The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights
Abstract
:1. Introduction
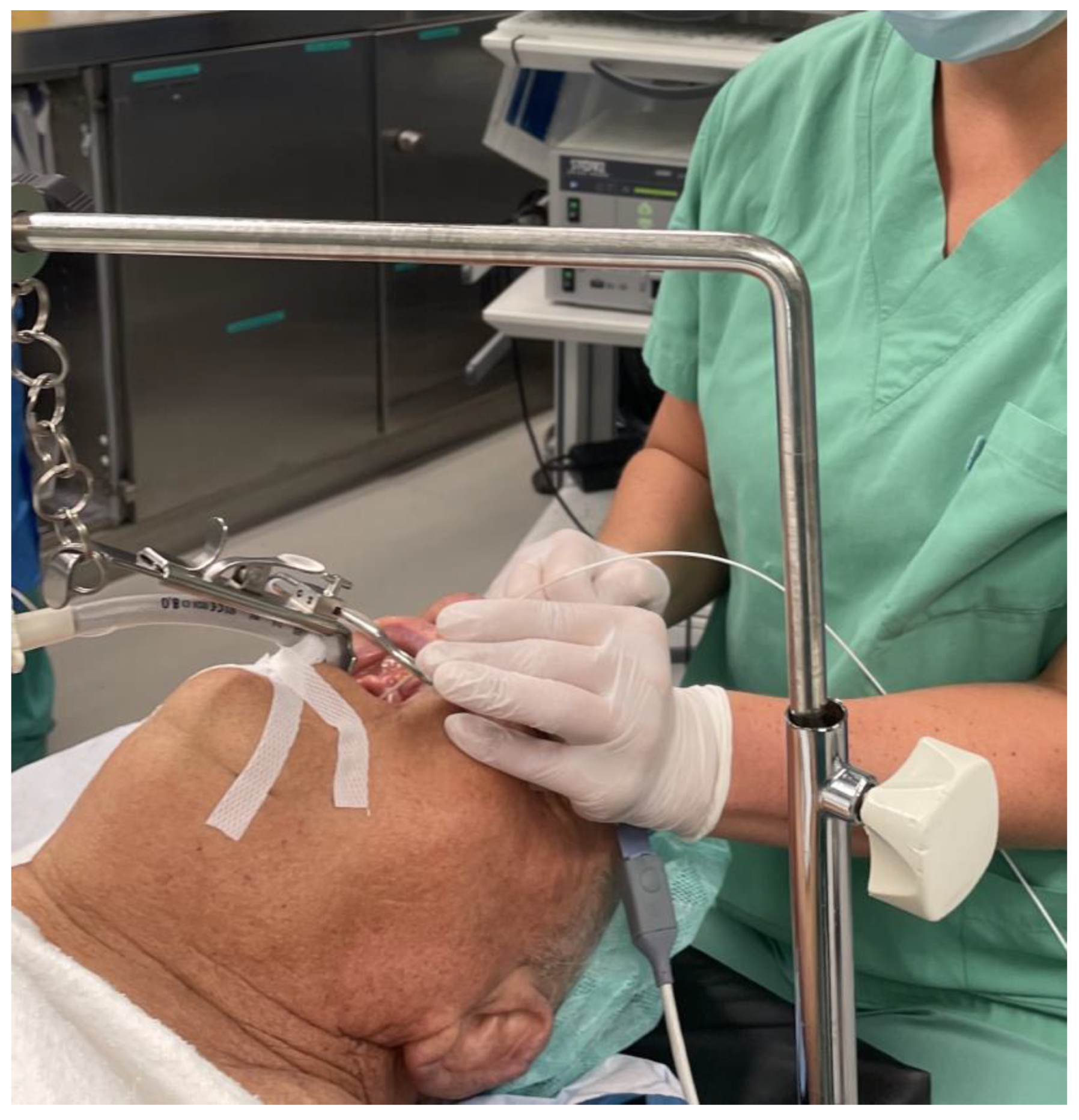
2. Materials and Methods
2.1. Oncologic Surgery
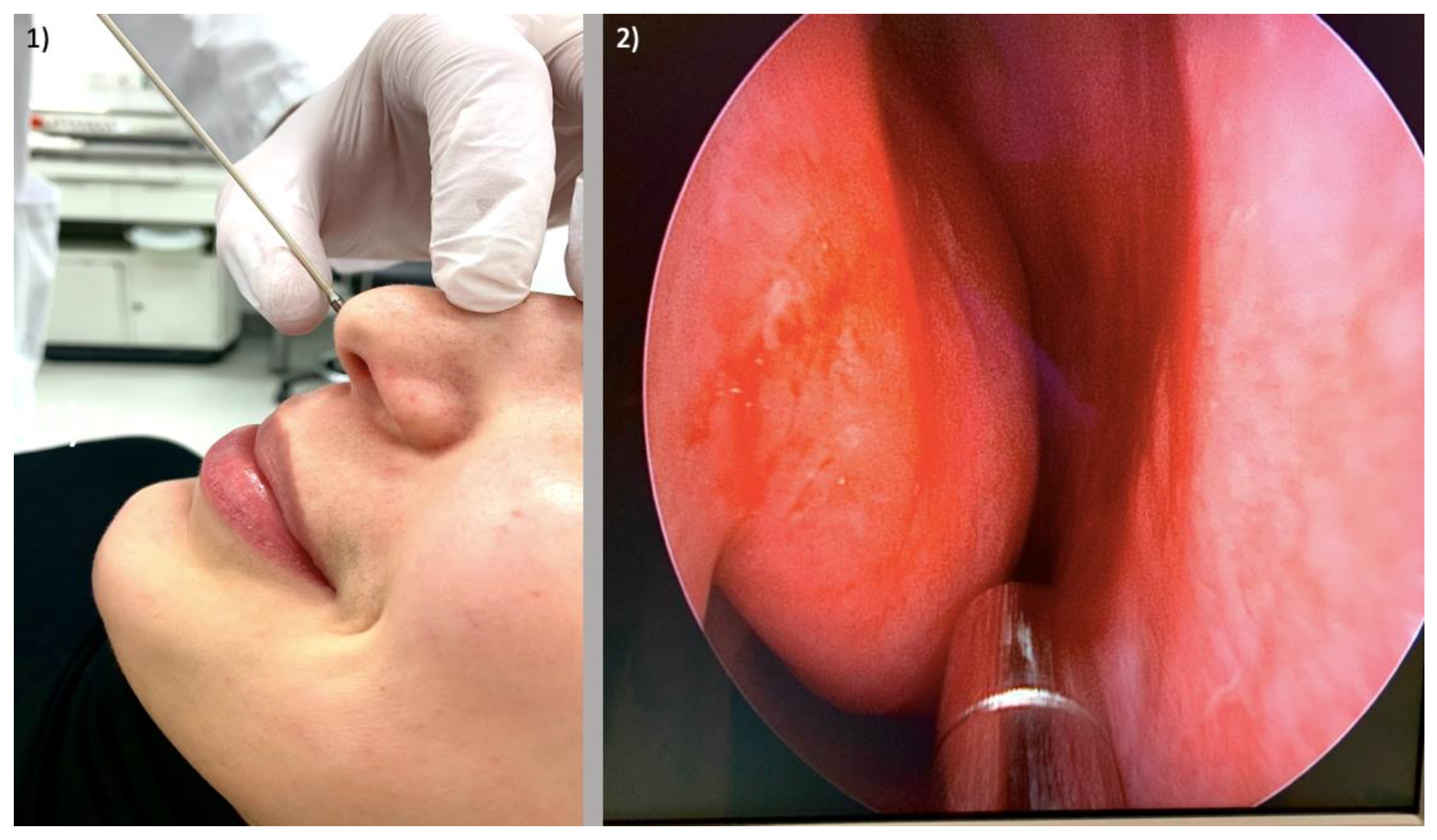
2.2. Nasal Provocation Testing in Allergic Rhinitis
3. Results
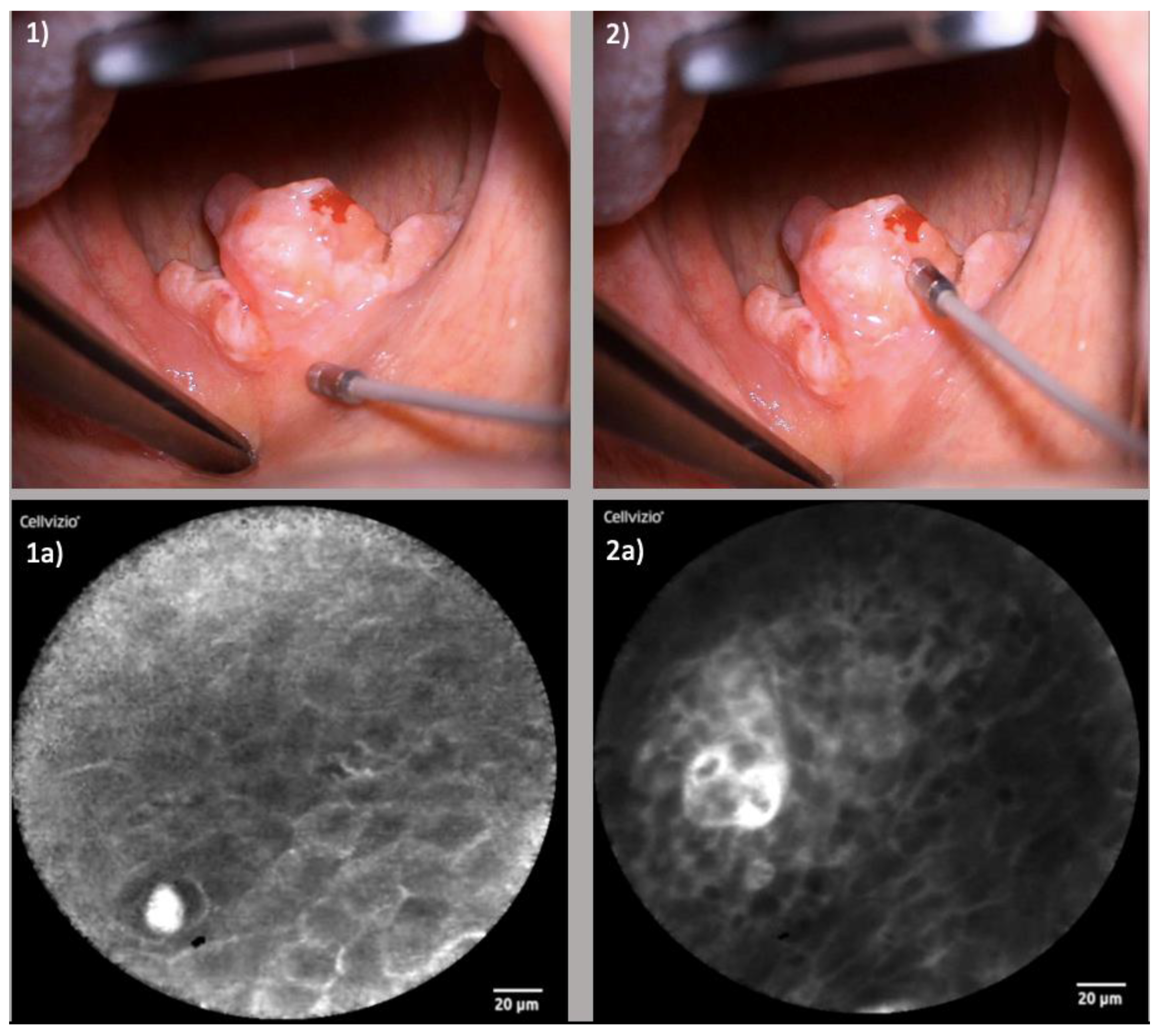
3.1. Oncologic Surgery
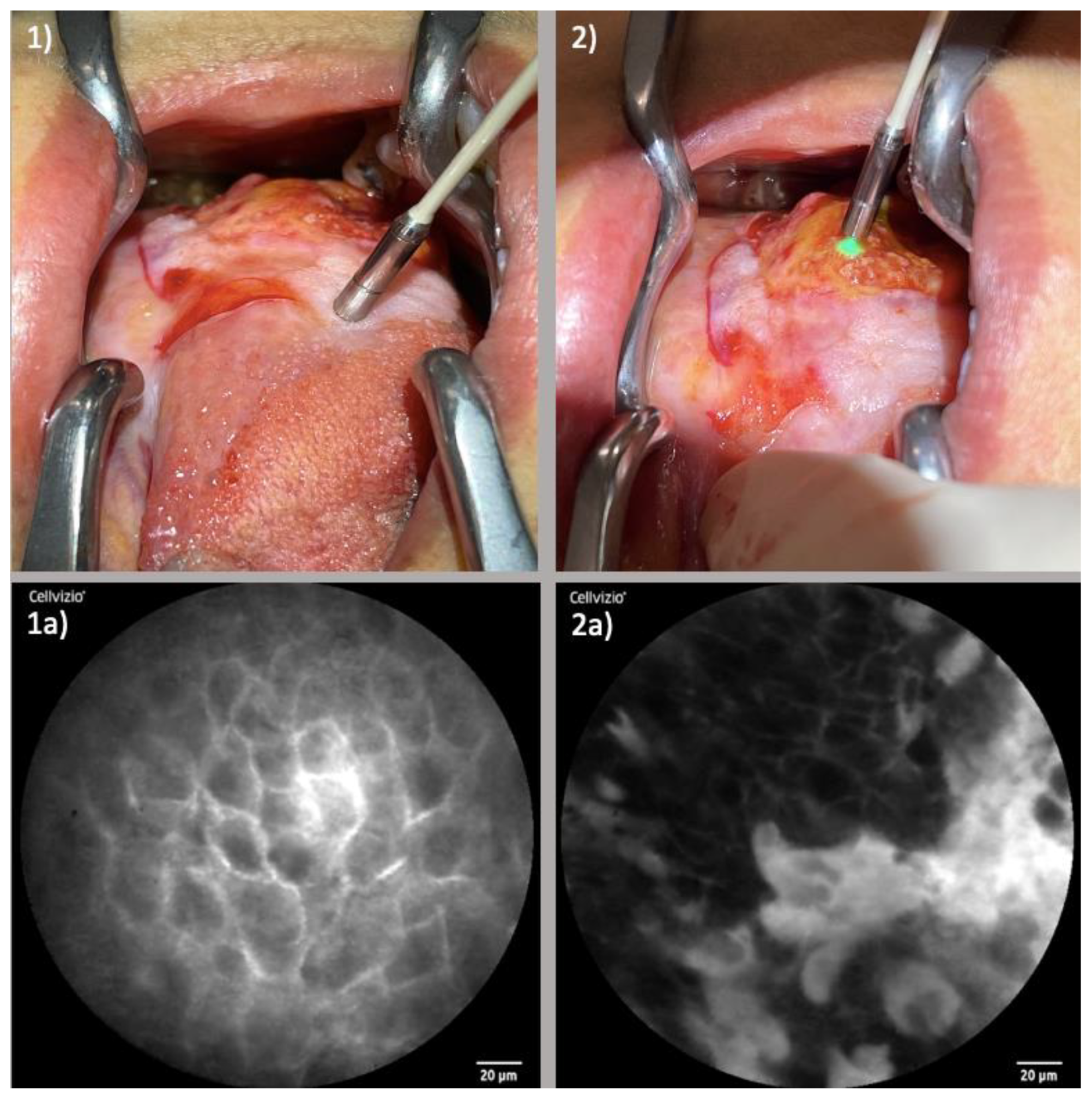
3.1.1. Endonasal Squamous Cell Carcinoma (ESCC)
3.1.2. Oropharyngeal Squamous Cell Carcinoma (OPSCC)
3.1.3. Oral Cavity Squamous Cell Carcinoma (OCSCC)
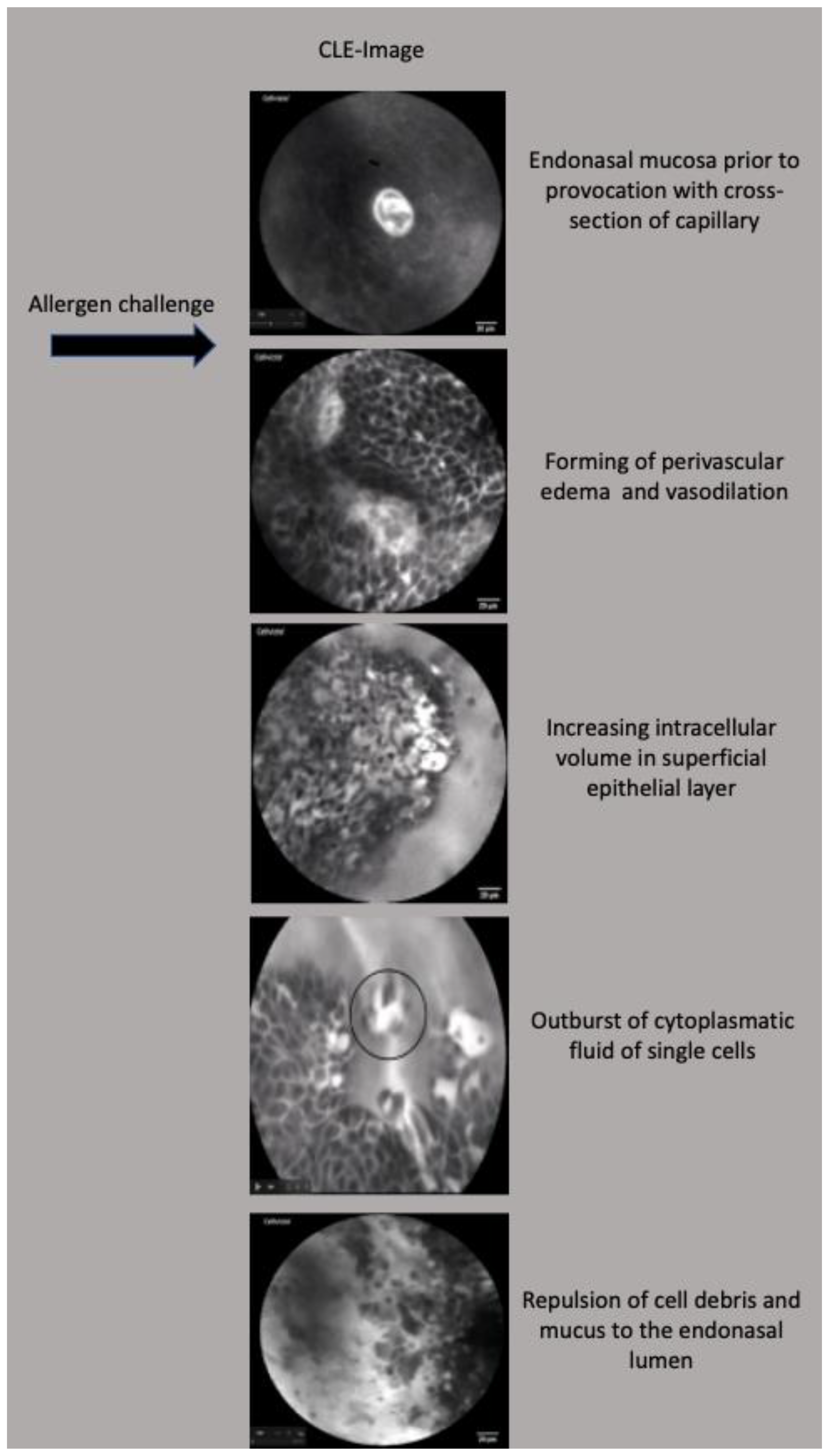
3.2. Functional Assessment of Allergic Rhinitis
4. Discussion
4.1. CLE in Functional Assessment of Allergic Rhinitis
4.2. CLE in Head and Neck Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiesslich, R.; Canto, M.I. Confocal laser endomicroscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, M.; Kadah, B.A.; Bozzato, A.; Bozzato, V.; Hasenfus, A.; Kim, Y.J.; Wagner, M.; Igressa, A.; Schick, B.; Charalampaki, P. Noninvasive histological imaging of head and neck squamous cell carcinomas using confocal laser endomicroscopy. Eur. Arch. Otorhinolaryngol. 