Resting-State Functional Connectivity Difference in Alzheimer’s Disease and Mild Cognitive Impairment Using Threshold-Free Cluster Enhancement
Abstract
:1. Introduction
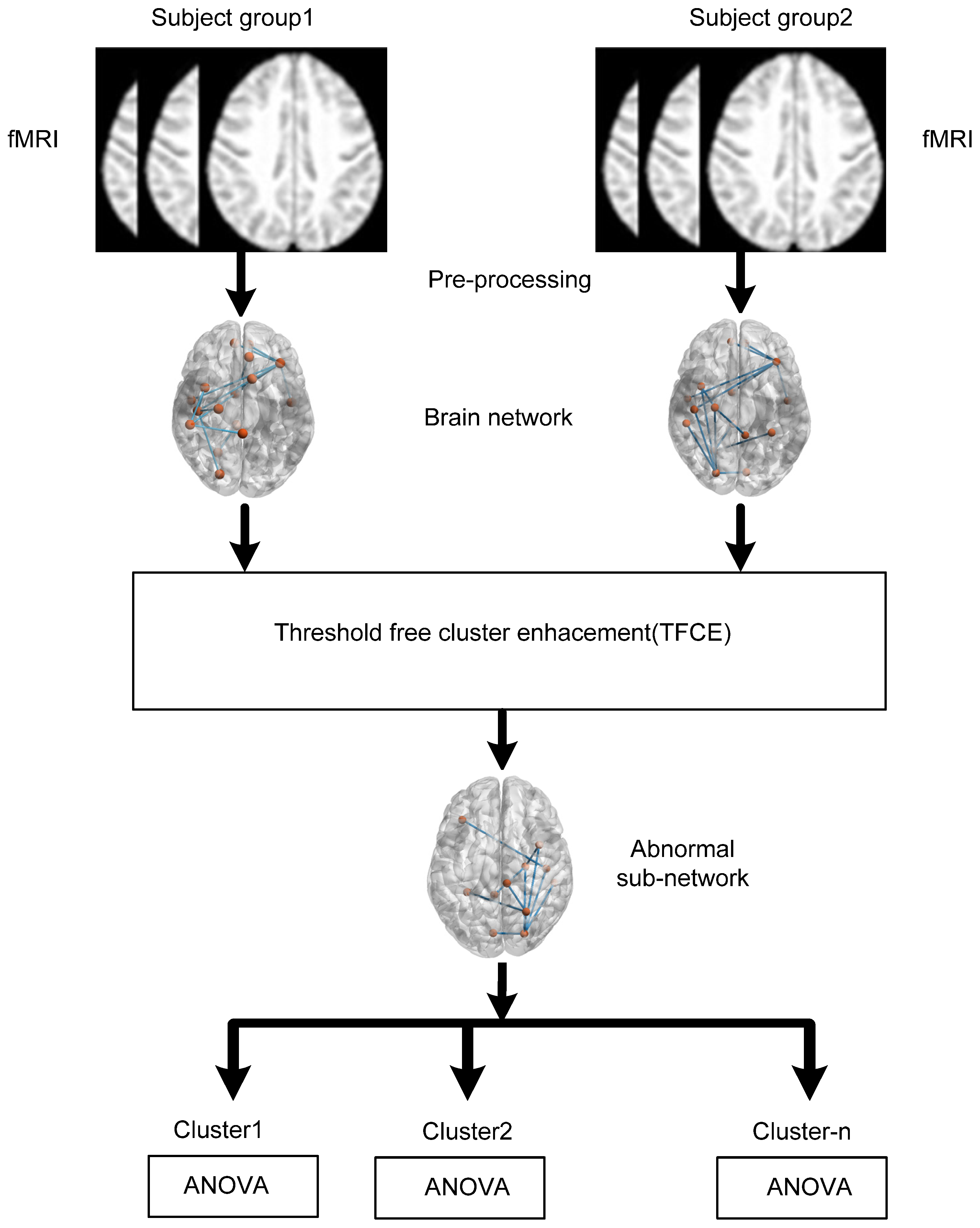
2. Materials and Methods
2.1. Subjects
2.2. Data Preprocessing
2.3. Brain Connectivity Estimation
3. Statistical Analysis
