Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections
Abstract
:1. Introduction
2. Materials and Methods
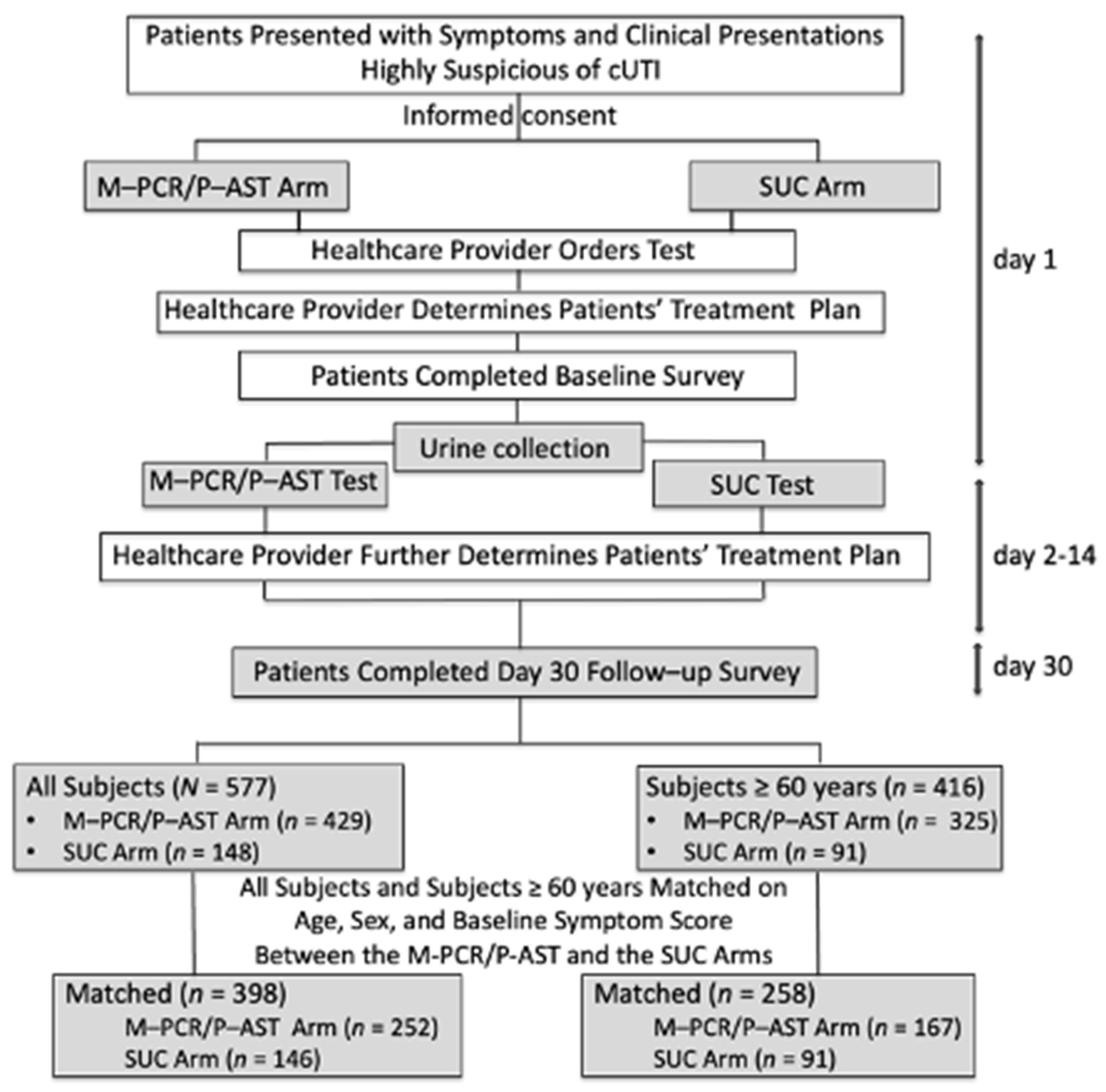
2.1. Study Design and Participants
2.2. Clinical Outcomes and Treatment Status
2.3. M-PCR/P-AST Test (Guidance® UTI, Offered by Pathnostics, Irvine, CA, USA)
2.4. Standard Urine Culture (SUC)
2.5. Subject Matching
2.6. Statistical Analysis
3. Results
3.1. Subject Demographics (Table 1)
| All Subjects (n = 398) | Subjects ≥60 Years of Age (n = 258) | |||||
|---|---|---|---|---|---|---|
| SUC (n = 146) | M-PCR/P-AST (n = 252) | p-Value | SUC (n = 91) | M-PCR/P-AST (n = 167) | p-Value | |
| Age | 0.39 | 0.92 | ||||
| Mean (SD) | 63.0 (14.8) | 64.3 (13.7) | 72.3 (7.6) | 72.2 (6.9) | ||
| Median (Min, Max) | 65.5 (20.6, 96.0) | 67.0 (22.2, 95.5) | 72.2 (60.3, 96.0) | 72.1 (60.5, 95.5) | ||
| Sex (n (%)) | 0.97 | 0.90 | ||||
| Male | 42 (28.8%) | 73 (29.0%) | 29 (31.9%) | 52 (31.1%) | ||
| Female | 104 (71.2%) | 179 (71.0%) | 62 (68.1%) | 115 (68.9%) | ||
| Mean Baseline Symptom Score (SD) | 5.0 (2.6) | 4.8 (2.4) | 0.45 | 5.4 (2.6) | 5.0 (2.4) | 0.18 |
3.2. Turnaround Time Comparisons between the SUC and the M-PCR/P-AST Arms
3.3. Clinical Outcome Comparisons between the SUC and the M-PCR/P-AST Arms
3.4. Outcomes for All Matched Subjects
3.5. Outcomes for Matched Subjects ≥60 Years of Age
3.6. Comparison of Percentages of Patients Who Received Empirical or Directed Antimicrobial Treatments in the SUC and M-PCR/P-AST Arms
3.7. Comparison of Percentages of Patients 60 Years of Age and Older Who Received Empirical or Directed Antimicrobial Treatments in the SUC and M-PCR/P-AST Arms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Descriptive Epidemiology and Outcomes of Hospitalizations with Complicated Urinary Tract Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab591. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Nash, S.; Zoorob, R.; Germanos, G.J.; Horsfield, M.S.; Khan, F.M.; Martin, L.; Trautner, B.W. Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics 2019, 8, 84. [Google Scholar] [CrossRef]
