Clinical Value of Glycan Changes in Cerebrospinal Fluid for Evaluation of Post-Neurosurgical Bacterial Meningitis with Hemorrhagic Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Sample Treatment
2.3. Profiling Glycosylation of Sera in ICH
2.4. Statistical Analysis
3. Results
3.1. Characteristics of PNBM Patients
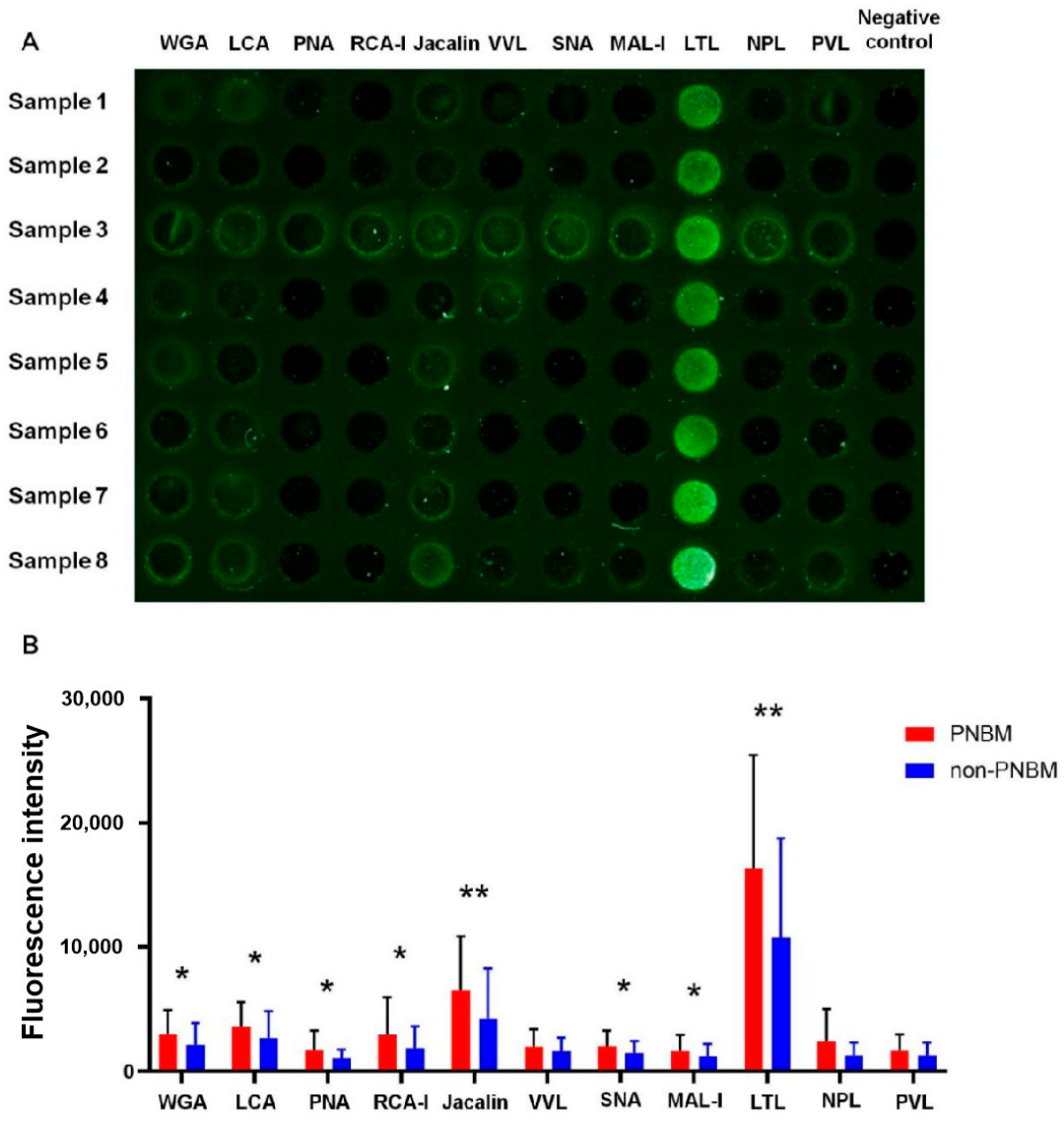
3.2. Glycosylation Profile of CSF
3.3. Diagnostic Values for PNBM
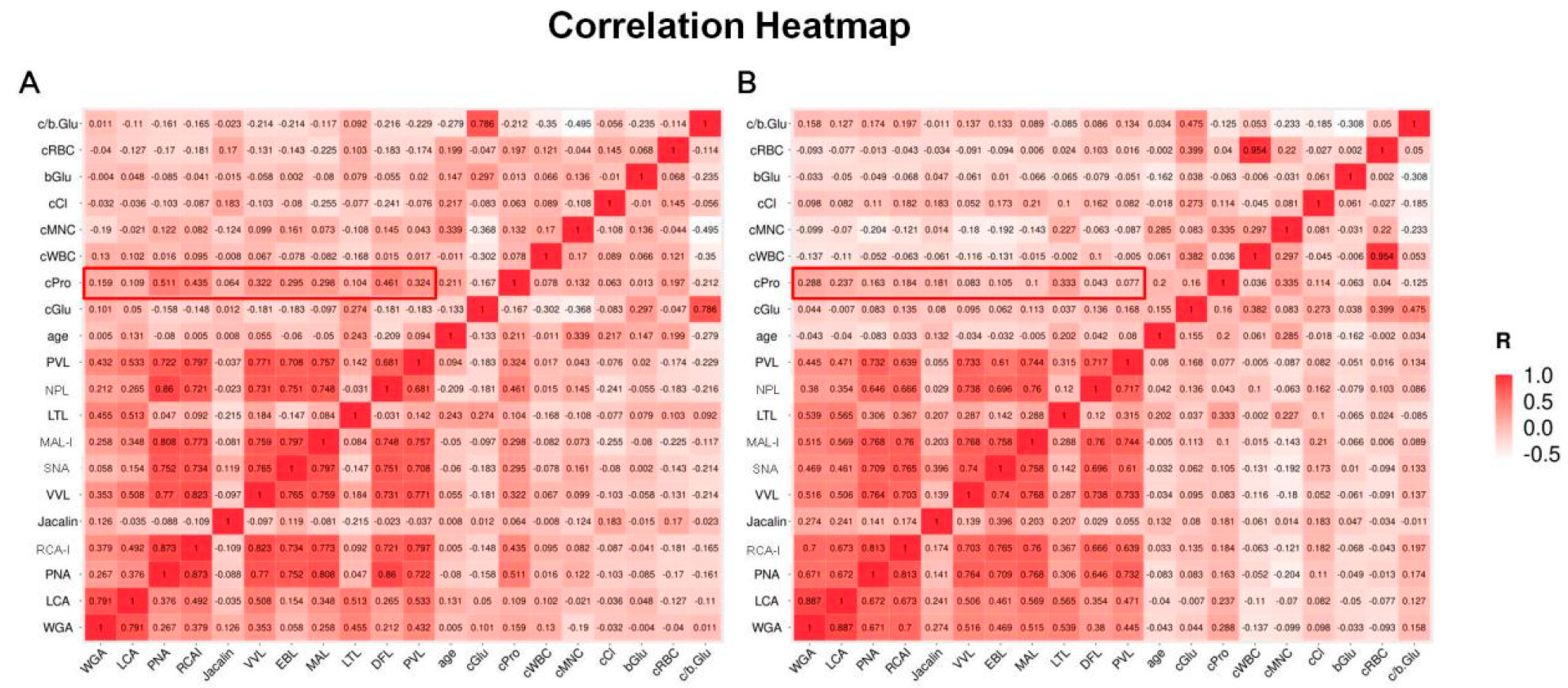
3.4. Correlation Analyses for Biochemical Parameters of CSF
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of post-neurosurgical meningitis: Narrative review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Blomstedt, G.C. Infections in neurosurgery: A retrospective study of 1143 patients and 1517 operations. Acta Neurochir. 1985, 78, 81–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Zhang, J.; Gao, Z.; Ji, N.; Zhang, L. Diagnostic accuracy of routine blood examinations and CSF lactate level for post-neurosurgical bacterial meningitis. Int. J. Infect. Dis. 2017, 59, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Han, Y.; Miao, G.; Jiang, L.; Xie, S.; Liu, B. CSF leukocyte, polykaryocyte, protein and glucose: Their cut-offs of judging whether post-neurosurgical bacterial meningitis has been cured. Clin. Neurol. Neurosurg. 2018, 174, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Alons, I.M.; Verheul, R.J.; Kuipers, I.; Jellema, K.; Wermer, M.J.; Algra, A.; Ponjee, G. Procalcitonin in cerebrospinal fluid in meningitis: A prospective diagnostic study. Brain. Behav. 2016, 6, e00545. [Google Scholar] [CrossRef]
- Lotfi, R.; Ines, B.; Aziz, D.M.; Mohamed, B. Cerebrospinal Fluid Lactate as an Indicator for Post-neurosurgical Bacterial Meningitis. Indian J. Crit. Care Med. 2019, 23, 127–130. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Montagnana, M.; Brocco, G.; Guidi, G.C. Influence of hemolysis on routine clinical chemistry testing. Clin. Chem. Lab. Med. 2006, 44, 311–316. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell. Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Trempel, F.; Kajiura, H.; Ranf, S.; Grimmer, J.; Westphal, L.; Zipfel, C.; Scheel, D.; Fujiyama, K.; Lee, J. Altered glycosylation of exported proteins, including surface immune receptors, compromises calcium and downstream signaling responses to microbe-associated molecular patterns in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 31. [Google Scholar] [CrossRef]
