Ultrasound of the Biceps Muscle in Idiopathic Parkinson’s Disease with Deep Brain Stimulation: Rigidity Can Be Quantified by Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
- DBS ON/MED OFF—medium rigidity
- DBS OFF/MED OFF—maximal rigidity
- DBS ON/MED ON—minimal rigidity
- MED OFF—maximal rigidity
- MED ON—minimal rigidity
2.3. Statistical Analyses
3. Results
3.1. SWV Varies among the Muscles, Positions, and Participant Groups
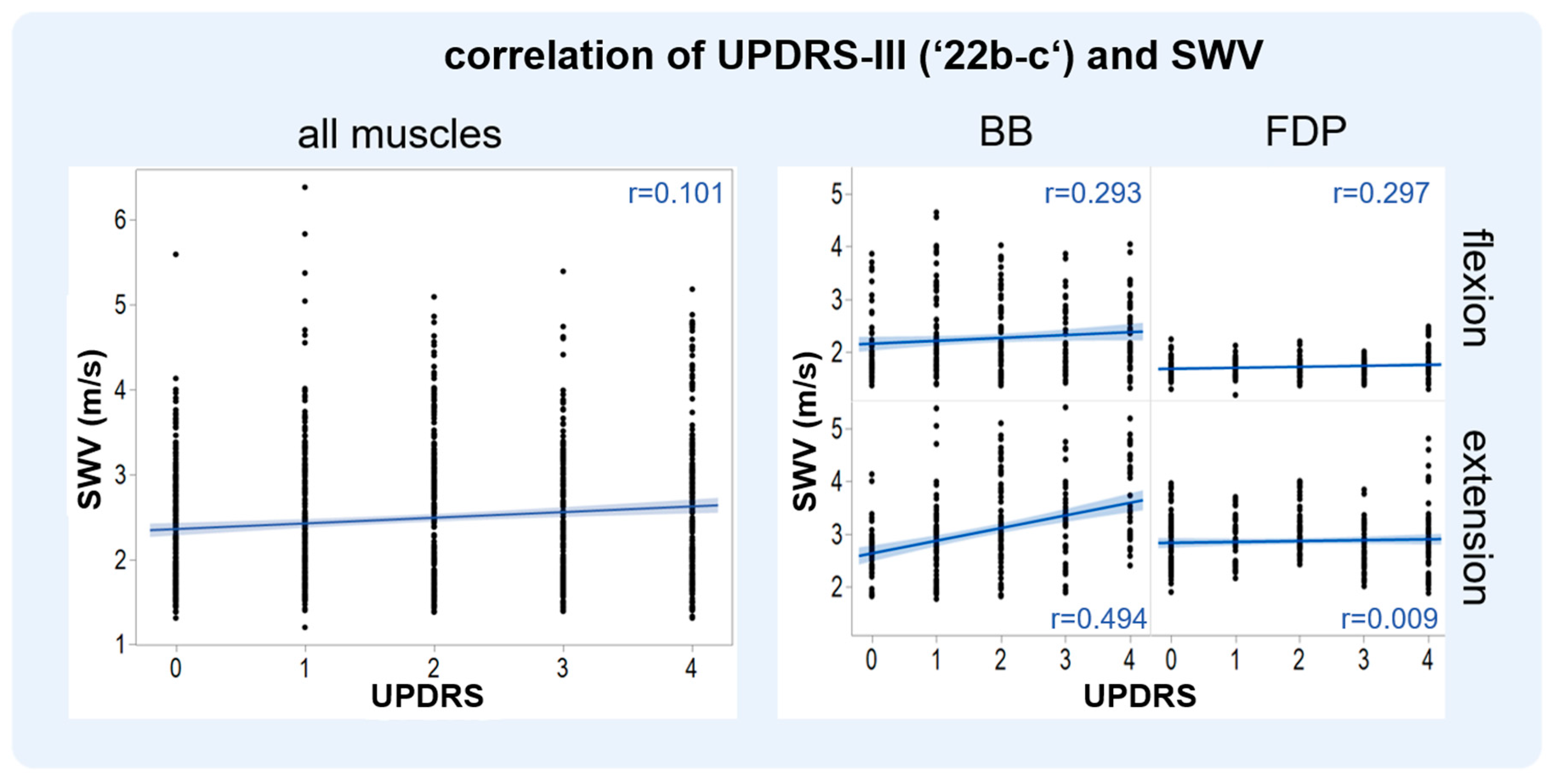
3.2. SWV and MDS-UPDRS-III (‘22b-c’) Show a Positive Correlation
3.3. SWV and MDS-UPDRS-III (‘22b-c’) of the BB Alter under Varying Therapeutic Conditions
3.4. The SWV Are Consistent with Previous Works
4. Discussion
4.1. Choice of Muscle, Technical Considerations for SWE
4.2. Comparison to SWE in Post-Stroke Spasticity
4.3. Comparison to Other Studies of SWE in PD
4.4. Other Technical Means and Relevance of the Rigidity Assessment in PD
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Barr, R.G. Shear wave liver elastography. Abdom. Radiol. 2018, 43, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Monpeyssen, H.; Tramalloni, J.; Poirée, S.; Hélénon, O.; Correas, J.-M. Elastography of the thyroid. Diagn. Interv. Imaging 2013, 94, 535–544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, B.; Zheng, Y.; Huang, G.; Lin, M.; Shan, Q.; Lu, Y.; Tian, W.; Xie, X. Breast Lesions: Quantitative Diagnosis Using Ultrasound Shear Wave Elastography—A Systematic Review and Meta-Analysis. Ultrasound Med. Biol. 2016, 42, 835–847. [Google Scholar] [CrossRef]
- Romano, A.; Staber, D.; Grimm, A.; Kronlage, C.; Marquetand, J. Limitations of Muscle Ultrasound Shear Wave Elastography for Clinical Routine—Positioning and Muscle Selection. Sensors 2021, 21, 8490. [Google Scholar] [CrossRef]
- Zimmer, M.; Kleiser, B.; Marquetand, J.; Ates, F. Shear Wave Elastography Characterizes Passive and Active Mechanical Properties of Biceps Brachii Muscle in Vivo; SSRN Scholarly Paper 4101072; Social Science Research Network: Rochester, NY, USA, 2022. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsui, P.H.; Lu, C.H.; Hung, Y.H.; Tsai, M.R.; Shieh, J.Y.; Weng, W.C. Quantifying Lower Limb Muscle Stiffness as Ambulation Function Declines in Duchenne Muscular Dystrophy with Acoustic Radiation Force Impulse Shear Wave Elastography. Ultrasound Med. Biol. 2021, 47, 2880–2889. [Google Scholar] [CrossRef] [PubMed]
- del Rosario Ferreira-Sánchez, M.; Moreno-Verdú, M.; Cano-de-la-Cuerda, R. Quantitative Measurement of Rigidity in Parkinson’s Disease: A Systematic Review. Sensors 2020, 20, 880. [Google Scholar] [CrossRef]
