Jpn. J. Radiol. 2021, 39, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhu, Y.; Liu, Z.; Yu, T.; He, C.; Jiang, W.; Kan, Y.; Dong, D.; Tian, J.; Luo, Y. Radiomic Nomogram for Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Eur. Radiol. 2019, 29, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
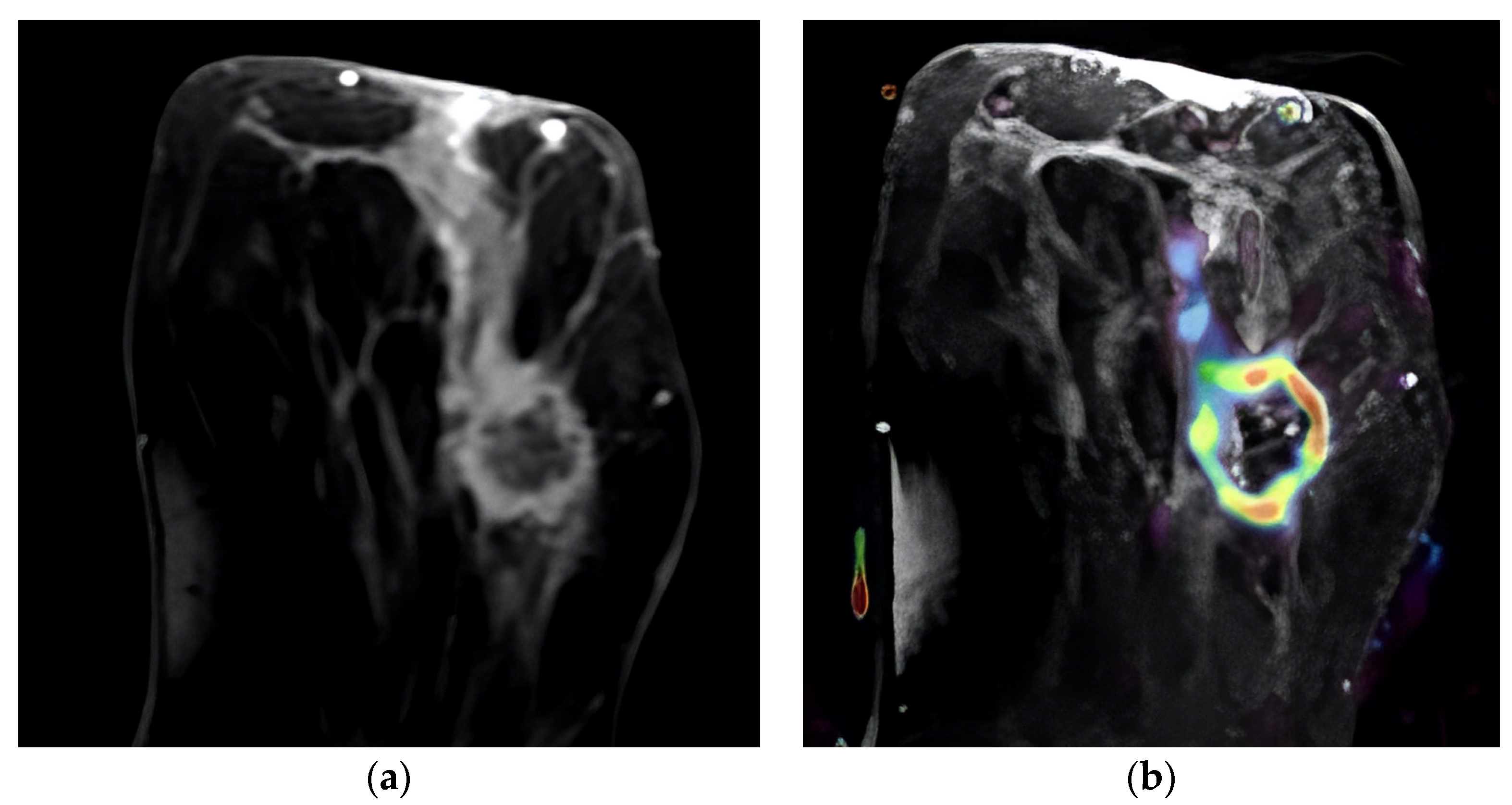
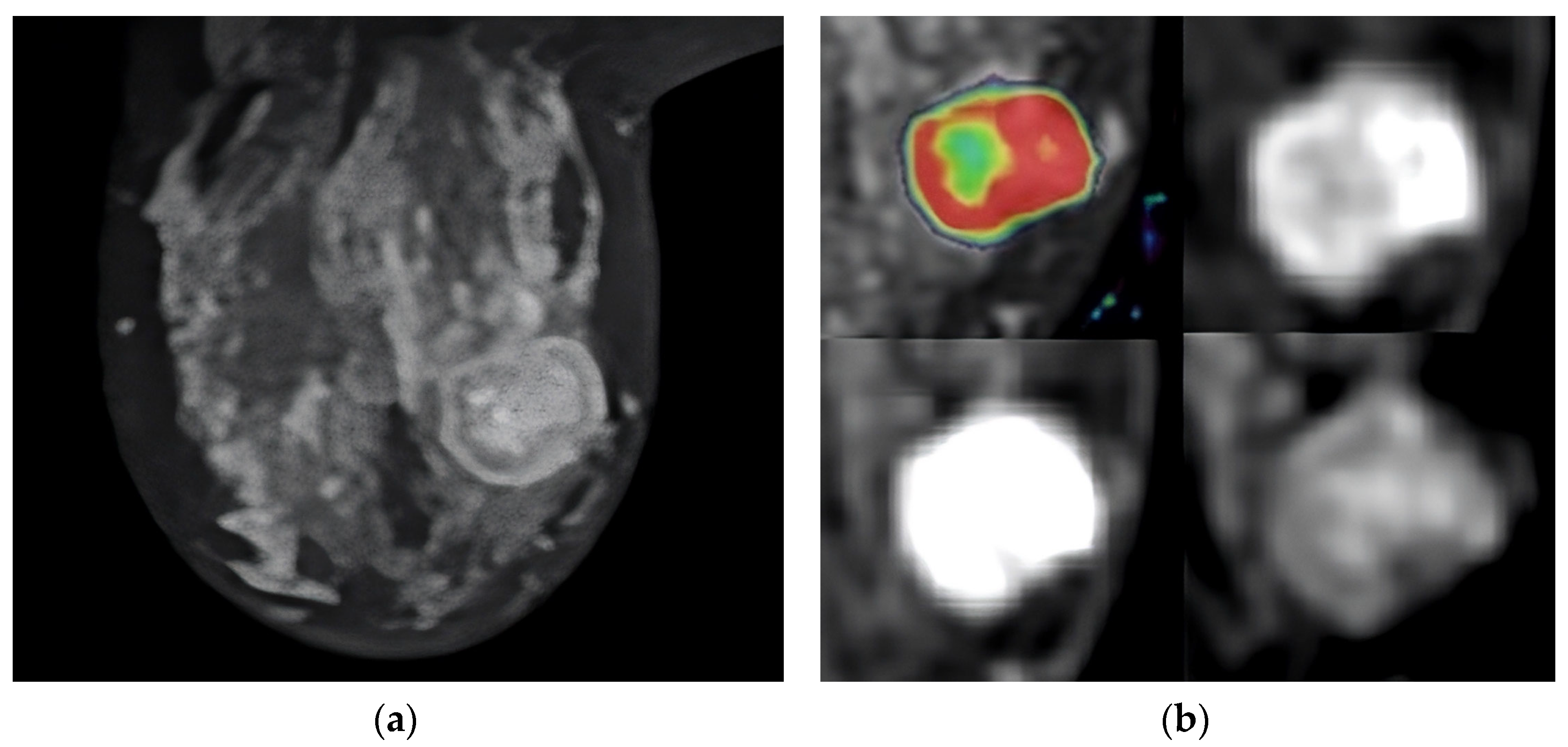

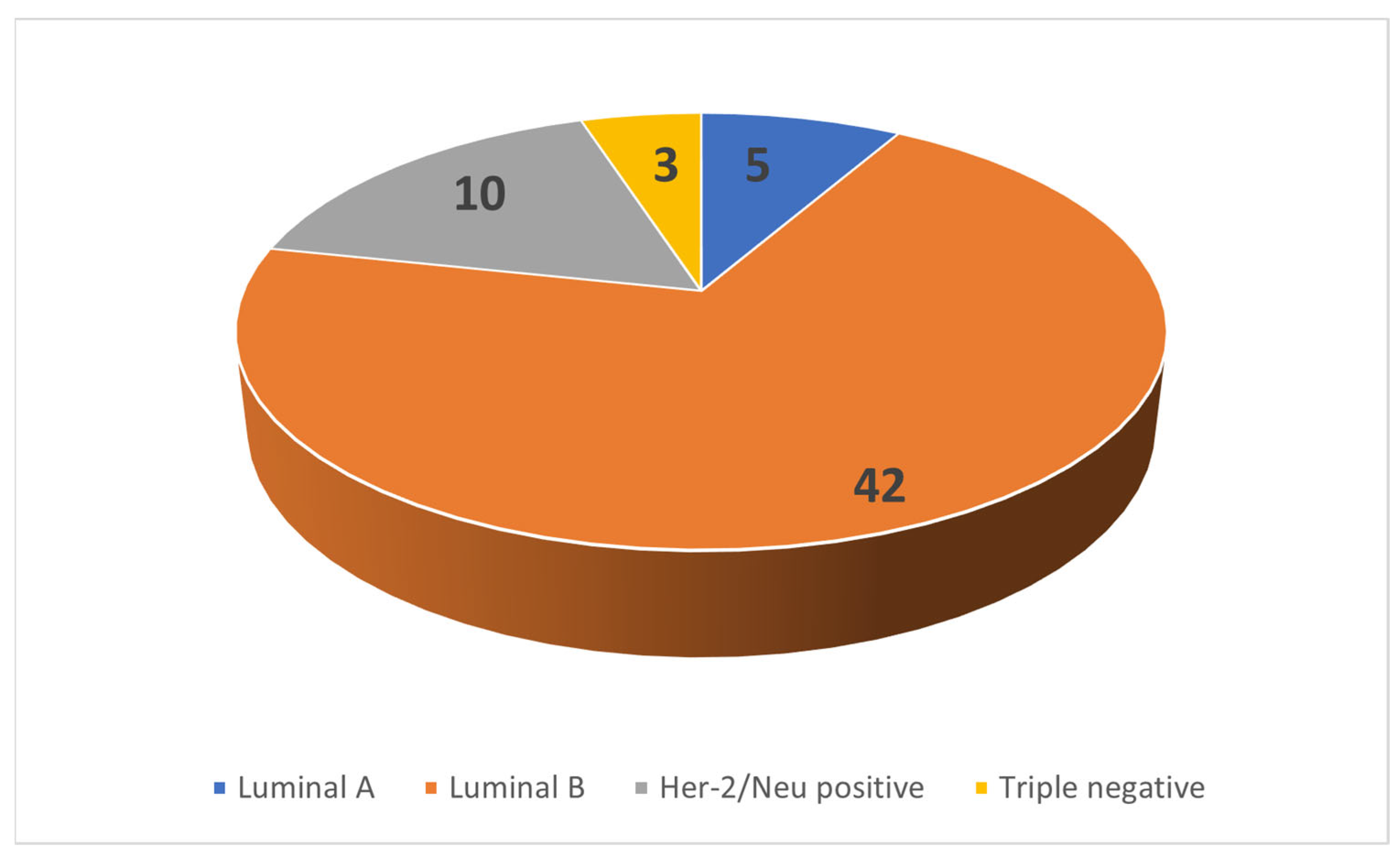

| Perfusion Parameter | N | Mean | Median | IQR |
|---|---|---|---|---|
| Ktrans | 60 | 1.320000 | 0.966 | 0.976 |
| Kep | 60 | 1.96800 | 1.412 | 1.624 |
| Ve | 60 | 0.771000 | 0.834 | 0.314 |
| IAUGC | 60 | 1.034000 | 0.906 | 0.641 |
| Enhancement Pattern | Number (% of Total) | Low Axillary Stage (%within Pattern) | High Axillary Stage (%within Pattern) | |
|---|---|---|---|---|