2016, 273, 4473–4483. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Iro, H.; Dittberner, A.; Agaimy, A.; Bohr, C. Value of confocal laser endomicroscopy in the diagnosis of vocal cord lesions. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3990–3997. [Google Scholar] [PubMed]
- De Palma, G.D. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J. Gastroenterol. 2009, 15, 5770–5775. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Stelzle, F.; Aubreville, M.; Mueller, S.K.; Eckstein, M.; Oetter, N.; Maier, A.; Mantsopoulos, K.; Iro, H.; Goncalves, M. Intraoperative free margins assessment of oropharyngeal squamous cell carcinoma with confocal laser endomicroscopy: A pilot study. Eur. Arch. Otorhinolaryngol. 2021, 278, 4433–4439. [Google Scholar] [CrossRef]
- Wenda, N.; Striedter, C.; Kiesslich, R.; Gosepath, J. ”Volcanic eruptions” and “mucosal avalanches”-real-time documentation of cellular reactions in allergic rhinitis visualized in vivo by confocal laser endomicroscopy. Int. Forum Allergy Rhinol. 2021, 11, 1269–1272. [Google Scholar] [CrossRef]
- Settipane, R.A. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001, 22, 185–189. [Google Scholar]
- Zhang, Y.; Lan, F.; Zhang, L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef]
- Hansen, I.; Klimek, L.; Mösges, R.; Hörmann, K. Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 159–163. [Google Scholar] [CrossRef]
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167. [Google Scholar] [CrossRef]
- Gawlik, R.; DuBuske, L. Mediator release of neuropeptides after nasal provocation in perennial allergic rhinitis patients. Rhinology 2010, 48, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Raap, U.; Holz, M.; Hörmann, K.; Klimek, L. Pathophysiology of itching and sneezing in allergic rhinitis. Swiss Med. Wkly. 2009, 139, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.G.; Neumann, J.; Hansen, T.; Goetz, M.; Hoffman, A.; Neurath, M.F.; Galle, P.R.; Chan, Y.H.; Kiesslich, R.; Watson, A.J. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yue, M.; Dai, Y.; Yu, C.; Chen, C. Confocal laser endomicroscopy reveals alterations in duodenal permeability in patients with acute pancreatitis. J. Int. Med. Res. 2019, 47, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2004, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.G.; Pai, M.S.; George, L.S. Quality