Diagnosis of Cholangiocarcinoma
Abstract
1. Introduction
2. Clinical Presentation
2.1. Signs and Symptoms
2.2. Physical Examination
3. Laboratory Findings
3.1. Carbohydrate Antigen 19-9
3.2. Carcinoembryonic Antigen
3.3. Alpha-Fetoprotein
3.4. Serum IgG4
4. Radiologic Findings
4.1. Transabdominal Ultrasound
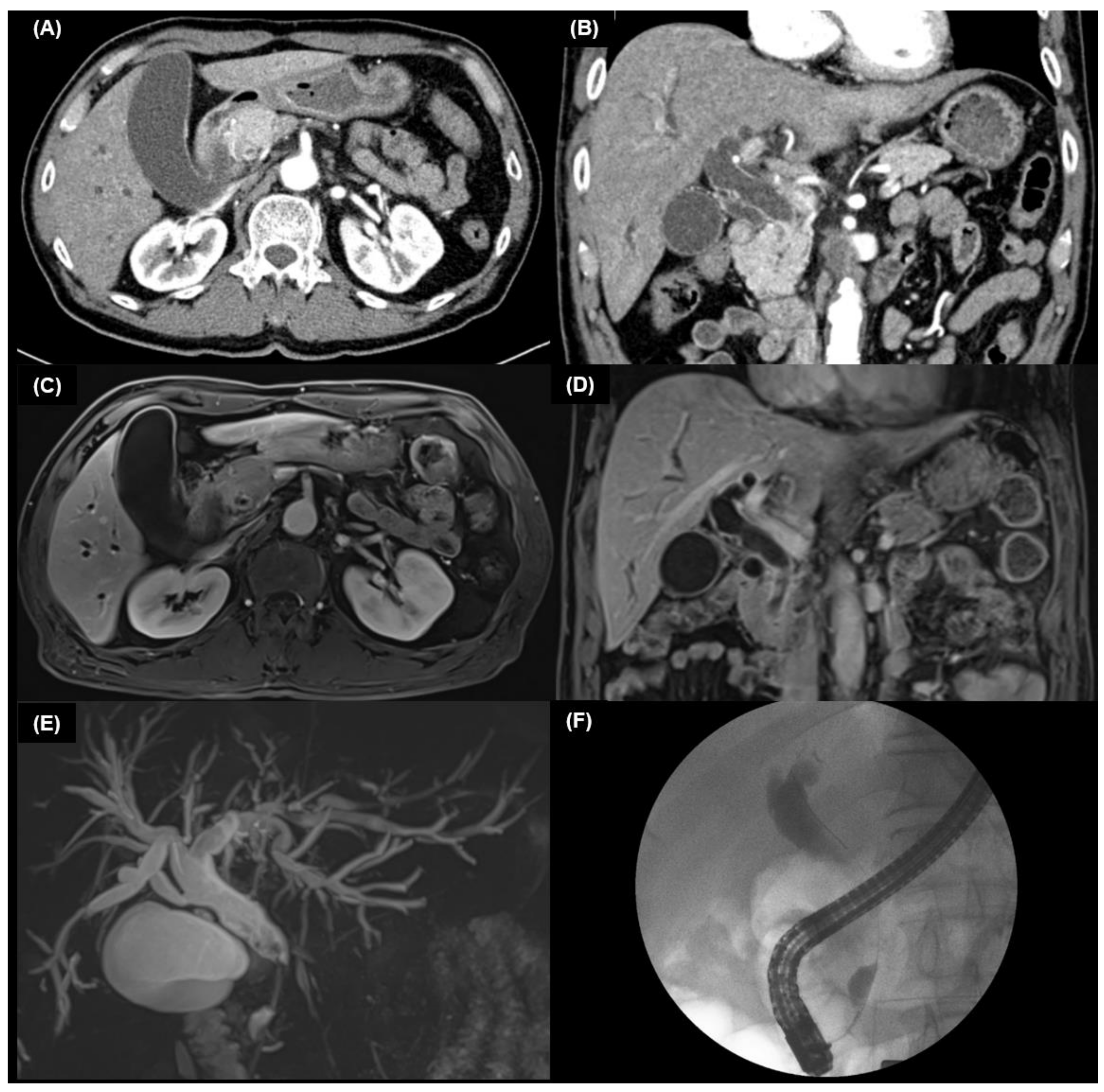
4.2. Multidetector Computed Tomography
4.2.1. Intrahepatic Cholangiocarcinoma
4.2.2. Extrahepatic Cholangiocarcinoma
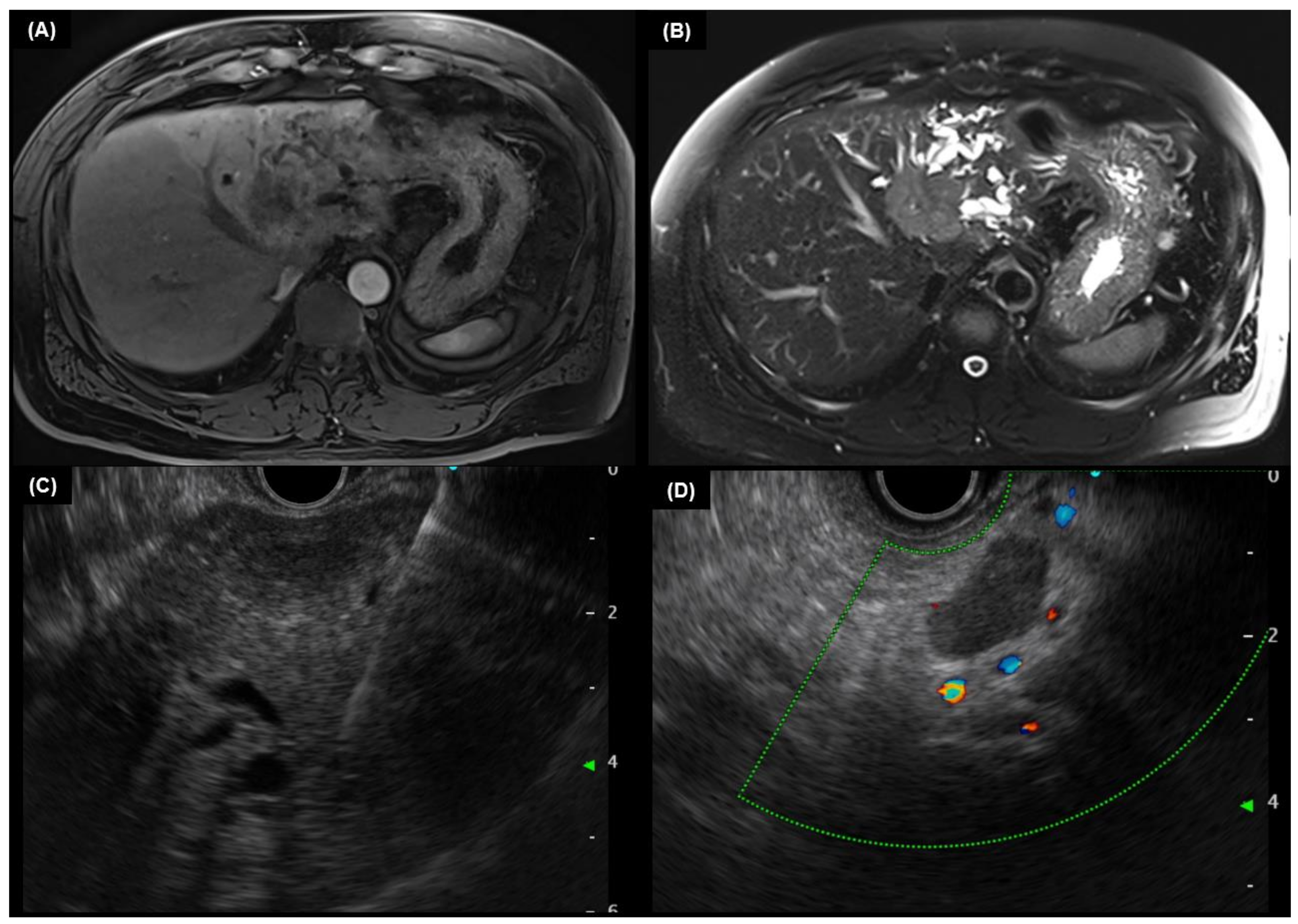
4.3. MRI and MRCP
4.4. PET Scan
4.5. Chest CT
5. Endoscopic Findings
5.1. Cholangiography
5.2. Endoscopic Ultrasound
5.3. Intraductal Ultrasound
5.4. Cholangioscopy
6. Pathologic Findings
7. Genomic Heterogeneity
8. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mukkamalla, S.K.R.; Naseri, H.M.; Kim, B.M.; Katz, S.C.; Armenio, V.A. Trends in Incidence and Factors Affecting Survival of Patients with Cholangiocarcinoma in the United States. J. Natl. Compr. Cancer Netw. 2018, 16, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Nagorney, D.M.; Chun, Y.S. Perihilar bile ducts. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; AJCC: Chicago, IL, USA, 2017; p. 311. [Google Scholar]
- Krasinskas, A.; Mino-Kenudson, M.; Vauthey, J.-N. Distal bile duct. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; AJCC: Chicago, IL, USA, 2017; p. 317. [Google Scholar]
- Aloia, T.; Pawlik, T.M.; Taouli, B. Intrahepatic bile ducts. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; AJCC: Chicago, IL, USA, 2017; p. 295. [Google Scholar]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Net-work for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Pan-Ngum, W.; Poovorawan, K.; Soonthornworasiri, N.; Treeprasertsuk, S.; Phaosawasdi, K. Characteristics and outcomes of cholangiocarcinoma by region in Thailand: A nationwide study. World J. Gastroenterol. 2017, 23, 7160–7167. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef]
