The Relationship between Intracranial Pressure and Visual Field Zones in Normal-Tension Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W. Under Pressure: Cellular and Molecular Responses during Glaucoma, a Common Neurodegeneration with Axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef]
- Wirostko, B.; Ehrlich, R.; Harris, A. The Vascular Theory in Glaucoma. Glaucoma Today 2009, 4, 25–27. [Google Scholar]
- Leske, M.C.; Wu, S.-Y.; Hennis, A.; Honkanen, R.; Nemesure, B. Risk Factors for Incident Open-angle Glaucoma: The Barbados Eye Studies. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Wang, N. Cerebrospinal Fluid Pressure and Glaucoma. J. Ophthalmic Vis. Res. 2013, 8, 257–263. [Google Scholar]
- Berdahl, J.P.; Allingham, R.R.; Johnson, D.H. Cerebrospinal Fluid Pressure Is Decreased in Primary Open-angle Glaucoma. Ophthalmology 2008, 115, 763–768. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Fautsch, M.P.; Stinnett, S.S.; Allingham, R.R. Intracranial Pressure in Primary Open Angle Glaucoma, Normal Tension Glaucoma, and Ocular Hypertension: A Case–Control Study. Investig. Opthalmol. Vis. Sci. 2008, 49, 5412–5418. [Google Scholar] [CrossRef]
- Jonas, J.B.; Nangia, V.; Wang, N.; Bhate, K.; Nangia, P.; Nangia, P.; Yang, D.; Xie, X.; Panda-Jonas, S. Trans-Lamina Cribrosa Pressure Difference and Open-Angle Glaucoma. The Central India Eye and Medical Study. PLoS ONE 2013, 8, e82284. [Google Scholar] [CrossRef]
- Morgan, W.H.; Chauhan, B.C.; Yu, D.-Y.; Cringle, S.J.; A Alder, V.; House, P.H. Optic disc movement with variations in intraocular and cerebrospinal fluid pressure. Investig. Opthalmol. Vis. Sci. 2002, 43, 3236–3242. [Google Scholar]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.-K.F.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Anatomic Relationship between Lamina Cribrosa, Intraocular Space, and Cerebrospinal Fluid Space. Investig. Opthalmol. Vis. Sci. 2003, 44, 5189–5195. [Google Scholar] [CrossRef]
- Ren, R.; Wang, N.; Zhang, X.; Cui, T.; Jonas, J.B. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B. Role of cerebrospinal fluid pressure in the pathogenesis of glaucoma. Acta Ophthalmol. 2011, 89, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The Difference in Translaminar Pressure Gradient and Neuroretinal Rim Area in Glaucoma and Healthy Subjects. J. Ophthalmol. 2014, 2014, 937360. [Google Scholar] [CrossRef] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Daveckaite, A.; Ragauskas, A.; Bartusis, L.; Kucinoviene, J.; Siesky, B.; Harris, A. Literature review and meta-analysis of translaminar pressure difference in open-angle glaucoma. Eye 2015, 29, 1242–1250. [Google Scholar] [CrossRef]