Threshold-Free Cluster Enhancement (TFCE)
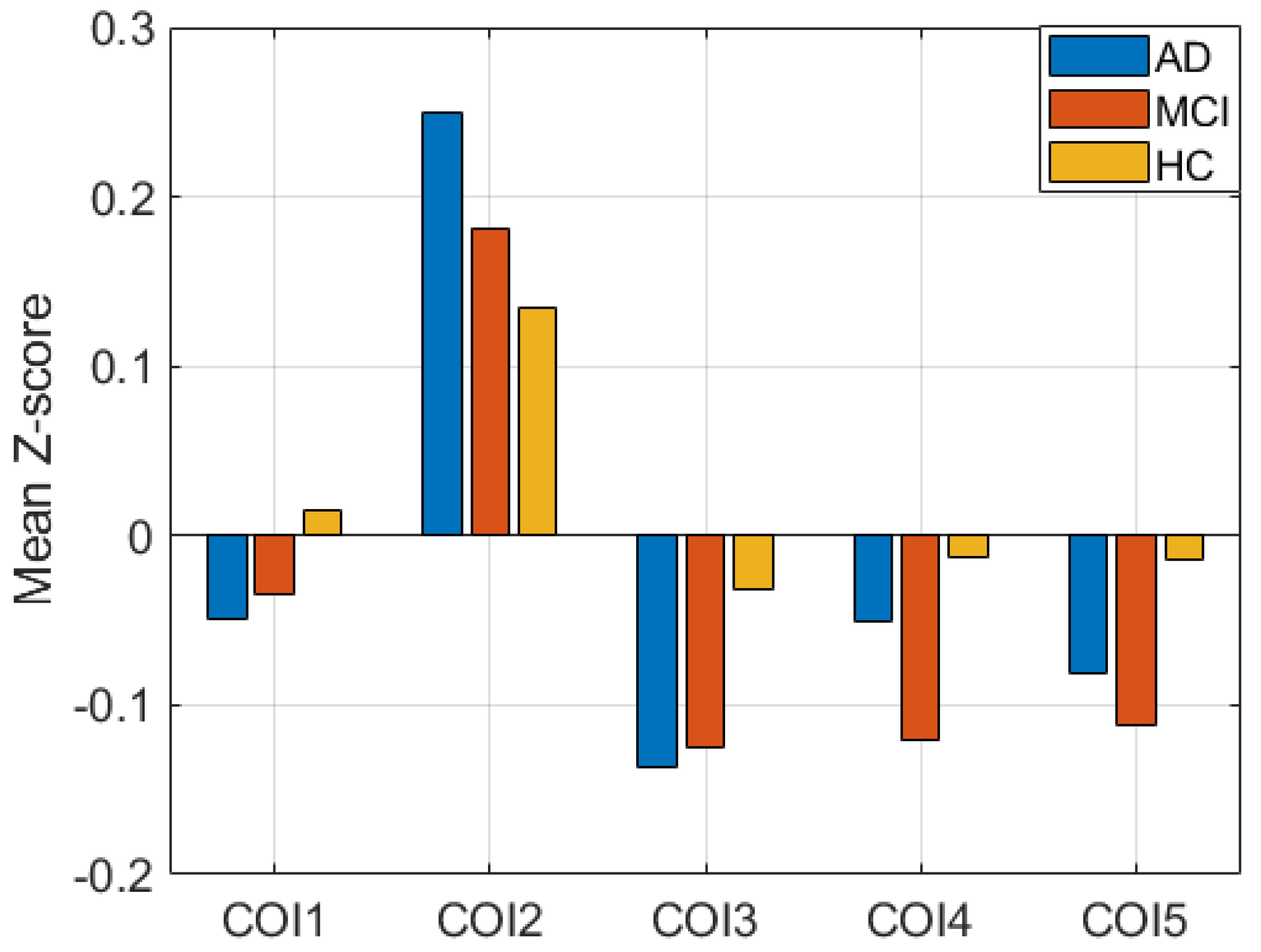
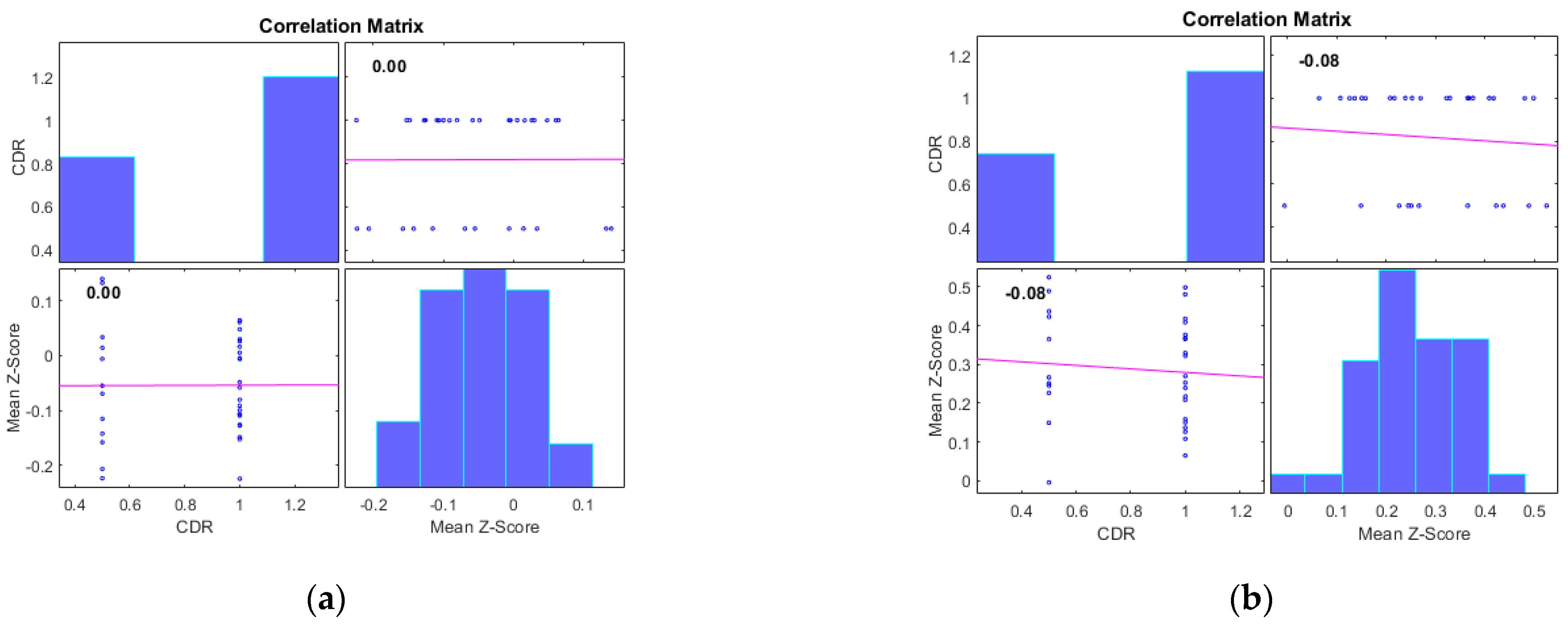
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Task Force on DSM-IV. In Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994; Volume xxv. [Google Scholar]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 5 June 2023).