- Brown, K.M.; Parmar, A.D.; Geller, D.A. Intrahepatic cholangiocarcinoma. Surg. Oncol. Clin. N Am. 2014, 23, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocar-cinoma. Liver Int. 2019, 39 (Suppl. S1), 98–107. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.E.; White, M.J.; Lobo, D.N. Courvoisier’s gallbladder: Law or sign? World J. Surg. 2009, 33, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, Y.; Toma, Y.; Terui, T. Sweet syndrome associated with intra-hepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br. J. Derm. 2006, 155, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Barrett, W.A.; Gilkes, J.; Rees, M.; Sarner, M.; Southcott, R.J. Oral acanthosis nigricans, the sign of Leser-Trélat and chol-angiocarcinoma. Br. J. Derm. 2001, 145, 506–507. [Google Scholar] [CrossRef]
- Sökmen, M.; Demirsoy, H.; Ersoy, O.; Gökdemir, G.; Akbayir, N.; Karaca, C.; Ozdil, K.; Kesici, B.; Calişkan, C.; Yilmaz, B. Paraneoplastic porphyria cutanea tarda associated with cholangiocarcinoma: Case report. Turk. J. Gastroenterol. 2007, 18, 200–205. [Google Scholar]
- La’Ulu, S.L.; Roberts, W.L. Performance Characteristics of Five Automated CA 19-9 Assays. Am. J. Clin. Pathol. 2007, 127, 436–440. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Paladina, I.; Giordano, M.; Malaguarnera, M.; Bertino, G.; Berretta, M. Serum markers of intrahepatic cholangiocarcinoma. Dis. Markers 2013, 34, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Harnois, D.M.; Klee, G.G.; LaRusso, N.F.; Gores, G.J. The utility of CA 19-9 in the diagnoses of cholangiocarcino-ma in patients without primary sclerosing cholangitis. Am. J. Gastroenterol. 2000, 95, 204–207. [Google Scholar] [CrossRef]
- Levy, C.; Lymp, J.; Angulo, P.; Gores, G.J.; LaRusso, N.; Lindor, K.D. The Value of Serum CA 19-9 in Predicting Cholangiocarcinomas in Patients with Primary Sclerosing Cholangitis. Dig. Dis. Sci. 2005, 50, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.R.; Ivanics, T.; Storlie, C.B.; Groeschl, R.T.; Tee, M.C.; Habermann, E.B.; Smoot, R.L.; Kendrick, M.L.; Farnell, M.B.; Roberts, L.R.; et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J. Surg. Oncol. 2016, 114, 475–482. [Google Scholar] [CrossRef]
- Chung, Y.J.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Kim, D.H. Prognostic factors following surgical resection of distal bile duct cancer. J. Korean Surg. Soc. 2013, 85, 212–218. [Google Scholar] [CrossRef]