of life of patients with head and neck cancer: A mixed method study. J. Cancer Res. Ther. 2019, 15, 638–644. [Google Scholar] [CrossRef]
- Tirelli, G.; Marcuzzo, A.V.; Boscolo Nata, F. Narrow-band imaging pattern classification in oral cavity. Oral. Dis. 2018, 24, 1458–1467. [Google Scholar] [CrossRef]
- Betz, C.S.; Kraft, M.; Arens, C.; Schuster, M.; Pfeffer, C.; Rühm, A.; Stepp, H.; Englhard, A.; Volgger, V. Optical diagnostic methods for early tumour diagnosis in the upper aerodigestive tract: Quo vadis? HNO 2016, 64, 41–48. [Google Scholar] [CrossRef]
- Piazza, C.; Dessouky, O.; Peretti, G.; Cocco, D.; De Benedetto, L.; Nicolai, P. Narrow-band imaging: A new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol. Ital. 2008, 28, 49–54. [Google Scholar]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral. Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef]
- Pogorzelski, B.; Hanenkamp, U.; Goetz, M.; Kiesslich, R.; Gosepath, J. Systematic intraoperative application of confocal endomicroscopy for early detection and resection of squamous cell carcinoma of the head and neck: A preliminary report. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 404–411. [Google Scholar]