- Waller, T.A.; Pantin, S.A.L.; Yenior, A.L.; Pujalte, G. Urinary Tract Infection Antibiotic Resistance in the United States. Prim. Care Clin. Office Pract. 2018, 45, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Siff, L.N. Recurrent Urinary Tract Infections. Deckermed. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Lichtenstern, C.; Rolfes, C.; Mayer, K.; Uhle, F.; Weidner, W.; Weigand, M.A. Diagnosis and Management for Urosepsis. Int. J. Urol. 2013, 20, 963–970. [Google Scholar] [CrossRef]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections; StatPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Balasubramanian, S.; Wang, X.; Sahil, S.; Cheng, A.; Sutkin, G.; Shepherd, J.P. Risk Factors for the Development of Acute Pyelonephritis in Women with a Positive Urine Culture. Neurourol. Urodyn. 2022, 41, 1582–1589. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2019, 136, 119–126. [Google Scholar] [CrossRef]
- Vollstedt, A.; Baunoch, D.; Wojno, K.; Luke, N.; Cline, K.; Belkoff, L.; Sirls, L. Multisite Prospective Comparison of Multiplex Polymerase Chain Reaction Testing with Urine Culture for Diagnosis of Urinary Tract Infections in Symptomatic Patients. J. Surg. Urol. 2020, 2020, JSU-102. [Google Scholar]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial Culture through Selective and Non-Selective Conditions: The Evolution of Culture Media in Clinical Microbiology. New Microbes New Infect. 2020, 34, 100622. [Google Scholar] [CrossRef]
- Kass, E.H. Bacteriuria and Pyelonephritis of Pregnancy. AMA Arch. Intern Med. 1960, 105, 194–198. [Google Scholar] [CrossRef]
- Price, T.K.; Hilt, E.E.; Dune, T.J.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Urine Trouble: Should We Think Differently about UTI? Int. Urogynecol. J. 2018, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.I.; Naboka, Y.L.; Ibishev, K.S.; Gudima, I.A.; Naber, K.G. Human Urine Is Not Sterile - Shift of Paradigm. Urol. Int. 2015, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W. On the Occurrence of Micro-Organisms in Fresh Urine. Br. Méd. J. 1881, 2, 623. [Google Scholar] [CrossRef]
- Anger, J.; Lee, U.; Ackerman, A.L.; Chou, R.; Chughtai, B.; Clemens, J.Q.; Hickling, D.; Kapoor, A.; Kenton, K.S.; Kaufman, M.R.; et al. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J. Urol. 2019, 202, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Moreland, R.B.; Choi, B.I.; Geaman, W.; Gonzalez, C.; Hochstedler-Kramer, B.R.; John, J.; Wolfe, A.J. Beyond the Usual Suspects: 2 Emerging Uropathogens in the Microbiome Age. Front. Urol. 2023, 3, 1212590. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Brubaker, L. “Sterile Urine” and the Presence of Bacteria. Eur. Urol. 2015, 68, 173–174. [Google Scholar] [CrossRef]