- Sinakos, E.; Saenger, A.K.; Keach, J.; Kim, W.R.; Lindor, K.D. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 434–439.e1. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.H.; Myung, S.J.; Lim, B.C.; Park, E.T.; Yoo, K.S.; Min, Y.I. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am. J. Gastroenterol. 1999, 94, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1875, 188409. [Google Scholar] [CrossRef]
- Siqueira, E.; Schoen, R.E.; Silverman, W.; Martini, J.; Rabinovitz, M.; Weissfeld, J.L.; Abu Elmaagd, K.; Madariaga, J.R.; Slivka, A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest. Endosc. 2002, 56, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screen-ing for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Primary Liver Cancer in Japan. Clinicopathologic features and results of surgi-cal treatment. Ann. Surg. 1990, 211, 277–287. [Google Scholar]
- Oseini, A.M.; Chaiteerakij, R.; Shire, A.M.; Ghazale, A.; Kaiya, J.; Moser, C.D.; Aderca, I.; Mettler, T.A.; Therneau, T.M.; Zhang, L.; et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 2011, 54, 940–948. [Google Scholar] [CrossRef]
- Fadahunsi, O.O.; Ibitoye, B.O.; Adisa, A.O.; Alatise, O.I.; Adetiloye, V.A.; Idowu, B.M. Diagnostic accuracy of ultrasonography in adults with obstructive jaundice. J. Ultrason. 2020, 20, e100–e105. [Google Scholar] [CrossRef]
- Sharma, M.P.; Ahuja, V. Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: A clinician’s perspective. Trop. Gastroenterol. 1999, 20, 167–169. [Google Scholar]
- Bloom, C.M.; Langer, B.; Wilson, S.R. Role of US in the detection, charac-terization, and staging of cholangiocarcinoma. Radiographics 1999, 19, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Saini, S. Imaging of the Hepatobiliary Tract. N. Engl. J. Med. 1997, 336, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J.; Lindor, K.D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011, 54, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Valls, C.; Gumà, A.; Puig, I.; Sanchez, A.; Andía, E.; Serrano, T.; Figueras, J. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom. Imaging 2000, 25, 490–496. [Google Scholar] [CrossRef]
- Choi, S.H.; Han, J.K.; Lee, J.M.; Lee, K.H.; Kim, S.H.; Lee, J.Y.; Choi, B.I. Differentiating Malignant from Benign Common Bile Duct Stricture with Multiphasic Helical CT. Radiology 2005, 236, 178–183. [Google Scholar] [CrossRef]