- Yang, D.; Fu, J.; Hou, R.; Liu, K.; Jonas, J.B.; Wang, H.; Chen, W.; Li, Z.; Sang, J.; Zhang, Z.; et al. Optic Neuropathy Induced by Experimentally Reduced Cerebrospinal Fluid Pressure in Monkeys. Investig. Opthalmol. Vis. Sci. 2014, 55, 3067–3073. [Google Scholar] [CrossRef]
- Lindén, C.; Qvarlander, S.; Jóhannesson, G.; Johansson, E.; Östlund, F.; Malm, J.; Eklund, A. Normal-Tension Glaucoma Has Normal Intracranial Pressure: A Prospective Study of Intracranial Pressure and Intraocular Pressure in Different Body Positions. Ophthalmology 2018, 125, 361–368. [Google Scholar] [CrossRef]
- Loiselle, A.R.; de Kleine, E.; van Dijk, P.; Jansonius, N.M. Noninvasive intracranial pressure assessment using otoacoustic emissions: An application in glaucoma. PLoS ONE 2018, 13, e0204939. [Google Scholar] [CrossRef]
- Ren, R.; Jonas, J.B.; Tian, G.; Zhen, Y.; Ma, K.; Li, S.; Wang, H.; Li, B.; Zhang, X.; Wang, N. Cerebrospinal Fluid Pressure in Glaucoma: A Prospective Study. Ophthalmology 2010, 117, 259–266. [Google Scholar] [CrossRef]
- Pircher, A.; Remonda, L.; Weinreb, R.N.; E Killer, H. Translaminar pressure in Caucasian normal tension glaucoma patients. Acta Ophthalmol. 2017, 95, e524–e531. [Google Scholar] [CrossRef]
- Susanna, R., Jr.; Vessani, R.M. Staging glaucoma patient: Why and how? Open Ophthalmol. J. 2009, 3, 59–64. [Google Scholar] [CrossRef]
- Ragauskas, A.; Šunokas, R.; Žakelis, R.; Matijošaitis, V. Clinical Assessment of the Accuracy of ICP Non-invasive Measurement. Elektron. Elektrotechnika 2010, 104, 31–33. [Google Scholar]
- Siaudvytyte, L.; Januleviciene, I.; Daveckaite, A.; Ragauskas, A.; Siesky, B.; Harris, A. Neuroretinal rim area and ocular haemodynamic parameters in patients with normal-tension glaucoma with differing intracranial pressures. Br. J. Ophthalmol. 2016, 100, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, C.; Waisbourd, M.; Ekici, F.; Erdem, E.; Wizov, S.S.; Hark, L.A.; Spaeth, G.L. The Impact of Visual Field Clusters on Performance-based Measures and Vision-Related Quality of Life in Patients With Glaucoma. Am. J. Ophthalmol. 2016, 163, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Katz, J. A comparison of the pattern- and total deviation-based Glaucoma Change Probability programs. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1012–1016. [Google Scholar]
- Klarica, M.; Radoš, M.; Draganić, P.; Erceg, G.; Orešković, D.; Maraković, J.; Bulat, M. Effect of Head Position on Cerebrospinal Fluid Pressure in Cats: Comparison with Artificial Model. Croat. Med. J. 2006, 47, 233–238. [Google Scholar] [PubMed]