| Homogenous | 17 (28.3) | 13 (76.5) | 4 (23.5) | |
| Heterogeneous | Complete enhancement | 23 (38.3) | 12 (52.2) | 11 (47.8) |
| Non-enhancing voxels | 11 (18.3) | 3 (27.3) | 8 (72.7) | |
| Peripheral | 9 (15) | 3 (33.3) | 6 (66.7) | |
| Total | 60 | 31 (51.7) | 29 (48.3) | |
| Filling Pattern | ||||
| Slow filling | 39 (65) | 24 (61.5) | 15 (38.5) | |
| Rapid filling | 8 (13.3) | 5 (62.5) | 3 (37.5) | |
| Rapid washout | 13 (21.7) | 2 (15.4) | 11 (24.6) | |
| Total | 60 | 100 | ||
| Perfusion Parameter | LN Stage | N | Mean | Median | IQR | 25–75 Percentiles | AUC | p Value * | Z Score * |
|---|---|---|---|---|---|---|---|---|---|
| Ktrans | Low (N0–1) | 31 | 1.173065 | 0.832 | 0.913 | 0.614–1.527 | 0.642 | 0.059 | 1.886 |
| High (N2–3) | 29 | 1.478069 | 1.129 | 0.957 | 0.834–1.792 | ||||
| Kep | Low (N0–1) | 31 | 1.71285 | 1.175 | 1.852 | 0.848–2.700 | 0.630 | 0.085 | 1.723 |