- Iavarone, M.; Piscaglia, F.; Vavassori, S.; Galassi, M.; Sangiovanni, A.; Venerandi, L.; Forzenigo, L.V.; Golfieri, R.; Bolondi, L.; Colombo, M. Contrast enhanced CT-scan to diagnose intrahepatic chol-angiocarcinoma in patients with cirrhosis. J. Hepatol. 2013, 58, 1188–1193. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, J.M.; Lee, K.B.; Kim, S.H.; Yoon, S.H.; Han, J.K.; Choi, B.I. Intrahepatic mass-forming cholangiocarcinomas: Enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern--correlation with clinicopathologic findings. Radiology 2011, 260, 148–157. [Google Scholar] [CrossRef]
- Fowler, K.J.; Sheybani, A.; Parker, R.A., 3rd; Doherty, S.; Brunt, E.M.; Chapman, W.C.; Menias, C.O. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: Imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am. J. Roentgenol. 2013, 201, 332–339. [Google Scholar] [CrossRef]
- Sanada, Y.; Shiozaki, S.; Aoki, H.; Takakura, N.; Yoshida, K.; Yamaguchi, Y. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol. Res. 2005, 32, 185–195. [Google Scholar] [CrossRef]
- Manfredi, R.; Barbaro, B.; Masselli, G.; Vecchioli, A.; Marano, P. Magnetic resonance imaging of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 155–164. [Google Scholar] [CrossRef]
- Potretzke, T.A.; Tan, B.R.; Doyle, M.B.; Brunt, E.M.; Heiken, J.P.; Fowler, K.J. Imaging Features of Biphenotypic Primary Liver Carcinoma (Hepatocholangiocarcinoma) and the Potential to Mimic Hepatocellular Carcinoma: LI-RADS Analysis of CT and MRI Features in 61 Cases. AJR Am. J. Roentgenol. 2016, 207, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, Y.K.; Park, M.J.; Lee, M.H.; Kim, S.H.; Lee, W.J.; Rhim, H.C. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J. Magn. Reason. Imaging 2012, 36, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.S.; Kim, Y.K.; Lee, M.W.; Kim, S.H.; Lee, W.J.; Rhim, H.C.; Lee, S.J. Differentiating mass-forming intrahepatic cholangiocarcinoma from atypical hepatocellular carcinoma using gadoxetic acid-enhanced MRI. Clin. Radiol. 2012, 67, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zidi, S.H.; Prat, F.; Le Guen, O.; Rondeau, Y.; Pelletier, G. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut 2000, 46, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Park, K.B.; Shin, Y.M.; Yoon, H.K.; Sung, K.B.; Kim, M.H.; Lee, S.G.; Kang, E.M. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: Comparison with intraarterial digital subtraction angiography. World J. Surg. 2003, 27, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, J.M.; Choi, J.Y.; Lee, M.W.; Kim, H.J.; Han, J.K.; Choi, B.I. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am. J. Roentgenol. 2008, 190, 396–405. [Google Scholar] [CrossRef]
- Rösch, T.; Meining, A.; Frühmorgen, S.; Zillinger, C.; Schusdziarra, V.; Hellerhoff, K.; Classen, M.; Helmberger, H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest. Endosc. 2002, 55, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.S.; Jan, Y.Y.; Tseng, J.H.; Chiu, C.T.; Chen, T.C.; Hwang, T.L.; Chen, M.F. Malignant perihilar biliary obstruction: Magnetic resonance cholangiopancreatographic findings. Am. J. Gastroenterol. 2000, 95, 432–440. [Google Scholar] [CrossRef]
- Freeman, M.L.; Sielaff, T.D. A modern approach to malignant hilar biliary obstruction. Rev. Gastroenterol. Disord 2003, 3, 187–201. [Google Scholar] [PubMed]
- Corvera, C.U.; Blumgart, L.H.; Akhurst, T.; DeMatteo, R.P.; D’Angelica, M.; Fong, Y.; Jarnagin, W.R. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J. Am. Coll. Surg. 2008, 206, 57–65. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yun, M.; Lee, W.J.; Kim, K.S.; Lee, J.D. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuehl, H.; Grabellus, F.; Müller, S.P.; Radunz, S.; Antoch, G.; Nadalin, S.; Broelsch, C.E.; Gerken, G.; Paul, A.; et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J. Surg Oncol. 2008, 98, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Alkhawaldeh, K.; Faltten, S.; Biersack, H.J.; Ezziddin, S. The value of F-18 FDG PET in patients with primary sclerosing cholangitis and cholangiocarcinoma using visual and semiquantitative analysis. Clin. Nucl. Med. 2011, 36, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Yamamoto, Y.; Kimura, N.; Miki, A.; Sasakawa, Y.; Wakabayashi, H.; Ohkawa, M. Comparison of early and delayed FDG PET for evaluation of biliary stricture. Nucl. Med. Commun. 2007, 28, 914–919. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls (accessed on 18 May 2022).
