Comparison of the 11-Day Adhesive ECG Patch Monitor and 24-h Holter Tests to Assess the Response to Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Study Protocol
2.3. Outcome Definition
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Detection Rates of Arrhythmias
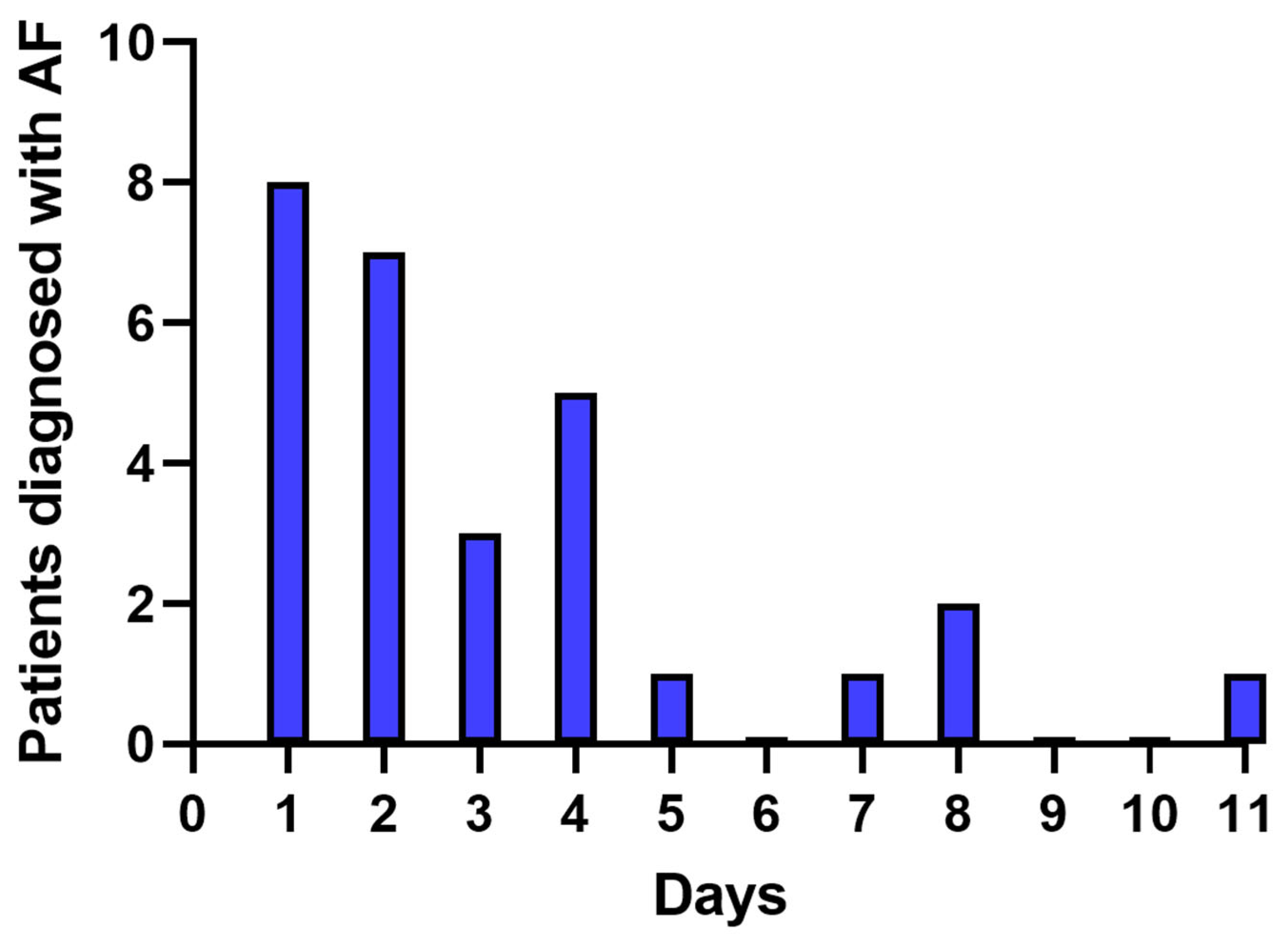
3.3. Temporal Trends in AF Detection on the ECG Patch Monitor
3.4. Treatment after AF Detection
3.5. Adverse Events
4. Discussion
4.1. Summary of the Results
4.2. Detection for Drug-Refractory AF by Extended Monitoring
4.3. Advantage and Limitation of the Adhesive Patch Monitor
4.4. Clinical Implication
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McManus, D.D.; Rienstra, M.; Benjamin, E.J. An update on the prognosis of patients with atrial fibrillation. Circulation 2012, 126, e143–e146. [Google Scholar] [CrossRef] [PubMed]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Hulme, O.L.; Khurshid, S.; Weng, L.C.; Choi, S.H.; Walkey, A.J.; Ashburner, J.M.; McManus, D.D.; Singer, D.E.; Atlas, S.J.; et al. Initial Precipitants and Recurrence of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007716. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Corley, S.D.; Epstein, A.E.; Dimarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kellen, J.C.; Klein, R.C.; Krahn, A.D.; et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef]
- Ziegler, P.D.; Koehler, J.L.; Mehra, R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006, 3, 1445–1452. [Google Scholar] [CrossRef]
- Tung, C.E.; Su, D.; Turakhia, M.P.; Lansberg, M.G. Diagnostic Yield of Extended Cardiac Patch Monitoring in Patients with Stroke or TIA. Front. Neurol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Suissa, L.; Lachaud, S.; Mahagne, M.H. Optimal timing and duration of continuous electrocardiographic monitoring for detecting atrial fibrillation in stroke patients. J. Stroke Cerebrovasc. Dis. 2013, 22, 991–995. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Cote, R.; et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Stahrenberg, R.; Weber-Kruger, M.; Seegers, J.; Edelmann, F.; Lahno, R.; Haase, B.; Mende, M.; Wohlfahrt, J.; Kermer, P.; Vollmann, D.; et al. Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke 2010, 41, 2884–2888. [Google Scholar] [CrossRef]
- Wachter, R.; Groschel, K.; Gelbrich, G.; Hamann, G.F.; Kermer, P.; Liman, J.; Seegers, J.; Wasser, K.; Schulte, A.; Jurries, F.; et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF(RANDOMISED)): An open-label randomised controlled trial. Lancet Neurol. 2017, 16, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Grond, M.; Jauss, M.; Hamann, G.; Stark, E.; Veltkamp, R.; Nabavi, D.; Horn, M.; Weimar, C.; Kohrmann, M.; Wachter, R.; et al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: A prospective multicenter cohort study. Stroke 2013, 44, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cris.nih.go.kr/cris/search/detailSearch.do/20389 (accessed on 26 August 2023).
- Bernstein, R.A.; Kamel, H.; Granger, C.B.; Piccini, J.P.; Sethi, P.P.; Katz, J.M.; Vives, C.A.; Ziegler, P.D.; Franco, N.C.; Schwamm, L.H.; et al. Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients with Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA 2021, 325, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Waalen, J.; Edwards, A.M.; Ariniello, L.M.; Mehta, R.R.; Ebner, G.S.; Carter, C.; Baca-Motes, K.; Felicione, E.; Sarich, T.; et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. JAMA 2018, 320, 146–155. [Google Scholar] [CrossRef]
- Halcox, J.P.J.; Wareham, K.; Cardew, A.; Gilmore, M.; Barry, J.P.; Phillips, C.; Gravenor, M.B. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation 2017, 136, 1784–1794. [Google Scholar] [CrossRef]