- Guzmán-Vélez, E.; Diez, I.; Schoemaker, D.; Pardilla-Delgado, E.; Vila-Castelar, C.; Fox-Fuller, J.T.; Baena, A.; Sperling, R.A.; Johnson, K.A.; Lopera, F.; et al. Amyloid-β and tau pathologies relate to distinctive brain dysconnectomics in preclinical autosomal-dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2113641119. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Lama, R.K.; Kwon, G.-R. Diagnosis of Alzheimer’s Disease Using Brain Network. Front. Neurosci. 2021, 15, 605115. [Google Scholar] [CrossRef] [PubMed]
- Khazaee, A.; Ebrahimzadeh, A.; Babajani-Feremi, A. Identifying patients with Alzheimer’s disease using resting-state fMRI and graph theory. Clin. Neurophysiol. 2015, 126, 2132–2141. [Google Scholar] [CrossRef]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V.; Raichle, M.E. Default-Mode Network Activity Distinguishes Alz-Heimer’s Disease from Healthy Aging: Evidence from Functional MRI. 2004. Available online: www.fmridc.org (accessed on 5 June 2023).
- Joo, S.H.; Lim, H.K.; Lee, C.U. Three Large-Scale Functional Brain Networks from Resting-State Functional MRI in Subjects with Different Levels of Cognitive Impairment. Psychiatry Investig. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Agosta, F.; Rocca, M.A.; Pagani, E.; Absinta, M.; Magnani; Marcone, A.; Filippi, M. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2010, 31, 515–525. [Google Scholar] [CrossRef]
- Agosta, F.; Pievani, M.; Geroldi, C.; Copetti, M.; Frisoni, G.B.; Filippi, M. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiol. Aging 2012, 33, 1564–1578. [Google Scholar] [CrossRef]
- Aoki, Y.; Takahashi, R.; Suzuki, Y.; Pascual-Marqui, R.D.; Kito, Y.; Hikida, S.; Maruyama, K.; Hata, M.; Ishii, R.; Iwase, M.; et al. EEG resting-state networks in Alzheimer’s disease associated with clinical symptoms. Sci. Rep. 2023, 13, 3964. [Google Scholar] [CrossRef]
- Chhatwal, J.P.; Schultz, A.P.; Johnson, K.; Benzinger, T.L.; Jack, C.; Ances, B.M.; Thompson, P.; Saykin, A.J.; Correia, S.; Marcus, D.S.; et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 2013, 81, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, R.; Fleisher, A.S.; Reiman, E.M.; Guan, X.; Zhang, Y.; Yao, L. Altered default mode network connectivity in Alzheimer’s disease—A resting functional MRI and Bayesian network study. Hum. Brain Mapp. 2011, 32, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Nielson, K.A.; Smith, J.C. Large-Scale Network Connectivity and Cognitive Function Changes After Ex-ercise Training in Older Adults with Intact Cognition and Mild Cognitive Impairment. J. Alzheimer’s Dis. Rep. 2023, 7, 399–413. [Google Scholar] [CrossRef]
- van Nifterick, A.M.; Gouw, A.A.; van Kesteren, R.E.; Scheltens, P.; Stam, C.J.; de Haan, W. A multiscale brain network model links Alzheimer’s disease-mediated neuronal hyperactivity to large-scale oscillatory slowing. Alzheimer’s Res. Ther. 2022, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Chen, P.; Wei, Y.; Si, J.; Zhou, Y.; Wang, D.; Alzheimer’s Disease Neuroimaging Initiative. Altered large-scale dynamic connectivity patterns in Alzheimer’s disease and mild cognitive impairment patients: A machine learning study. Hum. Brain Mapp. 2023, 44, 3467–3480. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wu, Y.; Liang, Y.; Zhang, D.; Xu, Z.; Yang, X.; Meng, L. A triple-network dynamic connection study in Alzheimer’s disease. Front. Psychiatry 2022, 13, 862958. [Google Scholar] [CrossRef]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. Neuroimage 2010, 53, 1197–1207. [Google Scholar] [CrossRef]
- Lama, R.K.; Lee, S.-W. White Matter Network Alterations in Alzheimer’s Disease Patients. Appl. Sci. 2020, 10, 919. [Google Scholar] [CrossRef]
- Baggio, H.C.; Abos, A.; Segura, B.; Campabadal, A.; Garcia-Diaz, A.; Uribe, C.; Junque, C. Statistical inference in brain graphs using threshold-free network-based statistics. Hum. Brain Mapp. 2018, 39, 2289–2302. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Mumford, J.A.; Nichols, T.E. Handbook of Functional MRI Data Analysis; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Available online: http://adni.loni.usc.edu/ (accessed on 27 October 2021).