- Wenda, N.; Kiesslich, R.; Gosepath, J. Confocal laser endomicroscopy—First application and validation of malignancy criteria. Laryngorhinootologie 2021, 100, 818–823. [Google Scholar] [PubMed]
- Wenda, N.; Kiesslich, R.; Gosepath, J. Technical Note: First Use of Endonasal Confocal Laser Endomicroscopy—Feasibility and Proof of Concept. Int. Arch. Otorhinolaryngol. 2021, 26, e396–e400. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Friedland, S.; Sahbaie, P.; Soetikno, R.; Hsiung, P.L.; Liu, J.T.; Crawford, J.M.; Contag, C.H. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin. Gastroenterol. Hepatol. 2007, 5, 1300–1305. [Google Scholar] [CrossRef]
- Lipson, B.K.; Yannuzzi, L.A. Complications of intravenous fluorescein injections. Int. Ophthalmol. Clin. 1989, 29, 200–205. [Google Scholar] [CrossRef]
- Abbaci, M.; Casiraghi, O.; Vergez, S.; Maillard, A.; Lakhdar, A.B.; De Leeuw, F.; Crestani, S.; Ngo, C.; Koscielny, S.; Ferchiou, M.; et al. Diagnostic accuracy of in vivo early tumor imaging from probe-based confocal laser endomicroscopy versus histologic examination in head and neck squamous cell carcinoma. Clin. Oral. Investig. 2022, 26, 1823–1833. [Google Scholar] [CrossRef]