- Thomas-White, K.; Brady, M.; Wolfe, A.J.; Mueller, E.R. The Bladder Is Not Sterile: History and Current Discoveries on the Urinary Microbiome. Curr. Bladder Dysfunct. Rep. 2016, 11, 18–24. [Google Scholar] [CrossRef]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Kuiken, M.V.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of Female Bladder Bacteria Reveals an Interconnected Urogenital Microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef]
- Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Female Urinary Microbiota. Curr. Opin. Urol. 2017, 27, 282–286. [Google Scholar] [CrossRef]
- Thomas-White, K.J.; Kliethermes, S.; Rickey, L.; Lukacz, E.S.; Richter, H.E.; Moalli, P.; Zimmern, P.; Norton, P.; Kusek, J.W.; Wolfe, A.J.; et al. Evaluation of the Urinary Microbiota of Women with Uncomplicated Stress Urinary Incontinence. Am. J. Obstet. Gynecol. 2017, 216, 55.e1–55.e16. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Gourdine, J.-P.F.; Siddiqui, N.Y.; Holland, A.; Halverson, T.; Limeria, R.; Pride, D.; Ackerman, L.; Forster, C.S.; Jacobs, K.M.; et al. Forming Consensus To Advance Urobiome Research. mSystems 2021, 6, e0137120. [Google Scholar] [CrossRef] [PubMed]
- Bajic, P.; Kuiken, M.E.V.; Burge, B.K.; Kirshenbaum, E.J.; Joyce, C.J.; Wolfe, A.J.; Branch, J.D.; Bresler, L.; Farooq, A.V. Male Bladder Microbiome Relates to Lower Urinary Tract Symptoms. Eur. Urol. Focus 2020, 6, 376–382. [Google Scholar] [CrossRef]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A.J. The New World of the Urinary Microbiota in Women. Am. J. Obstet. Gynecol. 2015, 213, 644–649. [Google Scholar] [CrossRef]
- Brubaker, L.; Wolfe, A. The Urinary Microbiota. Curr. Opin. Obstet. Gynecol. 2016, 28, 407–412. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Brubaker, L. Urobiome Updates: Advances in Urinary Microbiome Research. Nat. Rev. Urol. 2019, 16, 73–74. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Harding, C.; Rantell, A.; Cardozo, L.; Jacobson, S.K.; Anding, R.; Kirschner-Hermanns, R.; Greenwell, T.; Swamy, S.; Malde, S.; Abrams, P. How Can We Improve Investigation, Prevention and Treatment for Recurrent Urinary Tract Infections—ICI-RS 2018. Neurourol. Urodyn. 2019, 38, S90–S97. [Google Scholar] [CrossRef]