- Lama, R.K.; Kim, J.-I.; Kwon, G.-R. Classification of Alzheimer’s Disease Based on Core-Large Scale Brain Network Using Multilayer Extreme Learning Machine. Mathematics 2022, 10, 1967. [Google Scholar] [CrossRef]
- Andersson, J.L.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K.; Andersson, J.L.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K.; et al. Modeling Geometric Deformations in EPI Time Series. NeuroImage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Henson, R.N.A.; Buechel, C.; Josephs, O.; Friston, K.J. The slice-timing problem in event-related fMRI. NeuroImage 1999, 9, 125. [Google Scholar]
- Ashburner, J.; Friston, K.J.; Ashburner, J.; Friston, K.J.; Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Sperling, R.A. Large-Scale Functional Brain Network Abnormalities in Alzheimer’s Disease: Insights from Functional Neuroimaging. Behav. Neurol. 2009, 21, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Grieder, M.; Wang, D.J.J.; Dierks, T.; Wahlund, L.-O.; Jann, K. Default Mode Network Complexity and Cognitive Decline in Mild Alzheimer’s Disease. Front. Neurosci. 2018, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Qin, W.; Liu, Y.; Zhang, X.; Duan, Y.; Song, J.; Yu, C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2014, 35, 3446–3464. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Fleisher, A.S.; Reiman, E.M.; Chen, K.; Yao, L. Attention-related networks in Alzheimer’s disease: A resting functional MRI study. Hum. Brain Map. 2012, 35, 1076–1088. [Google Scholar] [CrossRef]