- Baneke, A.J.; Aubry, J.; Viswanathan, A.C.; Plant, G.T. The role of intracranial pressure in glaucoma and therapeutic implications. Eye 2020, 34, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.H.; Lilja-Cyron, A.; Andresen, M.; Juhler, M. The Relationship between Intracranial Pressure and Age—Chasing Age-Related Reference Values. World Neurosurg. 2018, 110, e119–e123. [Google Scholar] [CrossRef]
- Fleischman, D.; Berdahl, J.P.; Zaydlarova, J.; Stinnett, S.; Fautsch, M.P.; Allingham, R.R. Cerebrospinal Fluid Pressure Decreases with Older Age. PLoS ONE 2012, 7, e52664. [Google Scholar] [CrossRef]
- Ren, R.; Zhang, X.; Wang, N.; Li, B.; Tian, G.; Jonas, J.B. Cerebrospinal fluid pressure in ocular hypertension. Acta Ophthalmol. 2011, 89, e142–e148. [Google Scholar] [CrossRef]
- Bono, F.; Lupo, M.R.; Serra, P.; Cantafio, C.; Lucisano, A.; Lavano, A.; Fera, F.; Pardatscher, K.; Quattrone, A. Obesity does not induce abnormal CSF pressure in subjects with normal cerebral MR venography. Neurology 2002, 59, 1641–1643. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Fleischman, D.; Zaydlarova, J.; Stinnett, S.; Allingham, R.R.; Fautsch, M.P. Body Mass Index Has a Linear Relationship with Cerebrospinal Fluid Pressure. Investig. Opthalmol. Vis. Sci. 2012, 53, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; De Moraes, C.G.; Teng, C.C.; Tello, C.; Liebmann, J.M.; Ritch, R. Initial Parafoveal Versus Peripheral Scotomas in Glaucoma: Risk Factors and Visual Field Characteristics. Ophthalmology 2011, 118, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Ahrlich, K.G.; De Moraes, C.G.V.; Teng, C.C.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Visual Field Progression Differences between Normal-Tension and Exfoliative High-Tension Glaucoma. Investig. Opthalmol. Vis. Sci. 2010, 51, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kyung, H.; Shim, S.H.; Azarbod, P.; Caprioli, J. Location of Initial Visual Field Defects in Glaucoma and Their Modes of Deterioration. Investig. Opthalmol. Vis. Sci. 2015, 56, 7956–7962. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheung, C.Y.L.; Wong, T.Y.; Mitchell, P.; Aung, T. Influence of Height, Weight, and Body Mass Index on Optic Disc Parameters. Investig. Opthalmol. Vis. Sci. 2010, 51, 2998–3002. [Google Scholar] [CrossRef]
- Choi, K.R.; Kim, A.Y.; Han, K.E.; Jun, R.M. The Association between progression of visual field loss and body mass index in normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3915. [Google Scholar]
- Zingirian, M.; Calabria, G.; Gandolfo, E. The nasal step in normal and glaucomatous visual fields. Can. J. Ophthalmol. 1979, 14, 88–94. [Google Scholar]
- Spaeth, G.L. Terminolology and Guidelines for Glaucoma, 5th ed.; European Glaucoma Society: Florence, Italy, 2020. [Google Scholar]