- Borud, B.; Barnes, G.K.; Brynildsrud, O.B.; Fritzsonn, E.; Caugant, D.A. Genotypic and Phenotypic Characterization of the O-Linked Protein Glycosylation System Reveals High Glycan Diversity in Paired Meningococcal Carriage Isolates. J. Bacteriol. 2018, 200, e00794-17. [Google Scholar] [CrossRef]
- Deng, W.; Cao, J.; Chen, L.; McMullin, D.; Januzzi, J.L., Jr.; Buonanno, F.S.; Lo, E.H.; Ning, M.M. Plasma Glycoproteomic Study of Therapeutic Hypothermia Reveals Novel Markers Predicting Neurologic Outcome Post-cardiac Arrest. Transl. Stroke Res. 2018, 9, 64–73. [Google Scholar] [CrossRef]
- De, P.; Amin, A.G.; Flores, D.; Simpson, A.; Dobos, K.; Chatterjee, D. Structural implications of lipoarabinomannan glycans from global clinical isolates in diagnosis of Mycobacterium tuberculosis infection. J. Biol. Chem. 2021, 297, 101265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Ertelt, J.M. Fluorescent glycan fingerprinting of SARS2 spike proteins. Sci. Rep. 2021, 11, 20428. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.; Pathak, S.; Grzes, K.M.; Damerow, S.; Sinclair, L.V.; van Aalten, D.M.; Cantrell, D.A. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 2016, 17, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, K.V.; Scott, N.E.; Iwashkiw, J.A.; Thomson, E.L.; Foster, L.J.; Feldman, M.F.; Dennis, J.J. A general protein O-glycosylation system within the Burkholderia cepacia complex is involved in motility and virulence. Mol. Microbiol. 2014, 92, 116–137. [Google Scholar] [CrossRef]
- Mubaiwa, T.D.; Semchenko, E.A.; Hartley-Tassell, L.E.; Day, C.J.; Jennings, M.P.; Seib, K.L. The sweet side of the pathogenic Neisseria: The role of glycan interactions in colonisation and disease. Pathog. Dis. 2017, 75, ftx063. [Google Scholar] [CrossRef]
- Tan, F.Y.; Tang, C.M.; Exley, R.M. Sugar coating: Bacterial protein glycosylation and host-microbe interactions. Trends Biochem. Sci. 2015, 40, 342–350. [Google Scholar] [CrossRef]
- Vik, A.; Aas, F.E.; Anonsen, J.H.; Bilsborough, S.; Schneider, A.; Egge-Jacobsen, W.; Koomey, M. Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 2009, 106, 4447–4452. [Google Scholar] [CrossRef]