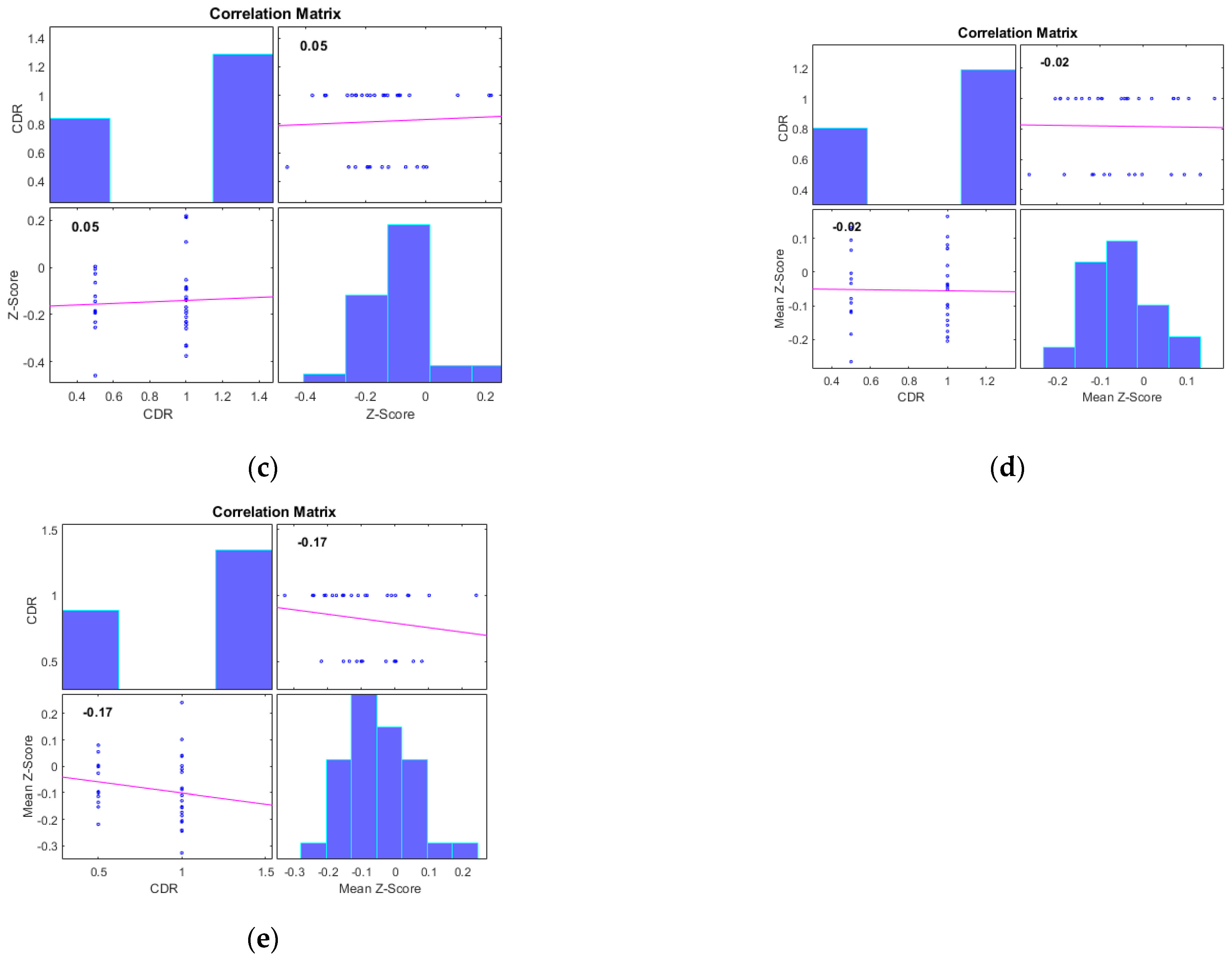
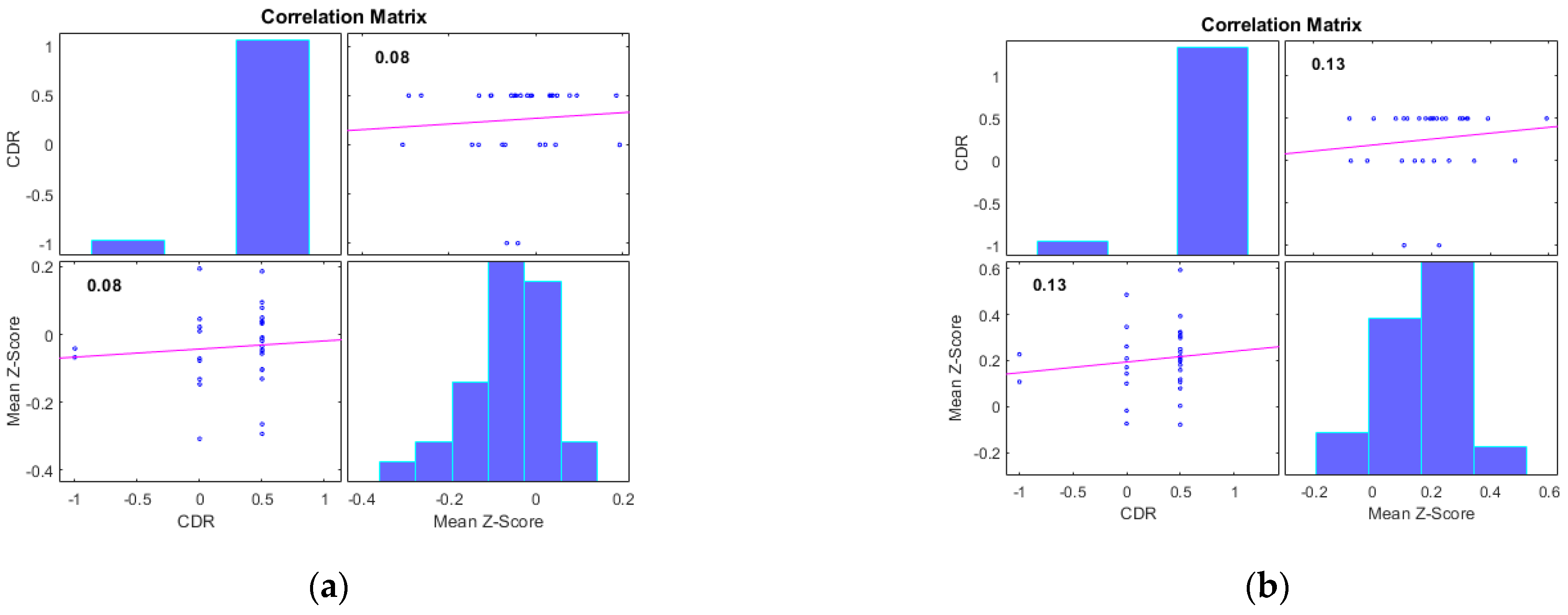
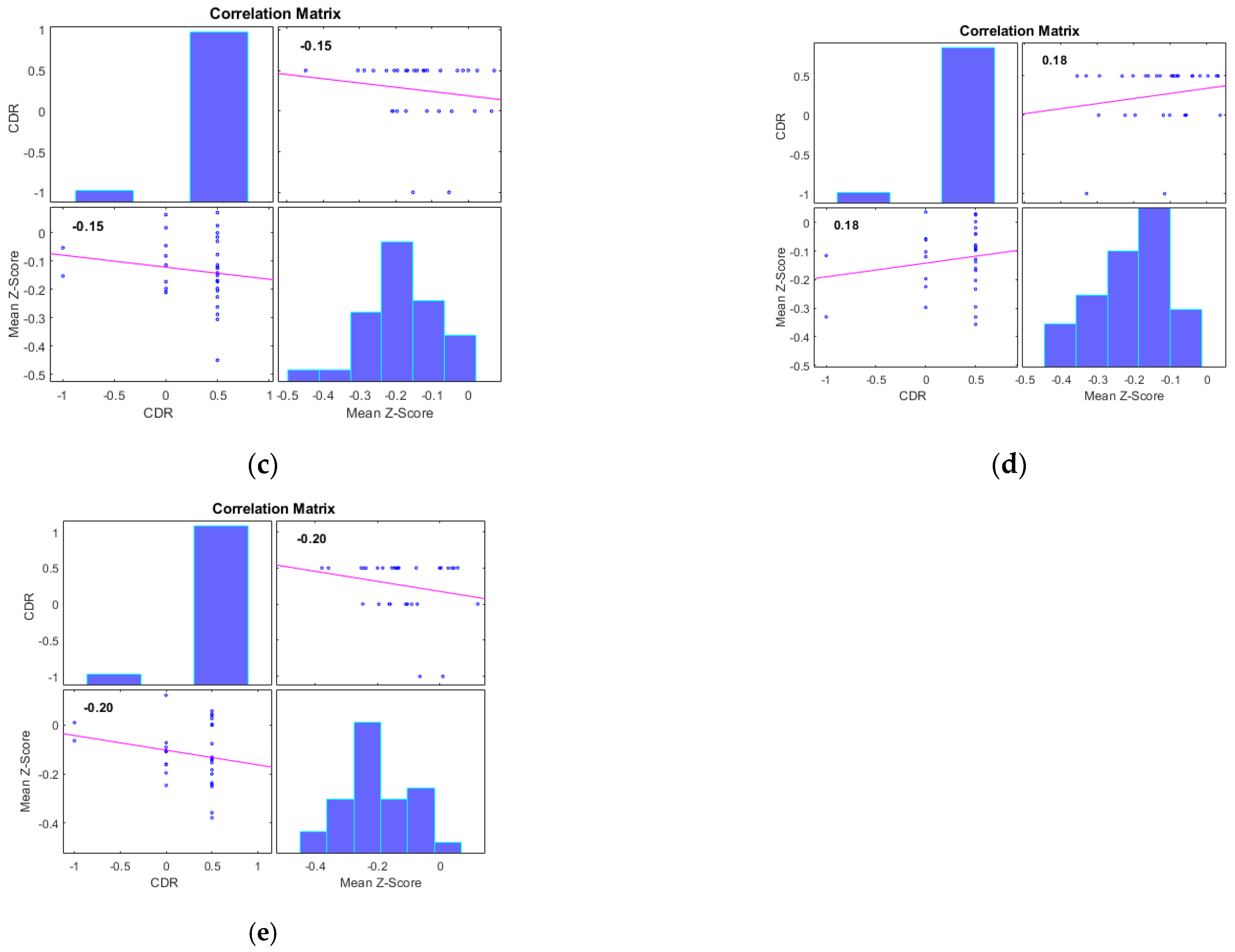
| Number of Subjects | HC (n = 31) | MCI (n = 31) | AD (n = 33) |
|---|---|---|---|
| Age (years) | 73.9 ± 5.4 | 74.5 ± 5.0 | 72.7 ± 7.0 |
| Global CDR | 0.04 ± 0.13 | 0.5 ± 0.18 | 0.95 ± 0.30 |
| MMSE | 28.9 ± 1.65 | 27.5 ± 2.02 | 20.87 ± 3.6 |
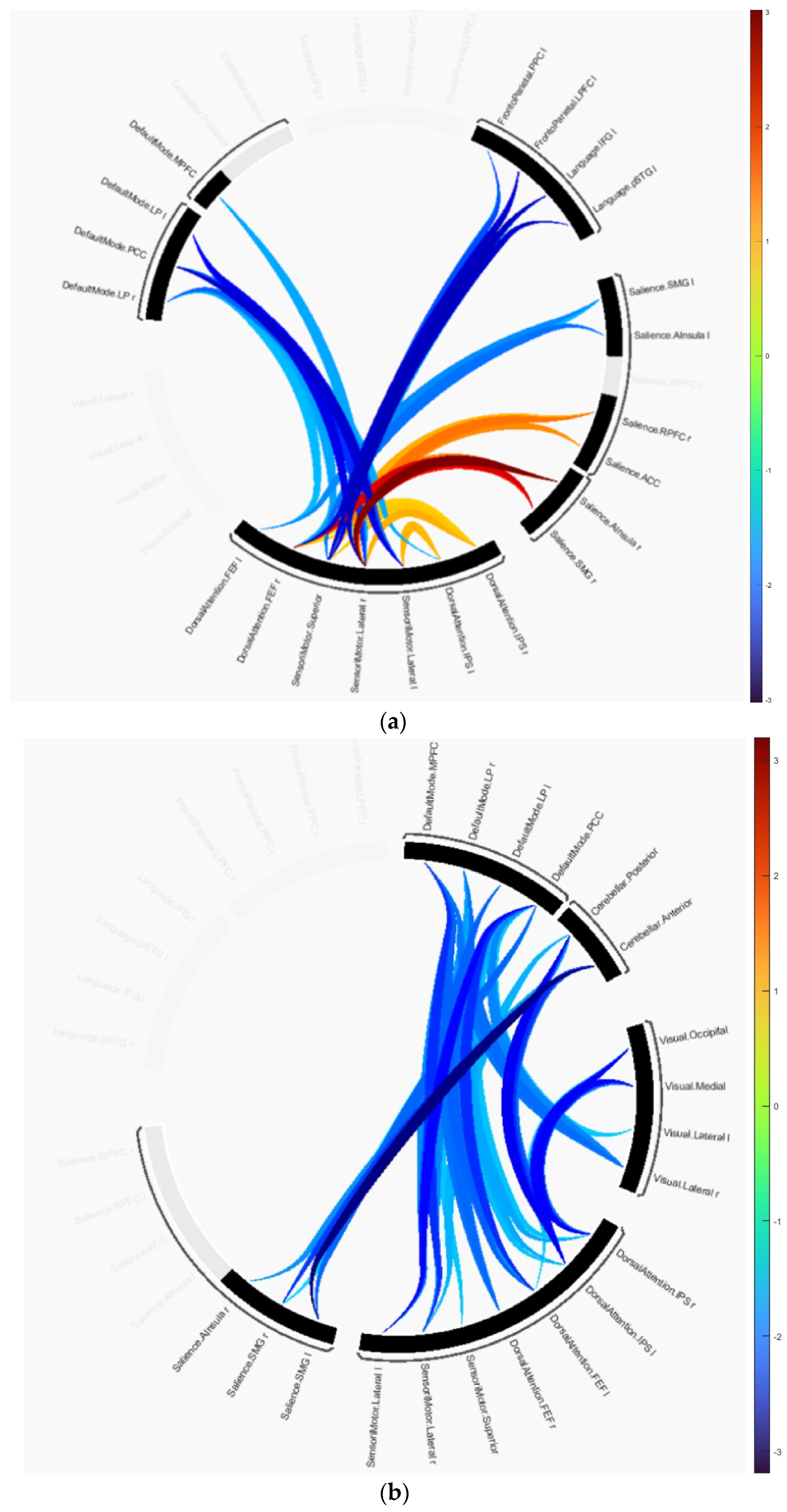
| Cluster 1 | Statistic | |
|---|---|---|
| SensoriMotor.Lateral (R) | Language.IFG (L) | p-uncorrected = 0.022849 |
| SensoriMotor.Superior | Language.pSTG (L) | Mass = 105.17 |
| DorsalAttention.FEF (R) | FrontoParietal.LPFC (L) | |
| SensoriMotor.Lateral (R) | FrontoParietal.LPFC (L) | |
| Language.IFG (L) | SensoriMotor.Superior | |
| DorsalAttention.FEF (R) | Language.pSTG (L) | |
| DorsalAttention.FEF (R) | Language.IFG (L) | |
| SensoriMotor.Superior | FrontoParietal.PPC (L) | |
| Salience.AInsula (L) | SensoriMotor.Superior | |
| FrontoParietal.LPFC (L) | SensoriMotor.Superior | |
| Salience.SMG (L) | DorsalAttention.FEF (L) | |
| SensoriMotor.Lateral (R) | Language.pSTG (L) | |
| SensoriMotor.Lateral (R) | FrontoParietal.PPC (L) | |
| Salience.SMG (L) | SensoriMotor.Superior | |
| Cluster 2 | Statistic | |