- Buck, B.H.; Hill, M.D.; Quinn, F.R.; Butcher, K.S.; Menon, B.K.; Gulamhusein, S.; Siddiqui, M.; Coutts, S.B.; Jeerakathil, T.; Smith, E.E.; et al. Effect of Implantable vs Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients with Ischemic Stroke: The PER DIEM Randomized Clinical Trial. JAMA 2021, 325, 2160–2168. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Bittel, B.; Browsky, D.; Houghtaling, P.; Drummond, C.K.; Desai, M.Y.; Gillinov, A.M. Accuracy of Apple Watch for Detection of Atrial Fibrillation. Circulation 2020, 141, 702–703. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Wachter, R.; Schmalstieg-Bahr, K.; Quinn, F.R.; Hummers, E.; Ivers, N.; Marsden, T.; Thornton, A.; Djuric, A.; Suerbaum, J.; et al. Screening for atrial fibrillation in the older population: A randomized clinical trial. JAMA Cardiol. 2021, 6, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hwang, B.H.; Oh, G.C.; Kim, J.J.; Choo, E.; Kim, M.C.; Kim, J.; Jung, H.O.; Youn, H.J.; Chung, W.-S.; et al. Long-term maintenance of sinus rhythm is associated with favorable echocardiographic remodeling and improved clinical outcomes after transcatheter aortic valve replacement. J. Clin. Med. 2022, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Talajic, M.; Dorian, P.; Connolly, S.; Eisenberg, M.J.; Green, M.; Kus, T.; Lambert, J.; Dubuc, M.; Gagne, P.; et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000, 342, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, S.N.; Reda, D.J.; Tang, X.C.; Lopez, B.; Harris, C.L.; Fletcher, R.D.; Sharma, S.C.; Atwood, J.E.; Jacobson, A.K.; et al. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 2005, 352, 1861–1872. [Google Scholar] [CrossRef]
- Zimetbaum, P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012, 125, 381–389. [Google Scholar] [CrossRef]
- Choi, Y.; Lim, B.; Yang, S.-Y.; Yang, S.-H.; Kwon, O.S.; Kim, D.; Kim, Y.G.; Park, J.-W.; Yu, T.H.; Kim, T.-H.; et al. Clinical usefullness of virtual ablation guided catheter ablation of atrial fibrillation targeting restitution parameter-guided catheter ablation: CUVIA-REGAB prospective randomized study. Korean Circ. J. 2022, 52, 699–711. [Google Scholar] [CrossRef]