- van Sorge, N.M.; Quach, D.; Gurney, M.A.; Sullam, P.M.; Nizet, V.; Doran, K.S. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J. Infect. Dis. 2009, 199, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Nothaft, H.; Szymanski, C.M. Bacterial protein N-glycosylation: New perspectives and applications. J. Biol. Chem. 2013, 288, 6912–6920. [Google Scholar] [CrossRef]
- Fredriksen, L.; Moen, A.; Adzhubei, A.A.; Mathiesen, G.; Eijsink, V.G.; Egge-Jacobsen, W. Lactobacillus plantarum WCFS1 O-linked protein glycosylation: An extended spectrum of target proteins and modification sites detected by mass spectrometry. Glycobiology 2013, 23, 1439–1451. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Ye, L.; Fang, Y.S.; Li, X.X.; Gao, Y.; Liu, S.S.; Chen, Q.; Wu, Q.; Cheng, H.W.; Du, W.D. A simple lectin-based biochip might display the potential clinical value of glycomics in patients with spontaneous intracerebral hemorrhage. Ann. Transl. Med. 2021, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Imperiali, B. Bacterial carbohydrate diversity—A Brave New World. Curr. Opin. Chem. Biol. 2019, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Yuan, C.H.; Ding, Q.; Zhou, Y.; Pan, Q.; Zhang, X.L. Selection and identification of specific glycoproteins and glycan biomarkers of macrophages involved in Mycobacterium tuberculosis infection. Tuberculosis 2017, 104, 95–106. [Google Scholar] [CrossRef]
- Wang, G.; Glaser, L.; Scott, N.E.; Fathy Mohamed, Y.; Ingram, R.; Laroucau, K.; Valvano, M.A. A glycoengineered antigen exploiting a conserved protein O-glycosylation pathway in the Burkholderia genus for detection of glanders infections. Virulence 2021, 12, 493–506. [Google Scholar] [CrossRef]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Sahly, H.; Keisari, Y.; Ofek, I. Manno(rhamno)biose-containing capsular polysaccharides of Klebsiella pneumoniae enhance opsono-stimulation of human polymorphonuclear leukocytes. J. Innate Immun. 2009, 1, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Vimonish, R.; Capelli-Peixoto, J.; Johnson, W.C.; Hussein, H.E.; Taus, N.S.; Brayton, K.A.; Munderloh, U.G.; Noh, S.M.; Ueti, M.W. Anaplasma marginale Infection of Dermacentor andersoni Primary Midgut Cell Culture Is Dependent on Fucosylated Glycans. Front. Cell. Infect. Microbiol. 2022, 12, 877525. [Google Scholar] [CrossRef]