- Shim, C.S.; Neuhaus, H.; Tamada, K. Direct cholangioscopy. Endoscopy 2003, 35, 752–758. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.Y.; Kim, K.S.; Park, M.S.; Kim, M.J.; Park, Y.N.; Bang, S.; Song, S.Y.; Chung, J.B.; Park, S.W. Intraductal ultrasonography combined with percutaneous transhepatic cholangioscopy for the preoperative evaluation of longitudinal tumor extent in hilar cholangiocarcinoma. J. Gastroenterol. Hepatol. 2010, 25, 286–292. [Google Scholar] [CrossRef]
- Tamada, K.; Ido, K.; Ueno, N.; Kimura, K.; Ichiyama, M.; Tomiyama, T. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am. J. Gastroenterol. 1995, 90, 239–246. [Google Scholar]
- Matsumori, T.; Uza, N.; Shiokawa, M.; Maruno, T.; Nishikawa, Y.; Morita, T.; Kuwada, T.; Marui, S.; Okada, H.; Taura, K.; et al. Clinical impact of a novel device delivery system in the diagnosis of bile duct lesions: A single-center experience. J. Gastroenterol. Hepatol. 2022, 37, 1360–1366. [Google Scholar] [CrossRef]
- Matsumori, T.; Uza, N.; Shiokawa, M.; Maruno, T.; Kuwada, T.; Marui, S.; Seno, H. Mapping biopsy for bile duct cancer using a novel device delivery system. Endoscopy 2022, 54, e217–e219. [Google Scholar] [CrossRef]
- Yoon, S.B.; Moon, S.H.; Ko, S.W.; Lim, H.; Kang, H.S.; Kim, J.H. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig. Dis. Sci. 2022, 67, 3284–3297. [Google Scholar] [CrossRef]
- Malikowski, T.; Levy, M.J.; Gleeson, F.C.; Storm, A.C.; Vargas, E.J.; Topazian, M.D.; Abu Dayyeh, B.K.; Iyer, P.G.; Rajan, E.; Gores, G.J.; et al. Endoscopic Ultrasound/Fine Needle Aspiration Is Effective for Lymph Node Staging in Patients With Cholangiocarcinoma. Hepatology 2020, 72, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hamda, E.M.; Baron, T.H. Endoscopic management of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Eloubeidi, M.A.; Chen, V.K.; Jhala, N.C.; Eltoum, I.E.; Jhala, D.; Chhieng, D.C.; Syed, S.A.; Vickers, S.M.; Mel Wilcox, C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2004, 2, 209–213. [Google Scholar] [CrossRef]
- Lee, J.H.; Salem, R.; Aslanian, H.; Chacho, M.; Topazian, M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1069–1073. [Google Scholar] [CrossRef]
- Rösch, T.; Hofrichter, K.; Frimberger, E.; Meining, A.; Born, P.; Weigert, N.; Allescher, H.D.; Classen, M.; Barbur, M.; Schenck, U.; et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest. Endosc. 2004, 60, 390–396. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.; Misra, V.L.; Leblanc, J.K.; McHenry, L.; Sherman, S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest. Endosc. 2006, 64, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Broering, D.C.; Knoefel, W.T.; Rogiers, X.; Swain, P.; Thonke, F.; Bobrowski, C.; Topalidis, T.; Soehendra, N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am. J. Gastroenterol. 2004, 99, 45–51. [Google Scholar] [CrossRef]
- Chak, A.; Isenberg, G.; Kobayashi, K.; Wong, R.C.; Sivak, M.V., Jr. Prospective evaluation of an over-the-wire catheter US probe. Gastrointest. Endosc. 2000, 51, 202–205. [Google Scholar] [CrossRef]
- Tamada, K.; Ueno, N.; Tomiyama, T.; Oohashi, A.; Wada, S.; Nishizono, T.; Tano, S.; Aizawa, T.; Ido, K.; Kimura, K. Characterization of biliary strictures using intraductal ultrasonography: Comparison with percutaneous cholangioscopic biopsy. Gastrointest. Endosc. 1998, 47, 341–349. [Google Scholar] [CrossRef]