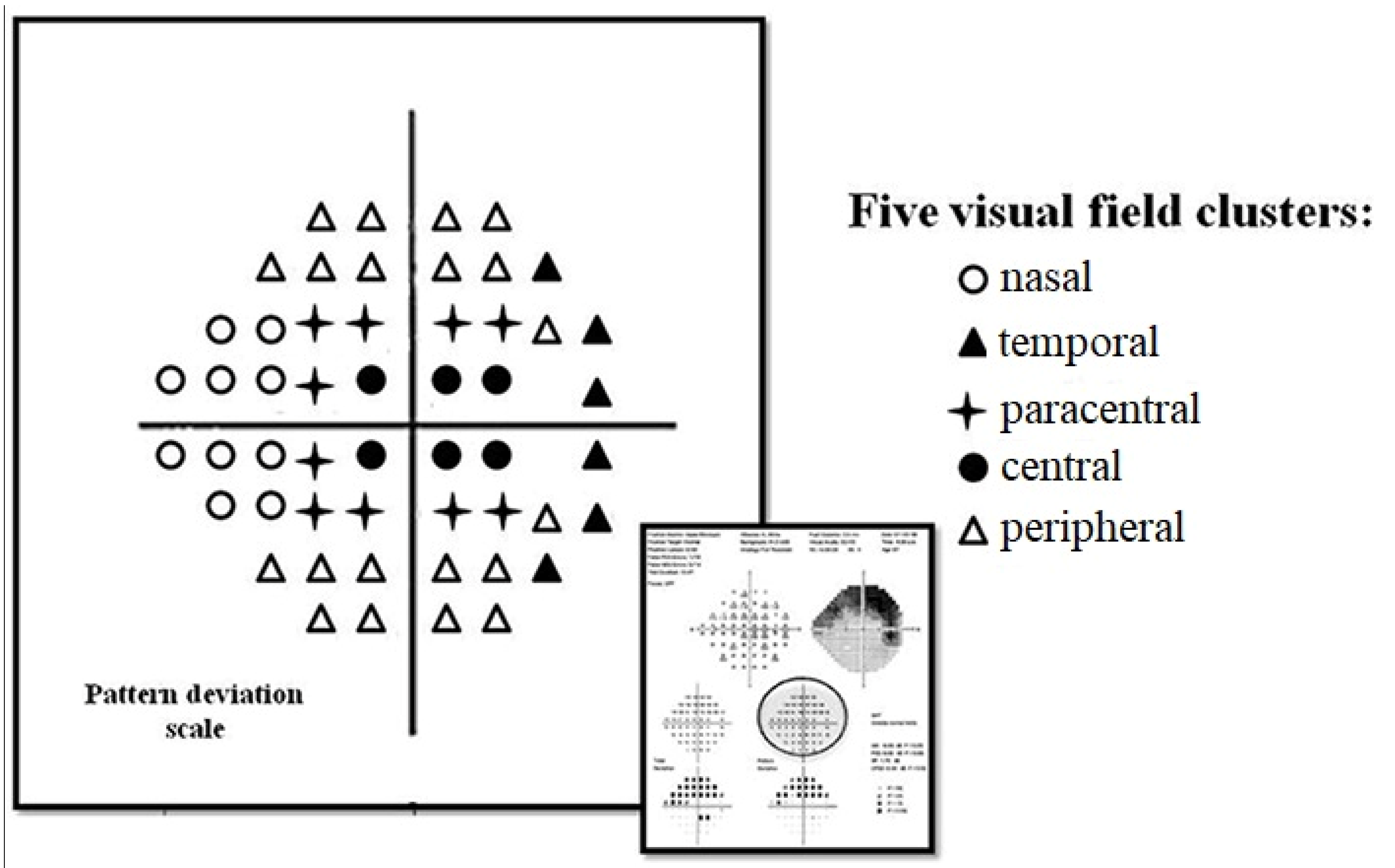
| NTG Patients (N = 80) Mean (SD) | |
|---|---|
| Male [N (%)] | 19 (24%) |
| Mean age [years] | 59.4 (11.6) |
| Mean body mass index [kg/m2] | 27.1 (4.8) |
| Mean glaucoma treatment [years] | 2.5 (3.5) |
Glaucoma medications [N (%)]:
| |
| 17 (21%) | |
| 43 (54%) | |
| 13 (16%) | |
| 1 (1%) | |
Systemic medications [N (%)]:
| |
| 7 (9%) | |
| 22 (28%) | |
| 25 (31%) | |
| 2 (3%) | |
| 35 (44%) | |
Systemic diseases [N (%)]:
| |
| 15 (19%) | |
| 40 (50%) | |
| 4 (5%) | |
| 8 (10%) | |
| 4 (5%) | |
Ophthalmological examination [mean]
| |
| 0.97 (0.1) | |
| 8.5 (2.4) | |
| 15.0 (2.3) | |
| 6.3 (2.5) | |
| 57.1 (7.6) | |
| 68.1 (8.6) | |
| 135.5 (16.4) | |
| 82.9 (9.9) | |
VF parameters [mean]
| |
| −1.8 (1.8) | |
| 2.4 (1.7) | |
| 97 (3) | |
Averaged PD scores within VF zones [mean]
| |
| −2.5 (2.1) | |
| −1.9 (2.0) | |
| −2.2 (1.9) | |
| −1.2 (0.7) | |
| −1.8 (1.0) |
| Age [years] | BMI [kg/m2] | Systolic BP [mmHg] | Diastolic BP [mmHg] | OPP [mmHg] | IOP [mmHg] | ||
|---|---|---|---|---|---|---|---|
| ICP [mmHg] | r | 0.12 | 0.07 | 0.26 | −0.03 | 0.05 | 0.39 |
| p | (0.26) | (0.56) | (0.02) * | (0.82) | (0.65) | (<0.001) * | |
| TPD [mmHg] | r | −0.14 | −0.14 | −0.20 | 0.03 | −0.16 | 0.41 |
| p | (0.21) | (0.21) | (0.07) | (0.78) | (0.16) | (<0.001) * | |
| ICP [mmHg] | TPD [mmHg] | IOP [mmHg] | Age [years] | BMI [kg/m2] | Systolic BP [mmHg] | Diastolic BP [mmHg] | OPP [mmHg] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VF zones | Nasal [dB] | r | 0.36 | −0.38 | −0.03 | −0.05 | −0.10 | −0.10 | −0.25 | −0.19 |
| p | (0.001) ** | (0.001) ** | (0.80) | (0.70) | (0.39) | (0.36) | (0.02) ** | (0.09) | ||
| Temporal [dB] | r | 0.03 | −0.14 | −0.20 | −0.08 | −0.03 | −0.02 | −0.18 | 0.02 | |
| p | (0.79) | (0.23) | (0.07) | (0.49) | (0.81) | (0.85) | (0.87) | (0.85) | ||
| Central [dB] | r | 0.003 | 0.12 | 0.16 | 0.07 | 0.15 | 0.29 | 0.27 | 0.26 | |
| p | (0.98) | (0.28) | (0.17) | (0.52) | (0.19) | (0.01) * | (0.02) * | (0.02) * | ||
| Paracentral [dB] | r | 0.04 | 0.05 | 0.10 | −0.09 | 0.31 | 0.10 | 0.15 | 0.13 | |
| p | (0.75) | (0.66) | (0.38) | (0.38) | (0.006) ** | (0.39) | (0.19) | (0.26) | ||
| Peripheral [dB] | r | 0.15 | −0.71 | −0.07 | −0.11 | −0.02 | −0.12 | −0.13 | −0.12 | |
| p | (0.20) | (0.13) | (0.56) | (0.34) | (0.86) | (0.27) | (−0.24) | (0.30) | ||
| VF parameters | MD [dB] | r | 0.19 | −0.27 | −0.07 | −0.53 | 0.15 | −0.04 | −0.12 | −0.07 |
| p | (0.10) | (0.01) * | (0.57) | (0.64) | (0.19) | (0.70) | (0.27) | (0.53) | ||
| PSD [dB] | r | −0.21 | 0.28 | −0.40 | 0.09 | 0.000 | 0.05 | 0.13 | 0.08 | |
| p | (0.06) | (0.01) ** | (0.72) | (0.42) | (1.00) | (0.63) | (0.27) | (0.48) | ||