- Sun, Y.H.; Luxardi, G.; Xu, G.; Zhu, K.; Reid, B.; Guo, B.P.; Lebrilla, C.B.; Maverakis, E.; Zhao, M. Surface Glycans Regulate Salmonella Infection-Dependent Directional Switch in Macrophage Galvanotaxis Independent of NanH. Infect. Immun. 2022, 90, e0051621. [Google Scholar] [CrossRef]
- Deng, Z.; Dai, T.; Zhang, W.; Zhu, J.; Luo, X.M.; Fu, D.; Liu, J.; Wang, H. Glyceraldehyde-3-Phosphate Dehydrogenase Increases the Adhesion of Lactobacillus reuteri to Host Mucin to Enhance Probiotic Effects. Int. J. Mol. Sci. 2020, 21, 9756. [Google Scholar] [CrossRef]
- Avery, O.T.; Heidelberger, M. Immunological Relationships of Cell Constituents of Pneumococcus. J. Exp. Med. 1923, 38, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Temme, J.S.; Butler, D.L.; Gildersleeve, J.C. Anti-glycan antibodies: Roles in human disease. Biochem. J. 2021, 478, 1485–1509. [Google Scholar] [CrossRef]
- Soliman, C.; Walduck, A.K.; Yuriev, E.; Richards, J.S.; Cywes-Bentley, C.; Pier, G.B.; Ramsland, P.A. Structural basis for antibody targeting of the broadly expressed microbial polysaccharide poly-N-acetylglucosamine. J. Biol. Chem. 2018, 293, 5079–5089. [Google Scholar] [CrossRef] [PubMed]
- Pier, G.B.; Boyer, D.; Preston, M.; Coleman, F.T.; Llosa, N.; Mueschenborn-Koglin, S.; Theilacker, C.; Goldenberg, H.; Uchin, J.; Priebe, G.P.; et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 2004, 173, 5671–5678. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.; Rothenberg, M.E.; Deng, R.; Lewin-Koh, N.; She, G.; Kamath, A.V.; Carrasco-Triguero, M.; Saad, O.; Castro, A.; Teufel, L.; et al. A Phase 1, Randomized, Single-Ascending-Dose Study to Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e02588-18. [Google Scholar] [CrossRef]

| PNBM (n = 53) | Non-PNBM (n = 83) | p Value | ||
|---|---|---|---|---|
| Age (years, mean ± SD) | 55.70 ± 17.09 | 57.65 ± 15.40 | 0.491 | |
| Gender | 0.568 | |||
| Male | 28 | 45 | ||
| Female | 25 | 38 | ||
| CSF | ||||
| Glucose (mmol/L, IQR) | 1.59 (1.26, 1.95) | 3.47 (2.90, 4.33) | <0.001 | |
| Protein (g/L, IQR) | 3.00 (2.00, 4.35) | 1.00 (0.63, 1.90) | <0.001 | |
| White blood cells (×106/L, IQR) | 972 (345, 4768) | 64 (18, 291) | <0.001 | |
| Red blood cells (×106/L, IQR) | 14,000 (550, 94,500) | 5000 (500, 16,000) | 0.038 | |
| Proportion of multinuclear cell (IQR) | 78.2 (58.6, 88.9) | 46.2 (20.0, 79.1) | <0.001 | |
| Chlorine (mmol/L, mean ± SD) | 120.30 ± 10.63 | 124.13 ± 8.94 | 0.025 | |