- Festa, R.A.; Luke, N.; Mathur, M.; Parnell, L.; Wang, D.; Zhao, X.; Magallon, J.; Remedios-Chan, M.; Nguyen, J.; Cho, T.; et al. A Test Combining Multiplex-PCR with Pooled Antibiotic Susceptibility Testing Has High Correlation with Expanded Urine Culture for Detection of Live Bacteria in Urine Samples of Suspected UTI Patients. Diagn. Microbiol. Infect. Dis. 2023, 107, 116015. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Baunoch, D.; Rehling, K.; Luke, N.; Campbell, M.; Cacdac, P.; Penaranda, M.; Opel, M.; Huang, S.; Zhao, X. Utilization of M-PCR and P-AST for Diagnosis and Management of Urinary Tract Infections in Home-Based Primary Care. JOJ Urol. Nephrol. 2020, 7, 555707. [Google Scholar]
- Barnes, H.C.; Wolff, B.; Abdul-Rahim, O.; Harrington, A.; Hilt, E.E.; Price, T.K.; Halverson, T.; Hochstedler, B.R.; Pham, T.; Acevedo-Alvarez, M.; et al. A Randomized Clinical Trial of Standard versus Expanded Cultures to Diagnose Urinary Tract Infections in Women. J. Urol. 2021, 206, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Korman, H.J.; Baunoch, D.; Luke, N.; Wang, D.; Zhao, X.; Levin, M.; Wenzler, D.L.; Mathur, M. A Diagnostic Test Combining Molecular Testing with Phenotypic Pooled Antibiotic Susceptibility Improved the Clinical Outcomes of Patients with Non-E. Coli or Polymicrobial Complicated Urinary Tract Infections. Res. Rep. Urol. 2023, 15, 141–147. [Google Scholar] [CrossRef]
- Brecher, S.M. Commentary: Complicated Urinary Tract Infections: What’s a Lab to Do? J. Clin. Microbiol. 2016, 54, 1189–1190. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Sathiananthamoorthy, S.; Malone-Lee, J.; Gill, K.; Tymon, A.; Nguyen, T.K.; Gurung, S.; Collins, L.; Kupelian, A.S.; Swamy, S.; Khasriya, R.; et al. Reassessment of Routine Midstream Culture in Diagnosis of Urinary Tract Infection. J. Clin. Microbiol. 2018, 57, e01452-18. [Google Scholar] [CrossRef]
- Baunoch, D.; Luke, N.; Wang, D.; Vollstedt, A.; Zhao, X.; Ko, D.S.C.; Huang, S.; Cacdac, P.; Sirls, L.T. Concordance between Antibiotic Resistance Genes and Susceptibility in Symptomatic Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 3275–3286. [Google Scholar] [CrossRef]
- Vollstedt, A.; Baunoch, D.; Wolfe, A.; Luke, N.; Wojno, K.J.; Cline, K.; Belkoff, L.; Milbank, A.; Sherman, N.; Haverkorn, R.; et al. Bacterial Interactions as Detected by Pooled Antibiotic Susceptibility Testing (P-AST) in Polymicrobial Urine Specimens. J. Surg. Urol. 2020, 1, 101. [Google Scholar]
- Food and Drug Administration (FDA). Uncomplicated Urinary Tract Infections: Developing Drugs for Treatment Guidance for Industry; FDA: Rockville, MD, USA, 2019.