- Sievert, M.; Mantsopoulos, K.; Mueller, S.K.; Eckstein, M.; Rupp, R.; Aubreville, M.; Stelzle, F.; Oetter, N.; Maier, A.; Iro, H.; et al. Systematic interpretation of confocal laser endomicroscopy: Larynx and pharynx confocal imaging score. Acta Otorhinolaryngol. Ital. 2022, 42, 26–33. [Google Scholar] [CrossRef]
- Sievert, M.; Oetter, N.; Mantsopoulos, K.; Gostian, A.O.; Mueller, S.K.; Koch, M.; Balk, M.; Thimsen, V.; Stelzle, F.; Eckstein, M.; et al. Systematic classification of confocal laser endomicroscopy for the diagnosis of oral cavity carcinoma. Oral. Oncol. 2022, 132, 105978. [Google Scholar] [CrossRef]
- Sethi, S.; Ju, X.; Logan, R.M.; Sambrook, P.; McLaughlin, R.A.; Jamieson, L.M. Diagnostic Accuracy of Confocal Laser Endomicroscopy for the Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2021, 18, 12390. [Google Scholar] [CrossRef]
- Guleria, S.; Shah, T.U.; Pulido, J.V.; Fasullo, M.; Ehsan, L.; Lippman, R.; Sali, R.; Mutha, P.; Cheng, L.; Brown, D.E.; et al. Deep learning systems detect dysplasia with human-like accuracy using histopathology and probe-based confocal laser endomicroscopy. Sci. Rep. 2021, 11, 5086. [Google Scholar] [CrossRef]
- Lee, T.C.; Angelina, C.L.; Kongkam, P.; Wang, H.P.; Rerknimitr, R.; Han, M.L.; Chang, H.T. Deep-Learning-Enabled Computer-Aided Diagnosis in the Classification of Pancreatic Cystic Lesions on Confocal Laser Endomicroscopy. Diagnostics 2023, 13, 1289. [Google Scholar] [CrossRef] [PubMed]
- Aubreville, M.; Stoeve, M.; Oetter, N.; Goncalves, M.; Knipfer, C.; Neumann, H.; Bohr, C.; Stelzle, F.; Maier, A. Deep learning-based detection of motion artifacts in probe-based confocal laser endomicroscopy images. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
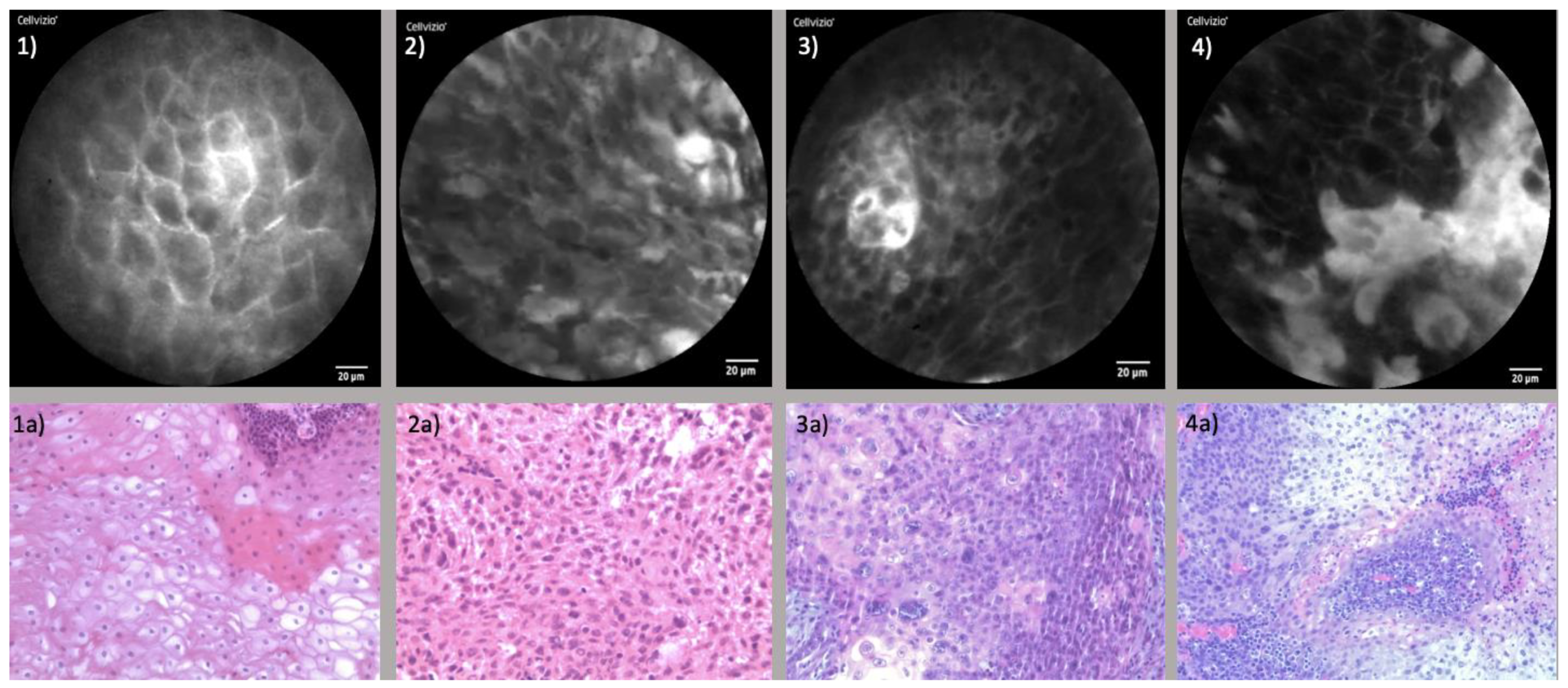

| Healthy Mucosa | Carcinoma | |
|---|---|---|
| Cellular Configuration | Regular, oval to polygonal architecture | Deformation of cellular shape |
| Cleary displayed boarders | Blurry to extinct boarders | |
| Capillaries | Small, round to oval shaped Individually displayable | Irregular shape Formation of vascular clusters |
| Absence of perivascular fluorescein leakage | Perivascular fluorescein leakage | |
| Fluorescein Uptake | Homogenous distribution | Inhomogeneous distribution with Brighter aspects in areas of leakage |
| Darker areas with reduced uptake |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenda, N.; Fruth, K.; Fisseler-Eckhoff, A.; Gosepath, J. The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights. Diagnostics 2023, 13, 3081. https://doi.org/10.3390/diagnostics13193081
Wenda N, Fruth K, Fisseler-Eckhoff A, Gosepath J. The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights. Diagnostics. 2023; 13(19):3081. https://doi.org/10.3390/diagnostics13193081
Chicago/Turabian StyleWenda, Nina, Kai Fruth, Annette Fisseler-Eckhoff, and Jan Gosepath. 2023. "The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights" Diagnostics 13, no. 19: 3081. https://doi.org/10.3390/diagnostics13193081
APA StyleWenda, N., Fruth, K., Fisseler-Eckhoff, A., & Gosepath, J. (2023). The Multifaceted Role of Confocal Laser Endomicroscopy in Head and Neck Surgery: Oncologic and Functional Insights. Diagnostics, 13(19), 3081. https://doi.org/10.3390/diagnostics13193081