- Hendrikx, T.; Rosenqvist, M.; Wester, P.; Sandstrom, H.; Hornsten, R. Intermittent short ECG recording is more effective than 24-hour Holter ECG in detection of arrhythmias. BMC Cardiovasc. Disor. 2014, 14, 41. [Google Scholar] [CrossRef]
- Rothman, S.A.; Laughlin, J.C.; Seltzer, J.; Walia, J.S.; Baman, R.I.; Siouffi, S.Y.; Sangrigoli, R.M.; Kowey, P.R. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J. Cardiovasc. Electrophysiol. 2007, 18, 241–247. [Google Scholar] [CrossRef]
- Roten, L.; Schilling, M.; Haberlin, A.; Seiler, J.; Schwick, N.G.; Fuhrer, J.; Delacretaz, E.; Tanner, H. Is 7-day event triggered ECG recording equivalent to 7-day Holter ECG recording for atrial fibrillation screening? Heart 2012, 98, 645–649. [Google Scholar] [CrossRef]
- Ackermans, P.A.; Solosko, T.A.; Spencer, E.C.; Gehman, S.E.; Nammi, K.; Engel, J.; Russell, J.K. A user-friendly integrated monitor-adhesive patch for long-term ambulatory electrocardiogram monitoring. J. Electrocardiol. 2012, 45, 148–153. [Google Scholar] [CrossRef]
- Turakhia, M.P.; Hoang, D.D.; Zimetbaum, P.; Miller, J.D.; Froelicher, V.F.; Kumar, U.N.; Xu, X.; Yang, F.; Heidenreich, P.A. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am. J. Cardiol. 2013, 112, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, S.R.; Choi, E.K.; Ahn, H.J.; Song, H.S.; Lee, Y.S.; Oh, S. Validation of Adhesive Single-Lead ECG Device Compared with Holter Monitoring among Non-Atrial Fibrillation Patients. Sensors 2021, 21, 3122. [Google Scholar] [CrossRef] [PubMed]