| Pandy tests (negative/positive) | 12/41 | 22/61 | 0.612 | |
| Blood glucose (mmol/L, IQR) | 6.43 (5.43, 7.55) | 7.08 (5.72, 9.22) | 0.296 | |
| cGlu/bGlu ratio (IQR) | 0.23 (0.16, 0.33) | 0.51 (0.39, 0.64) | <0.001 | |
| ICP (mmH2O, IQR) | 180 (115, 260) | 153 (110, 210) | 0.595 | |
| Primary disease | 0.264 | |||
| aSAH | 32 | 42 | ||
| ICH | 21 | 41 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| B | p Value | B | p Value | |
| WGA | −0.034 | 0.830 | −0.107 | 0.409 |
| LCA | 0.132 | 0.423 | 0.130 | 0.333 |
| PNA | −0.356 | 0.034 | −0.074 | 0.606 |
| RCA-I | 0.078 | 0.676 | 0.053 | 0.729 |
| Jacalin | −0.200 | 0.034 | −0.128 | 0.096 |
| SNA | −0.129 | 0.431 | −0.960 | 0.468 |
| MAL-I | 0.189 | 0.220 | 0.032 | 0.795 |
| LTL | −0.324 | 0.001 | −0.200 | 0.021 |
| CSF glucose | - | - | 0.394 | <0.001 |
| CSF protein | - | - | −0.108 | 0.198 |
| CSF white blood cells | - | - | −0.106 | 0.207 |
| CSF red blood cells | - | - | −0.036 | 0.661 |
| CSF proportion of multinuclear cell | - | - | −0.097 | 0.199 |
| CSF chlorine | - | - | 0.100 | 0.146 |
| cGlu/bGlu ratio | - | - | 0.107 | 0.306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Ji, X.; Song, Z.; Guan, L.; Zhao, L.; Wang, W.; Du, W. Clinical Value of Glycan Changes in Cerebrospinal Fluid for Evaluation of Post-Neurosurgical Bacterial Meningitis with Hemorrhagic Stroke Patients. Diagnostics 2023, 13, 187. https://doi.org/10.3390/diagnostics13020187
Ye L, Ji X, Song Z, Guan L, Zhao L, Wang W, Du W. Clinical Value of Glycan Changes in Cerebrospinal Fluid for Evaluation of Post-Neurosurgical Bacterial Meningitis with Hemorrhagic Stroke Patients. Diagnostics. 2023; 13(2):187. https://doi.org/10.3390/diagnostics13020187
Chicago/Turabian StyleYe, Lei, Xuefei Ji, Zijian Song, Liao Guan, Liang Zhao, Wenwen Wang, and Weidong Du. 2023. "Clinical Value of Glycan Changes in Cerebrospinal Fluid for Evaluation of Post-Neurosurgical Bacterial Meningitis with Hemorrhagic Stroke Patients" Diagnostics 13, no. 2: 187. https://doi.org/10.3390/diagnostics13020187
APA StyleYe, L., Ji, X., Song, Z., Guan, L., Zhao, L., Wang, W., & Du, W. (2023). Clinical Value of Glycan Changes in Cerebrospinal Fluid for Evaluation of Post-Neurosurgical Bacterial Meningitis with Hemorrhagic Stroke Patients. Diagnostics, 13(2), 187. https://doi.org/10.3390/diagnostics13020187