| High (N2–3) | 29 | 2.24086 | 1.485 | 1.437 | 1.234–2.676 | ||||
| Ve | Low (N0–1) | 31 | 0.749968 | 0.791 | 0.288 | 0.618–0.906 | 0.618 | 0.115 | 1.575 |
| High (N2–3) | 29 | 0.793252 | 0.909 | 0.348 | 0.632–0.980 | ||||
| IAUGC | Low (N0–1) | 31 | 0.841161 | 0.654 | 0.657 | 0.408–1.070 | 0.686 | 0.013 | 2.478 |
| High (N2–3) | 29 | 1.241069 | 0.985 | 0.893 | 0.761–1.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uncu, U.Y.; Aydin Aksu, S. Correlation of Perfusion Metrics with Ki-67 Proliferation Index and Axillary Involvement as a Prognostic Marker in Breast Carcinoma Cases: A Dynamic Contrast-Enhanced Perfusion MRI Study. Diagnostics 2023, 13, 3260. https://doi.org/10.3390/diagnostics13203260
Uncu UY, Aydin Aksu S. Correlation of Perfusion Metrics with Ki-67 Proliferation Index and Axillary Involvement as a Prognostic Marker in Breast Carcinoma Cases: A Dynamic Contrast-Enhanced Perfusion MRI Study. Diagnostics. 2023; 13(20):3260. https://doi.org/10.3390/diagnostics13203260
Chicago/Turabian StyleUncu, Ulas Yalim, and Sibel Aydin Aksu. 2023. "Correlation of Perfusion Metrics with Ki-67 Proliferation Index and Axillary Involvement as a Prognostic Marker in Breast Carcinoma Cases: A Dynamic Contrast-Enhanced Perfusion MRI Study" Diagnostics 13, no. 20: 3260. https://doi.org/10.3390/diagnostics13203260
APA StyleUncu, U. Y., & Aydin Aksu, S. (2023). Correlation of Perfusion Metrics with Ki-67 Proliferation Index and Axillary Involvement as a Prognostic Marker in Breast Carcinoma Cases: A Dynamic Contrast-Enhanced Perfusion MRI Study. Diagnostics, 13(20), 3260. https://doi.org/10.3390/diagnostics13203260