- Nault, I.; Andre, P.; Plourde, B.; Leclerc, F.; Sarrazin, J.F.; Philippon, F.; O’Hara, G.; Molin, F.; Steinberg, C.; Roy, K.; et al. Validation of a novel single lead ambulatory ECG monitor—Cardiostat—Compared to a standard ECG Holter monitoring. J. Electrocardiol. 2019, 53, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jimmelreich, J.C.L.; Karregat, E.P.M.; Lucassen, W.A.M.; van Weert, H.; de Groot, J.R.; Handoko, M.L.; Nijveldt, R.; Harskamp, R.E. Diagnostic accuracy of a smartphone-operated, single-lead electrocardiography device for detection of rhythm and conduction abnormalities in primary care. Ann. Fam. Med. 2019, 17, 403–411. [Google Scholar] [CrossRef]
- Witvliet, M.P.; Karregat, E.P.M.; Himmelreich, J.C.L.; de Jong, J.; Lucassen, W.A.M.; Harskamp, R.E. Usefulness, pitfalls and interpretation of handheld single-lead electrocardiograms. J. Electrocardiol. 2021, 66, 33–37. [Google Scholar] [CrossRef]
- Smith, W.M.; Riddell, F.; Madon, M.; Gleva, M.J. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. Am. Heart J. 2017, 185, 67–73. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.-S.; Park, H.W.; Choi, E.-K.; Park, J.-K.; Kim, J.-B.; Kang, K.-W.; Shim, J.; Joung, B.; Park, K.-M. Clinical outcomes of rhythm control strategies for asymptomatic atrial fibrillation according to the quality-of-life score: The CODE-AF (Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation) Registry. J. Am. Heart Assoc. 2022, 11, e025956. [Google Scholar] [CrossRef]
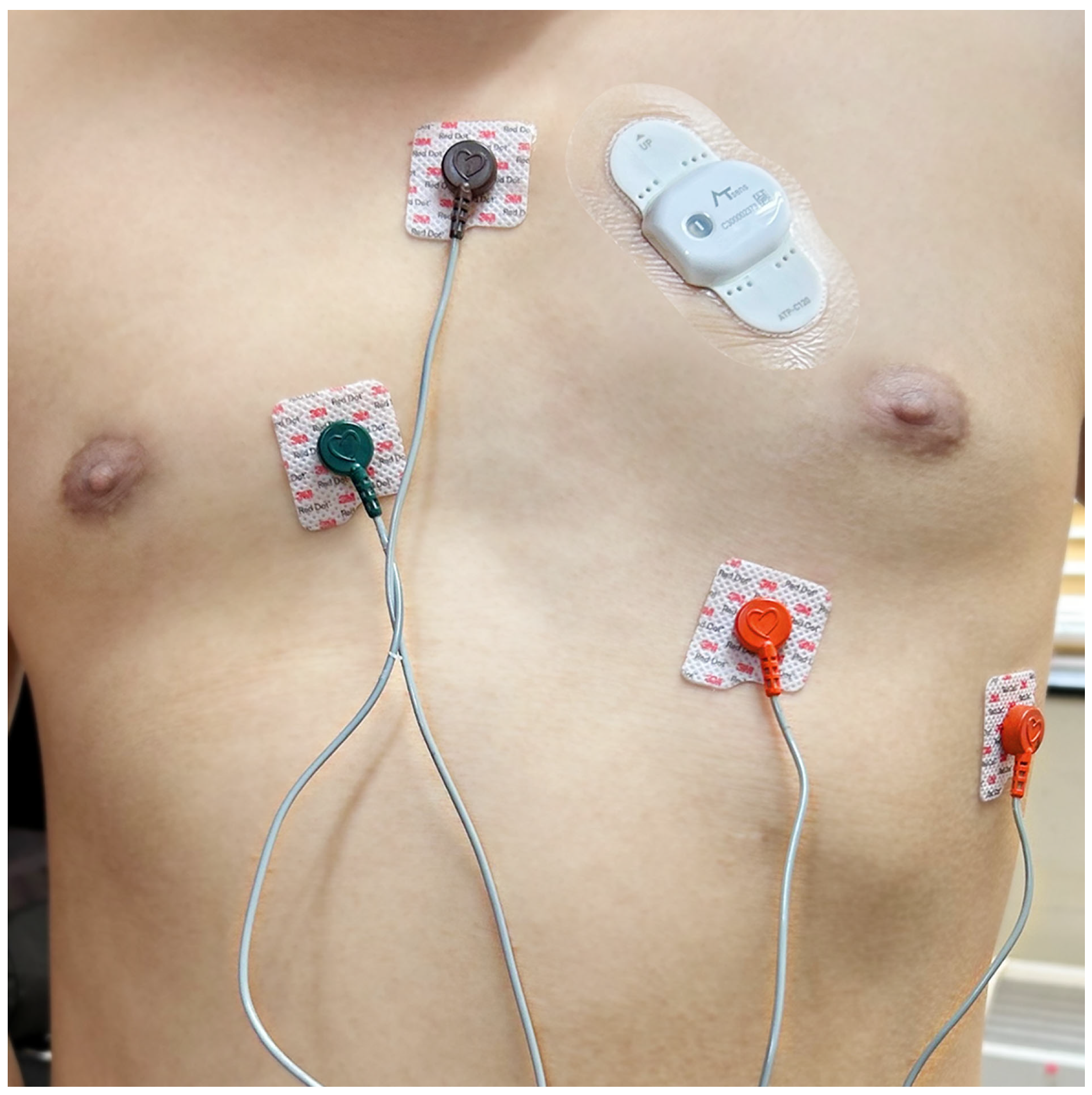
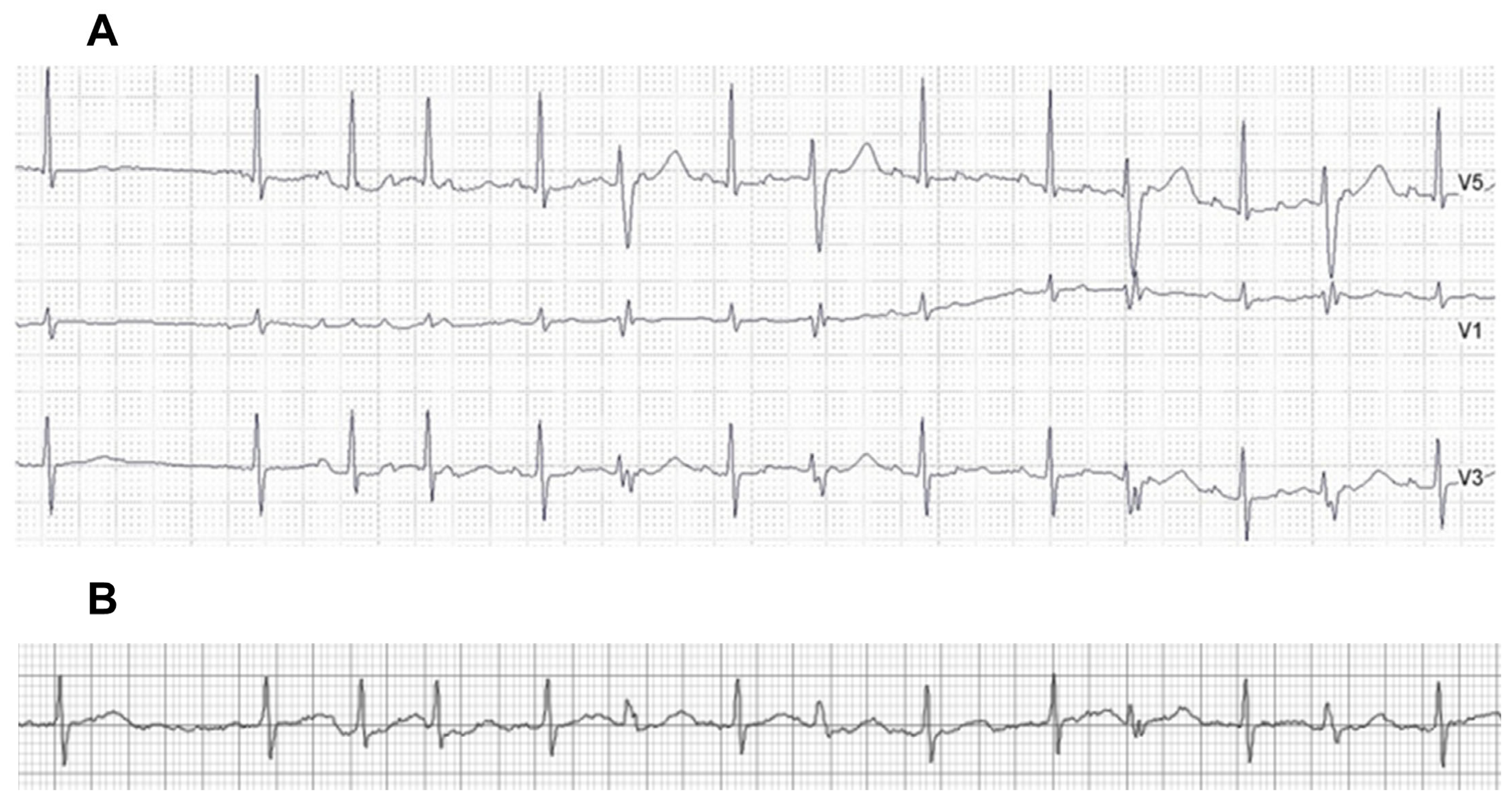
| Variables | Total N = 59 |
|---|---|
| Age, years | 67.0 ± 10.5 |
| Female, n (%) | 31 (52.5%) |
| Heart rate, beats/min | 71 ± 14 |
| CHA2DS2-VASc score | 2.8 ± 1.5 |
| AF duration, months | 34.2 (15–74) |
| Patch wearing time, days | 10.6 ± 1.48 |
| Comorbidities, n (%) | |
| Hypertension | 51 (86.4%) |
| Diabetes | 11 (18.6%) |
| Prior stroke | 26 (44.1%) |
| Dyslipidemia | 44 (74.6%) |
| Heart failure | 8 (13.6%) |
| Coronary artery disease | 2 (3.4%) |
| Type of anticoagulant, n (%) | |
| DOAC | 44 (74.6%) |
| Warfarin | 1 (1.7%) |
| Antiplatelet | 3 (5.1%) |
| Type of antiarrhythmic drugs, n (%) | |
| Propafenone | 7 (11.9%) |
| Flecainide | 3 (5.1%) |