| SensoriMotor.Lateral (R) | Salience.AInsula (R) | p-uncorrected = 0.03837 |
| DorsalAttention.FEF (R) | Salience.SMG (R) | Mass = 74.73 |
| Salience.SMG (R) | SensoriMotor.Lateral (R) | |
| SensoriMotor.Lateral (R) | Salience.RPFC (R) | |
| DorsalAttention.FEF (R) | Salience.AInsula (R) | |
| Salience.SMG (R) | SensoriMotor.Superior | |
| Salience.ACC | SensoriMotor.Lateral (R) | |
| Salience.RPFC (R) | SensoriMotor.Superior | |
| SensoriMotor.Lateral (R) | DorsalAttention.IPS (R) | |
| DorsalAttention.FEF (R) | Salience.RPFC (R) | |
| DorsalAttention.IPS (L) | SensoriMotor.Lateral (L) | |
| DorsalAttention.IPS (R) | SensoriMotor.Superior | |
| Cluster 3 | Statistic | |
| SensoriMotor.Lateral (L) | DefaultMode.PCC | p-uncorrected = 0.04207 |
| DefaultMode.LP (L) | SensoriMotor.Superior | Mass = 70.06 |
| SensoriMotor.Lateral (L) | DefaultMode.LP (L) | |
| SensoriMotor.Lateral (R) | DefaultMode.LP (L) | |
| SensoriMotor.Lateral (R) | DefaultMode.PCC | |
| DefaultMode.LP (R) | SensoriMotor.Lateral (L) | |
| DefaultMode.LP (L) | DorsalAttention.FEF (R) | |
| SensoriMotor.Lateral (R) | DefaultMode.MPFC | |
| DefaultMode.LP (R) | DorsalAttention.IPS (L) | |
| DorsalAttention.FEF (R) | DefaultMode.PCC | |
| DefaultMode.LP (R) | DorsalAttention.FEF (L) |
| Cluster 1 | Statistic | |
|---|---|---|
| Cerebellar.Posterior | DorsalAttention.IPS (L) | p-uncorrected = 0.016848 |
| Visual.Occipital | DorsalAttention.IPS (R) | Mass = 160.07 |