- Alidjanov, J.F.; Overesch, A.; Abramov-Sommariva, D.; Hoeller, M.; Steindl, H.; Wagenlehner, F.M.; Naber, K.G. Acute Cystitis Symptom Score Questionnaire for Measuring Patient-Reported Outcomes in Women with Acute Uncomplicated Cystitis: Clinical Validation as Part of a Phase III Trial Comparing Antibiotic and Nonantibiotic Therapy. Investig. Clin. Urol. 2020, 61, 498–507. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Abdufattaev, U.A.; Makhsudov, S.A.; Pilatz, A.; Akilov, F.A.; Naber, K.G.; Wagenlehner, F.M.E. The Acute Cystitis Symptom Score for Patient-Reported Outcome Assessment. Urol. Int. 2016, 97, 402–409. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Naber, K.G.; Pilatz, A.; Wagenlehner, F.M. Validation of the American English Acute Cystitis Symptom Score. Antibiotics 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Chai, T.C.; Horsley, H.; Khasriya, R.; Moreland, R.B.; Wolfe, A. Tarnished Gold—The “Standard” Urine Culture: Reassessing the Characteristics of a Criterion Standard for Detecting Urinary Microbes. Front. Urol. 2023, 3, 1206046. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Johansen, T.E.B.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, Definition and Treatment of Complicated Urinary Tract Infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Xu, F.; Wang, Z.; Ren, Y.; Han, D.; Lyu, J.; Yin, H. Construction and Evaluation of a Sepsis Risk Prediction Model for Urinary Tract Infection. Front. Med. 2021, 8, 671184. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef]
- Heytens, S.; Sutter, D.A.; Coorevits, L.; Cools, P.; Boelens, J.; Simaey, V.L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with Symptoms of a Urinary Tract Infection but a Negative Urine Culture: PCR-Based Quantification of Escherichia Coli Suggests Infection in Most Cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef]

| SUC | M-PCR/P-AST | p-Value | |
|---|---|---|---|
| Mean Days (SD) | |||
| Full sample matched (n = 248 M-PCR/P-AST; n = 131 SUC) | 2.87 (1.14) | 1.45 (0.55) | <0.0001 |
| Sample of age 60+ matched (n = 164 M-PCR/P-AST; n = 81 SUC) | 2.94 (1.13) | 1.48 (0.56) | <0.0001 |
| SUC | M-PCR/P-AST | p-Value | |
|---|---|---|---|
| Mean Days (SD) | |||
| Full sample matched (n = 197 M-PCR/P-AST; n = 53 SUC) | 3.56 (1.14) | 1.52 (0.55) | <0.0001 |
| Sample of age 60+ matched (n = 134 M-PCR/P-AST; n = 42 SUC) | 3.54 (1.08) | 1.55 (0.56) | <0.0001 |
| Overall | |||
|---|---|---|---|
| SUC | M-PCR/P-AST | p-Value (SUC vs. M-PCR/P-AST) | |
| n/Total (%) | n/Total (%) | ||
| Recurrence of UTI symptoms | 31/146 (21.2%) | 42/252 (16.7%) | 0.26 |
| Medical provider visit (for UTI) | 49/146 (33.6%) | 55/252 (21.8%) | 0.010 |
| Hospital, ER, or UC visit (for UTI) | 26/146 (17.8%) | 27/252 (10.75%) | 0.045 |
| Total n = 398 | SUC n = 146 n (%) | M-PCR/P-AST n = 252 n (%) | p-Value |
|---|---|---|---|
| Any UTI-related hospital visits | 13 (8.9%) | 11 (4.4%) | 0.07 |
| Any UTI-related ER visits | 19 (13.0%) | 18 (7.1%) | 0.052 |
| Any UTI-related UC visits | 19 (13.0%) | 14 (5.6%) | 0.009 |
| UTI-related hospital visits (number of occurrences) | 0.08 | ||
| 0 | 133 (91.1%) | 241 (95.6%) | |
| 1 | 7 (4.8%) | 7 (2.8%) | |
| 2 | 3 (2.1%) | 0 (0%) | |
| 3+ | 3 (2.1%) | 4 (1.6%) | |
| UTI-related ER visits (number of occurrences) | 0.16 | ||
| 0 | 127 (87.0%) | 234 (92.9%) | |