| Pilsicainide | 43 (72.9%) |
| Dronedarone | 4 (6.8%) |
| Other medications | |
| ACEi/ARB | 49 (83.1%) |
| Beta Blocker | 49 (83.1%) |
| Diuretics | 5 (8.5%) |
| CCB | 39 (66.1%) |
| Holter | |||
|---|---|---|---|
| No | Yes | ||
| Patch ECG monitor | No | 31 | 0 |
| Yes | 20 | 8 | |
| Arrhythmias, n (%) | Patch Monitor | Holter |
|---|---|---|
| AF or AT | 28 (47.5%) | 8 (13.6%) |
| AF | 28 (47.5%) | 4 (6.8%) |
| AT | 4 (6.8%) | 4 (6.8%) |
| Non-sustained VT | 1 (1.7%) | 0 |
| Sinus node dysfunction * | 4 (6.8%) | 2 (3.4%) |
| 2nd-degree AV block | 2 (3.4%) | 2 (3.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Choi, Y.; Lee, K.; Kim, S.-H.; Kim, H.; Shin, S.; Park, S.; Oh, Y.-S. Comparison of the 11-Day Adhesive ECG Patch Monitor and 24-h Holter Tests to Assess the Response to Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation. Diagnostics 2023, 13, 3078. https://doi.org/10.3390/diagnostics13193078
Kim S, Choi Y, Lee K, Kim S-H, Kim H, Shin S, Park S, Oh Y-S. Comparison of the 11-Day Adhesive ECG Patch Monitor and 24-h Holter Tests to Assess the Response to Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation. Diagnostics. 2023; 13(19):3078. https://doi.org/10.3390/diagnostics13193078
Chicago/Turabian StyleKim, Soohyun, Young Choi, Kichang Lee, Sung-Hwan Kim, Hwajung Kim, Sanghoon Shin, Soyoon Park, and Yong-Seog Oh. 2023. "Comparison of the 11-Day Adhesive ECG Patch Monitor and 24-h Holter Tests to Assess the Response to Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation" Diagnostics 13, no. 19: 3078. https://doi.org/10.3390/diagnostics13193078
APA StyleKim, S., Choi, Y., Lee, K., Kim, S.-H., Kim, H., Shin, S., Park, S., & Oh, Y.-S. (2023). Comparison of the 11-Day Adhesive ECG Patch Monitor and 24-h Holter Tests to Assess the Response to Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation. Diagnostics, 13(19), 3078. https://doi.org/10.3390/diagnostics13193078