- Inui, K.; Miyoshi, H. Cholangiocarcinoma and intraductal sonography. Gastrointest. Endosc. Clin. N. Am. 2005, 15, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Adler, D.G.; Conway, J.D.; Diehl, D.L.; Farraye, F.A.; Kantsevoy, S.V.; Kwon, R.; Mamula, P.; Rodriguez, S.; Wong Kee Song, L.M.; et al. Cholangiopancreatoscopy. Gastrointest. Endosc. 2008, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Langer, D.A.; Antillon, M.R.; Chen, Y.K. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin. Gastroenterol. Hepatol. 2006, 4, 219–225. [Google Scholar] [CrossRef]
- Piraka, C.; Shah, R.J.; Awadallah, N.S.; Langer, D.A.; Chen, Y.K. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin. Gastroenterol. Hepatol. 2007, 5, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Etoh, K.; Haba, S.; Yamato, H.; Shinada, K.; Nakanishi, Y.; Tanaka, E.; Hirano, S.; Kondo, S.; et al. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy 2009, 41, 959–964. [Google Scholar] [CrossRef]
- Chan, E.S.; Yeh, M.M. The use of immunohistochemistry in liver tumors. Clin. Liver Dis. 2010, 14, 687–703. [Google Scholar] [CrossRef]
- Al-Muhannadi, N.; Ansari, N.; Brahmi, U.; Satir, A.A. Differential diagnosis of malignant epithelial tumours in the liver: An immunohistochemical study on liver biopsy material. Ann. Hepatol. 2011, 10, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Pawlik, T.M.; Anders, R.A.; Selaru, F.M.; Streppel, M.M.; Lucas, D.J.; Niknafs, N.; Guthrie, V.B.; Maitra, A.; Argani, P.; et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013, 45, 1470–1473. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.C.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Boulter, L.; Guest, R.V.; Kendall, T.J.; Wilson, D.H.; Wojtacha, D.; Robson, A.J.; Ridgway, R.A.; Samuel, K.; Van Rooijen, N.; Barry, S.T.; et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Investig. 2015, 125, 1269–1285. [Google Scholar] [CrossRef]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; Allen, G.E.; Ng, C.C.; Wong, B.H.; et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Losic, B.; Moeini, A.; Cabellos, L.; Hao, K.; Revill, K.; Bonal, D.; Miltiadous, O.; Zhang, Z.; Hoshida, Y.; et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat. Commun. 2015, 6, 6087. [Google Scholar] [CrossRef] [PubMed]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.D.; et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, Y.J.; Wang, X.Y.; Guo, W.J.; Gao, S.; Wei, L.; Shi, J.Y.; Shi, G.M.; Wang, Z.C.; Zhang, Y.N.; et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014, 146, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Chan-On, W.; Nairismägi, M.L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.; Nguyen, H.H.; et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef]

| Advantages | Limitations | |
|---|---|---|
| ERCP |
|
|
| EUS |
|
|
| SOC |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.W.; Moon, S.-H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. https://doi.org/10.3390/diagnostics13020233
Shin DW, Moon S-H, Kim JH. Diagnosis of Cholangiocarcinoma. Diagnostics. 2023; 13(2):233. https://doi.org/10.3390/diagnostics13020233
Chicago/Turabian StyleShin, Dong Woo, Sung-Hoon Moon, and Jong Hyeok Kim. 2023. "Diagnosis of Cholangiocarcinoma" Diagnostics 13, no. 2: 233. https://doi.org/10.3390/diagnostics13020233
APA StyleShin, D. W., Moon, S.-H., & Kim, J. H. (2023). Diagnosis of Cholangiocarcinoma. Diagnostics, 13(2), 233. https://doi.org/10.3390/diagnostics13020233