- de Deus Fonticoba, T.; García, D.S.; Arribí, M.M. Inter-rater variability in motor function assessment in Parkinson’s disease between experts in movement disorders and nurses specialising in PD management. Neurología 2019, 34, 520–526. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Metman, L.V.; Myre, B.; Verwey, N.; Hassin-Baer, S.; Arzbaecher, J.; Sierens, D.; Bakay, R. Test-retest reliability of UPDRS-III, dyskinesia scales, and timed motor tests in patients with advanced Parkinson’s disease: An argument against multiple baseline assessments. Mov. Disord. 2004, 19, 1079–1084. [Google Scholar] [CrossRef]
- Siderowf, A.; McDermott, M.; Kieburtz, K.; Blindauer, K.; Plumb, S.; Shoulson, I. Test-retest reliability of the unified Parkinson’s disease rating scale in patients with early Parkinson’s disease: Results from a multicenter clinical trial. Mov. Disord. 2002, 17, 758–763. [Google Scholar] [CrossRef]
- Bočková, M.; Rektor, I. Impairment of brain functions in Parkinson’s disease reflected by alterations in neural connectivity in EEG studies: A viewpoint. Clin. Neurophysiol. 2019, 130, 239–247. [Google Scholar] [CrossRef]
- Kehnemouyi, Y.M.; Wilkins, K.B.; Anidi, C.M.; Anderson, R.W.; Afzal, M.F.; Bronte-Stewart, H.M. Modulation of beta bursts in subthalamic sensorimotor circuits predicts improvement in bradykinesia. Brain 2021, 144, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, T.-J.; Li, Y.; Gao, Y. Application of real-time shear wave elastography in the assessment of torsional cervical dystonia. Quant. Imaging Med. Surg. 2019, 9, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, W.; Cheng, L.; Li, S.; Pan, Y.; Gao, J. Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson’s disease: A primary observation. Clin. Imaging 2016, 40, 1075–1080. [Google Scholar] [CrossRef]
- Ding, C.W.; Song, X.; Fu, X.Y.; Zhang, Y.C.; Mao, P.; Sheng, Y.J.; Yang, M.; Wang, C.S.; Zhang, Y.; Chen, X.F.; et al. Shear wave elastography characteristics of upper limb muscle in rigidity-dominant Parkinson’s disease. Neurol. Sci. 2021, 42, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Du, L.; Li, Y.; Xiao, Y.; Zhang, S.; Ma, H.; He, W. Quantitative Evaluation of Gastrocnemius Medialis Stiffness During Passive Stretching Using Shear Wave Elastography in Patients with Parkinson’s Disease: A Prospective Preliminary Study. Korean J. Radiol. 2021, 22, 1841–1849. [Google Scholar] [CrossRef]
- Zaidel, A.; Arkadir, D.; Israel, Z.; Bergman, H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr. Opin. Neurol. 2009, 22, 387. [Google Scholar] [CrossRef]
- Kronlage, C.; Grimm, A.; Romano, A.; Stahl, J.H.; Martin, P.; Winter, N.; Marquetand, J. Muscle Ultrasound Shear Wave Elastography as a Non-Invasive Biomarker in Myotonia. Diagnostics 2021, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Gao, J.; Hu, Y.; Liu, Y.; Li, W.; Chen, S.; Liu, F. Assessment of ultrasound shear wave elastography within muscles using different region of interest sizes, manufacturers, probes and acquisition angles: An ex vivo study. Quant Imaging Med. Surg. 2022, 12, 3227–3237. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; O’Connor, P.; Hensor, E.; Tan, A.L.; Emery, P.; Wakefield, R.J. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J. Clin. Ultrasound 2018, 46, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 2019, 31, 1755–1763. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ho, Y.-C.; Hsiao, M.-Y.; Chen, W.-S.; Wang, T.-G. Evaluation of Post-Stroke Spastic Muscle Stiffness Using Shear Wave Ultrasound Elastography. Ultrasound Med. Biol. 2017, 43, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Mera, T.O.; Johnson, M.D.; Rothe, D.; Zhang, J.; Xu, W.; Ghosh, D.; Vitek, J.; Alberts, J.L. Objective quantification of arm rigidity in MPTP-treated primates. J. Neurosci. Methods 2009, 177, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Coste, J.; Lemaire, J.J.; Schkommodau, E.; Taub, E.; Guzman, R.; Derost, P.; Hemm, S. A novel assistive method for rigidity evaluation during deep brain stimulation surgery using acceleration sensors. J. Neurosurg. 2017, 127, 602–612. [Google Scholar] [CrossRef]
- Journee, H.L.; Postma, A.A.; Staal, M.J. Intraoperative neurophysiological assessment of disabling symptoms in DBS surgery. Neurophysiol. Clin. 2007, 37, 467–475. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.K.; Sheng, S.Y.; Liang, S.M.; Lu, H.; Chen, R.Y.; Pan, M.; Wen, Z.B. Quantitative evaluation of passive muscle stiffness by shear wave elastography in healthy individuals of different ages. Eur. Radiol. 2021, 31, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Şendur, H.N.; Cindil, E.; Cerit, M.N.; Kılıç, P.; Gültekin, I.İ.; Oktar, S.Ö. Evaluation of effects of aging on skeletal muscle elasticity using shear wave elastography. Eur. J. Radiol. 2020, 128, 109038. [Google Scholar] [CrossRef]
- Akagi, R.; Yamashita, Y.; Ueyasu, Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound Med. Biol. 2015, 41, 2906–2912. [Google Scholar] [CrossRef] [PubMed]



| ID | Age (yrs) | Gender | BMI (kg/m2) | Hoehn and Yahr | MDS- UPDRS III | LEDD, mg | Type | More Affected Side | Disease Duration, (yrs) | Time with DBS, (yrs) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| On | Off | ||||||||||
| DBS1 | 73 | F | 23.7 | 3 | 39 | 52 | 963 | ET | L | 21 | 13 |
| DBS2 | 73 | F | 33.2 | 2.5 | 12 | 34 | 595 | AR | E | 10 | 1 |
| DBS3 | 71 | F | 22.5 | 2.5 | 16 | 40 | 415 | ET | L | 16 | 1 |
| DBS4 | 66 | M | 22.8 | 3 | 39 | 53 | 979 | AR | L | 12 | 4 |
| DBS5 | 78 | M | 21.7 | 4 | 32 | 83 | 1650 | AR | R | 17 | 4 |
| MED1 | 75 | M | 32.7 | 2 | 10 | 41 | 1200 | AR | R | 11 | n/a |
| MED2 | 58 | M | 23.4 | 3 | n/a | n/a | 730 | ET | L | 13 | n/a |
| MED3 | 74 | M | 22.1 | 4 | 43 | 68 | 325 | ET | R | 2 | n/a |
| MED4 | 69 | M | 18.8 | 2 | 25 | 47 | 563 | ET | R | 13 | n/a |
| MED5 | 54 | M | 32.1 | 3 | 11 | 24 | 1656 | ET | L | 7 | n/a |
| HC | All PD Patients (MED + DBS) | HC vs. MED + DBS | MED | DBS | |
|---|---|---|---|---|---|