| Visual.Occipital | DorsalAttention.IPS (L) | |
| DorsalAttention.FEF (L) | DefaultMode.PCC | |
| SensoriMotor.Lateral (R) | DefaultMode.MPFC | |
| Cerebellar.Anterior | DorsalAttention.IPS (L) | |
| Cerebellar.Posterior | DorsalAttention.IPS (R) | |
| DefaultMode.MPFC | DorsalAttention.FEF (L) | |
| DefaultMode.LP (L) | DorsalAttention.FEF (R) | |
| Cerebellar.Anterion | DorsalAttention.IPS (R) | |
| DefaultMode.LP (R) | Visual.Lateral (R) | |
| Visual.Medial | DorsalAttention.IPS (R) | |
| DorsalAttention.FEF (R) | DefaultMode.MPFC | |
| Visual.Lateral (R) | DefaultMode.MPFC | |
| DorsalAttention.FEF (R) | DefaultMode.PCC | |
| Visual.Lateral (R) | DefaultMode.PCC | |
| DefaultMode.MPFC | SensoriMotor.Lateral (L) | |
| DefaultMode.LP (R) | DorsalAttention.FEF (L) | |
| DefaultMode.LP (R) | DorsalAttention.IPS (R) | |
| DorsalAttention.IPS (L) | DefaultMode.PCC | |
| DefaultMode.LP (R) | DorsalAttention.IPS (L) | |
| DorsalAttention.IPS (R) | DefaultMode.PCC | |
| DefaultMode.MPFC | SensoriMotor.Superior | |
| Visual.Occipital | DorsalAttention.FEF (L) | |
| Visual.Lateral (L) | DefaultMode.PCC | |
| Cluster 2 | Statistic | |
| SensoriMotor.Lateral (L) | DefaultMode.PCC | p-uncorrected = 0.047229 |
| Cerebellar.Anterior | Salience.SMG (R) | Mass = 77.93 |
| SensoriMotor.Lateral (L) | DefaultMode.LP (L) | |
| Salience.SMG (L) | DefaultMode.PCC | |