| VFI [%] | r | 0.13 | −0.19 | −0.76 | −0.11 | −0.03 | −0.03 | −0.09 | −0.04 | |
| p | (0.25) | (0.10) | (0.50) | (0.35) | (0.79) | (0.82) | (0.44) | (0.72) | ||
| Multivariate | Age Adjusted | BMI Adjusted | ||||
|---|---|---|---|---|---|---|
| Parameter | B [95% CI] | p-Value | B [95% CI] | p-Value | B [95% CI] | p-Value |
| ICP | ||||||
| Age, years | −0.28 [−0.77–0.2] | 0.25 | – | – | −0.28 [−0.77–0.2] | 0.25 |
| BMI, kg/m2 | 0.12 [−0.94–0.12] | 0.85 | 0.01 [−0.1–0.11] | 0.9 | – | – |
| Systolic BP, mmHg | 0.11 [0.06–0.17] | <0.001 | 0.1 [0.05–0.14] | <0.001 | 0.11 [0.06–0.17] | <0.001 |
| Diastolic BP, mmHg | −0.15 [−0.22–0.07] | <0.001 | −0.13 [−0.2–−0.06] | <0.001 | −0.14 [−0.21–−0.07] | <0.001 |
| IOP, mmHg | 0.42 [0.18–0.65] | 0.001 | 0.43 [0.24–0.61] | <0.001 | 0.43 [0.25–0.62] | <0.001 |
| TPD | ||||||
| Age, years | 0.28 [−0.02–0.08] | 0.56 | – | – | 0.03 [−0.02–0.08] | 0.08 |
| BMI, kg/m2 | −0.11 [−0.12–0.09] | 0.11 | −0.01 [–0.11–0.1] | 0.9 | – | – |
| Systolic BP, mmHg | −0.09 [−0.16–−0.03] | 0.003 | −0.09 [−0.14–−0.05] | <0.001 | −0.11 [−0.17–−0.06] | <0.001 |
| Diastolic BP, mmHg | 0.12 [0.03–0.21] | 0.007 | 0.13 [0.06–0.2] | <0.001 | 0.14 [0.07–0.21] | <0.001 |
| IOP, mmHg | 0.57 [0.39–0.75] | <0.001 | 0.57 [0.39–0.76] | <0.001 | 0.57 [0.39–0.75] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoskuviene, A.; Siaudvytyte, L.; Januleviciene, I.; Vaitkus, A.; Simiene, E.; Bakstyte, V.; Ragauskas, A.; Antman, G.; Siesky, B.; Harris, A. The Relationship between Intracranial Pressure and Visual Field Zones in Normal-Tension Glaucoma Patients. Diagnostics 2023, 13, 174. https://doi.org/10.3390/diagnostics13020174
Stoskuviene A, Siaudvytyte L, Januleviciene I, Vaitkus A, Simiene E, Bakstyte V, Ragauskas A, Antman G, Siesky B, Harris A. The Relationship between Intracranial Pressure and Visual Field Zones in Normal-Tension Glaucoma Patients. Diagnostics. 2023; 13(2):174. https://doi.org/10.3390/diagnostics13020174
Chicago/Turabian StyleStoskuviene, Akvile, Lina Siaudvytyte, Ingrida Januleviciene, Antanas Vaitkus, Evelina Simiene, Viktorija Bakstyte, Arminas Ragauskas, Gal Antman, Brent Siesky, and Alon Harris. 2023. "The Relationship between Intracranial Pressure and Visual Field Zones in Normal-Tension Glaucoma Patients" Diagnostics 13, no. 2: 174. https://doi.org/10.3390/diagnostics13020174
APA StyleStoskuviene, A., Siaudvytyte, L., Januleviciene, I., Vaitkus, A., Simiene, E., Bakstyte, V., Ragauskas, A., Antman, G., Siesky, B., & Harris, A. (2023). The Relationship between Intracranial Pressure and Visual Field Zones in Normal-Tension Glaucoma Patients. Diagnostics, 13(2), 174. https://doi.org/10.3390/diagnostics13020174