| Mean SWV [m/s] | 2.2 ± 0.5 (1.4–3.8) 1.7/2.2/2.7 | 2.5 ± 0.8 (1.2–6.4) 1.8/2.4/3 | p < 0.001 | 2.4 ± 0.9 (1.3–6.4) 1.8/2.2/2.9 | 2.5 ± 0.7 (1.2–4.9) 1.8/2.6/3 |
| SWV of BB in flexion [m/s] | 1.8 ± 0.4 (1.4–3.0) 1.6/1.7/1.9 | 2.3 ± 0.7 (1.3–4.7) 1.7/2/2.7 | p < 0.001 | 2.1 ± 0.6 (1.3–4.6) 1.6/1.9/2.4 | 2.4 ± 0.7 (1.4–4.0) 1.8/2.2/3.1 |
| SWV of BB in extension [m/s] | 2.6 ± 0.3 (1.9–3.2) 2.4/2.5/2.7 | 3.1 ± 0.9 (1.8–6.4) 2.5/2.9/3.4 | p < 0.001 | 3.1 ± 1.1 (1.8–6.4) 2.1/3/4 | 3.0 ± 0.5 (2.2–4.9) 2.7/2.9/3.1 |
| SWV of FDP in flexion [m/s] | 1.7 ± 0.1 (1.5–2.2) 1.7/1.7/1.8 | 1.7 ± 0.2 (1.2–2.5) 1.6/1.7/1.8 | p = 0.078 | 1.7 ± 0.2 (1.3–2.4) 1.6/1.7/1.8 | 1.7 ± 0.2 (1.2–2.5) 1.6/1.7/1.8 |
| SWV of FDP in extension [m/s] | 2.8 ± 0.3 (2.0–3.8) 2.6/2.8/3 | 2.9 ± 0.5 (1.9–4.8) 2.5/2.8/3.1 | p = 0.083 | 2.7 ± 0.5 (1.9–4.8) 2.3/2.6/3 | 3.0 ± 0.4 (2.0–4.1) 2.8/3/3.3 |
| All PD Patients (MED + DBS) | |
|---|---|
| BB in flexion | r = 0.294; p < 0.001 |
| BB in extension | r = 0.494; p < 0.001 |
| FDP in flexion | r = 0.297; p < 0.001 |
| FDP in extension | r = 0.009; p = 0.91 |
| This Study | Ding et al. (2021) [17] | Du et al. (2016) [16] | |
|---|---|---|---|
| Therapeutic condition | Off and On | On | Off |
| Cohort | N = 10 | N = 63 | N = 46 |
| SWE of BB in extension in m/s | DBS: 2.7 ± 0.69 MED: 2.62 ± 1.03 | 3.65 ± 0.46 | 3.99 ± 2.83 4.28 ± 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppold, J.; Breu, M.-S.; Gharabaghi, A.; Grimm, A.; Del Grosso, N.A.; Hormozi, M.; Kleiser, B.; Klocke, P.; Kronlage, C.; Weiß, D.; et al. Ultrasound of the Biceps Muscle in Idiopathic Parkinson’s Disease with Deep Brain Stimulation: Rigidity Can Be Quantified by Shear Wave Elastography. Diagnostics 2023, 13, 213. https://doi.org/10.3390/diagnostics13020213
Oppold J, Breu M-S, Gharabaghi A, Grimm A, Del Grosso NA, Hormozi M, Kleiser B, Klocke P, Kronlage C, Weiß D, et al. Ultrasound of the Biceps Muscle in Idiopathic Parkinson’s Disease with Deep Brain Stimulation: Rigidity Can Be Quantified by Shear Wave Elastography. Diagnostics. 2023; 13(2):213. https://doi.org/10.3390/diagnostics13020213
Chicago/Turabian StyleOppold, Julia, Maria-Sophie Breu, Alireza Gharabaghi, Alexander Grimm, Nicholas A. Del Grosso, Mohammad Hormozi, Benedict Kleiser, Philipp Klocke, Cornelius Kronlage, Daniel Weiß, and et al. 2023. "Ultrasound of the Biceps Muscle in Idiopathic Parkinson’s Disease with Deep Brain Stimulation: Rigidity Can Be Quantified by Shear Wave Elastography" Diagnostics 13, no. 2: 213. https://doi.org/10.3390/diagnostics13020213
APA StyleOppold, J., Breu, M.-S., Gharabaghi, A., Grimm, A., Del Grosso, N. A., Hormozi, M., Kleiser, B., Klocke, P., Kronlage, C., Weiß, D., & Marquetand, J. (2023). Ultrasound of the Biceps Muscle in Idiopathic Parkinson’s Disease with Deep Brain Stimulation: Rigidity Can Be Quantified by Shear Wave Elastography. Diagnostics, 13(2), 213. https://doi.org/10.3390/diagnostics13020213