| 1 | 10 (6.9%) | 11 (4.4%) | |
| 2 | 3 (2.1%) | 1 (0.4%) | |
| 3+ | 6 (4.1%) | 6 (2.4%) | |
| UTI-related UC visits (number of occurrences) | 0.03 | ||
| 0 | 127 (87.0%) | 238 (94.4%) | |
| 1 | 9 (6.2%) | 4 (1.6%) | |
| 2 | 2 (1.4%) | 4 (1.6%) | |
| 3+ | 8 (5.5%) | 6 (2.4%) |
| ≥60 Years | |||
|---|---|---|---|
| SUC | M-PCR/P-AST | p-Value (SUC vs. M-PCR/P-AST) | |
| n/Total (%) | n/Total (%) | ||
| Recurrence of UTI symptoms | 22/91 (24.2%) | 23/167 (13.8%) | 0.035 |
| Medical provider visit (for UTI) | 29/91 (31.9%) | 34/167 (20.4%) | 0.040 |
| Hospital, ER, or UC visit (for UTI) | 20/91 (22.0%) | 14/167 (8.4%) | 0.002 |
| Total n = 258 | SUC n = 91 n (%) | M-PCR/P-AST n = 167 n (%) | p-Value |
|---|---|---|---|
| Any UTI-related hospital visits | 9 (9.9%) | 6 (3.6%) | 0.039 |
| Any UTI-related ER visits | 14 (15.4%) | 12 (7.2%) | 0.037 |
| Any UTI-related UC visits | 14 (15.4%) | 6 (3.6%) | 0.0007 |
| UTI-related hospital visits (number of occurrences) | 0.041 | ||
| 0 | 82 (90.1%) | 161 (96.4%) | |
| 1 | 5 (5.5%) | 2 (1.2%) | |
| 2 | 2 (2.2%) | 0 (0%) | |
| 3+ | 2 (2.2%) | 4 (2.4%) | |
| UTI-related ER visits (number of occurrences) | 0.084 | ||
| 0 | 77 (84.6%) | 155 (92.8%) | |
| 1 | 8 (8.8%) | 7 (4.2%) | |
| 2 | 2 (2.2%) | 0 (0%) | |
| 3+ | 4 (4.4%) | 5 (3.0%) | |
| UTI-related UC visits (number of occurrences) | 0.001 | ||
| 0 | 77 (84.6%) | 161 (96.4%) | |
| 1 | 7 (7.7%) | 1 (0.6%) | |
| 2 | 2 (2.2%) | 3 (1.7%) | |
| 3+ | 5 (5.5%) | 2 (1.2%) |
| Total (n = 398) | SUC Arm (n = 146) | M-PCR/P-AST Arm (n = 252) | p-Value |
|---|---|---|---|
| Not treated with antimicrobial agents (n, %) | 47, 32.2% | 74, 29.4% | 0.55 |
| Treated with antimicrobial agents (n, %) | 99, 67.8% | 178, 70.6% | |
| Of those treated with antimicrobial agents (n = 277) | SUC Arm (n = 99) | M-PCR/P-AST Arm (n = 178) | |
| Empirical treatment (n, %) | 66, 66.7% | 51, 28.7% | <0.0001 |
| Directed treatment (n, %) | 33, 33.3% | 127, 71.4% |
| Total (n = 258) | SUC Arm (n = 91) | M-PCR/P-AST Arm (n = 167) | p-Value |
|---|---|---|---|
| Not treated with antimicrobial agents (n, %) | 22, 24.2% | 45, 27.0% | 0.63 |
| Treated with antimicrobial agents (n, %) | 69, 75.8% | 122, 73.1% | |
| Of those treated with antimicrobial agents (n = 191) | SUC Arm (n = 69) | M-PCR/P-AST Arm (n = 122) | |
| Empirical treatment (n, %) | 48, 69.6% | 37, 30.3% | <0.0001 |
| Directed treatment (n, %) | 21, 30.4% | 85, 69.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haley, E.; Luke, N.; Korman, H.; Baunoch, D.; Wang, D.; Zhao, X.; Mathur, M. Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections. Diagnostics 2023, 13, 3060. https://doi.org/10.3390/diagnostics13193060
Haley E, Luke N, Korman H, Baunoch D, Wang D, Zhao X, Mathur M. Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections. Diagnostics. 2023; 13(19):3060. https://doi.org/10.3390/diagnostics13193060
Chicago/Turabian StyleHaley, Emery, Natalie Luke, Howard Korman, David Baunoch, Dakun Wang, Xinhua Zhao, and Mohit Mathur. 2023. "Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections" Diagnostics 13, no. 19: 3060. https://doi.org/10.3390/diagnostics13193060
APA StyleHaley, E., Luke, N., Korman, H., Baunoch, D., Wang, D., Zhao, X., & Mathur, M. (2023). Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections. Diagnostics, 13(19), 3060. https://doi.org/10.3390/diagnostics13193060