| SensoriMotor.Lateral (R) | DefaultMode.PCC | |
| Cerebellar.Anterior | Salience.AInsula (R) | |
| SensoriMotor.Lateral (R) | DefaultMode.LP (L) | |
| Cerebellar.Posterior | Salience.AInsula (R) | |
| Cerebellar.Posterior | SensoriMotor.Lateral (R) | |
| Cerebellar.Posterior | Salience.SMG (L) | |
| Salience.SMG (R) | DefaultMode.PCC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lama, R.K.; Kwon, G.-R. Resting-State Functional Connectivity Difference in Alzheimer’s Disease and Mild Cognitive Impairment Using Threshold-Free Cluster Enhancement. Diagnostics 2023, 13, 3074. https://doi.org/10.3390/diagnostics13193074
Lama RK, Kwon G-R. Resting-State Functional Connectivity Difference in Alzheimer’s Disease and Mild Cognitive Impairment Using Threshold-Free Cluster Enhancement. Diagnostics. 2023; 13(19):3074. https://doi.org/10.3390/diagnostics13193074
Chicago/Turabian StyleLama, Ramesh Kumar, and Goo-Rak Kwon. 2023. "Resting-State Functional Connectivity Difference in Alzheimer’s Disease and Mild Cognitive Impairment Using Threshold-Free Cluster Enhancement" Diagnostics 13, no. 19: 3074. https://doi.org/10.3390/diagnostics13193074
APA StyleLama, R. K., & Kwon, G.-R. (2023). Resting-State Functional Connectivity Difference in Alzheimer’s Disease and Mild Cognitive Impairment Using Threshold-Free Cluster Enhancement. Diagnostics, 13(19), 3074. https://doi.org/10.3390/diagnostics13193074








