The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Considering the Studies for This Review
2.2. Search Methods for Identification of Studies
2.3. Study Selection
2.4. Data Extraction
2.5. Evidence Synthesis and Meta-Analysis
2.6. Publication Bias and Sensitivity Analysis
3. Results
3.1. Study Selection Process
3.2. Study Characteristics
3.2.1. Characteristics of RT-PCR Techniques
3.2.2. The Diagnostic Parameters of RT-PCR in Various Samples
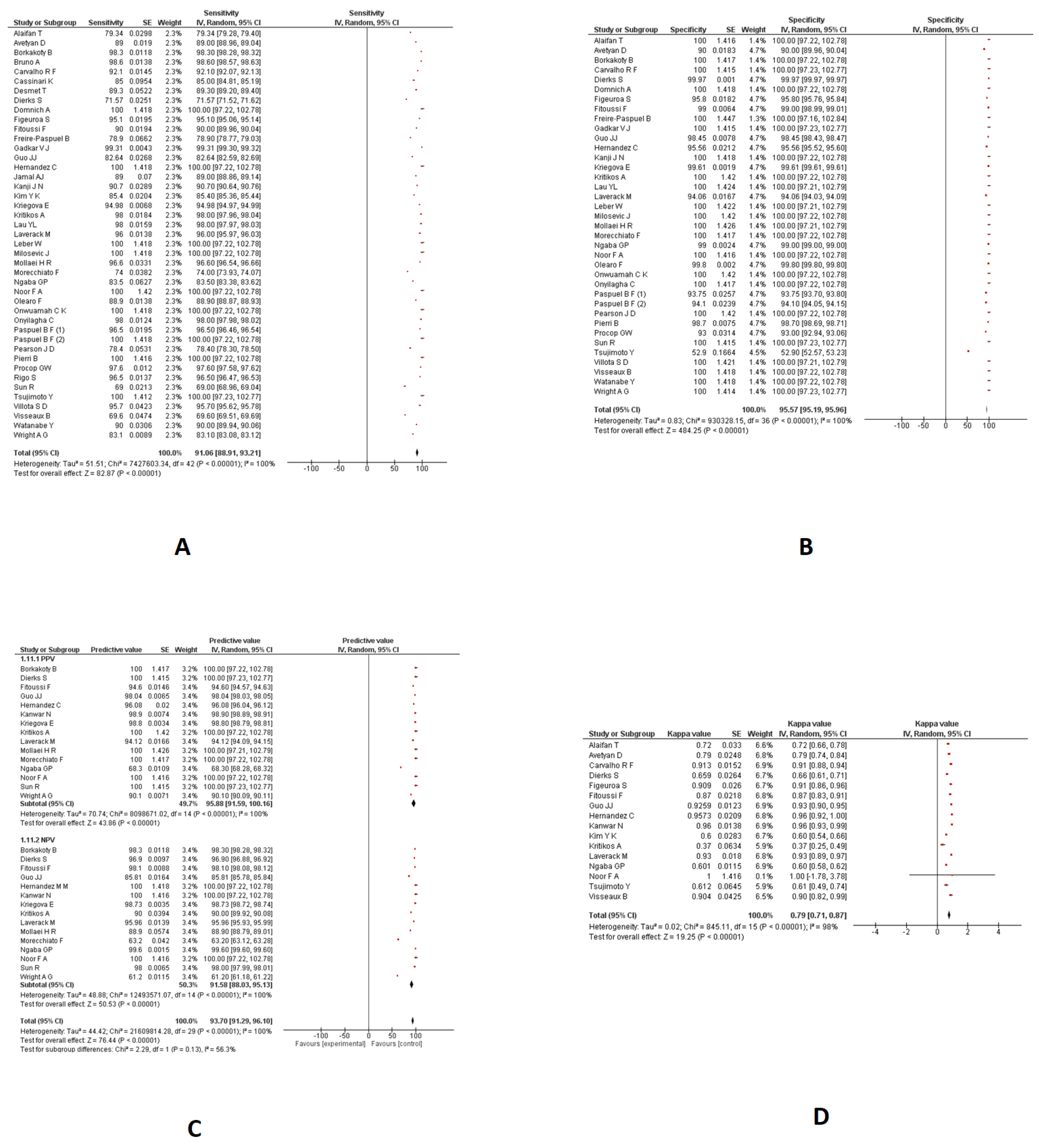
Nasopharyngeal Swabs
- Sensitivity and specificity
- PPV and NPV
- Kappa coefficient
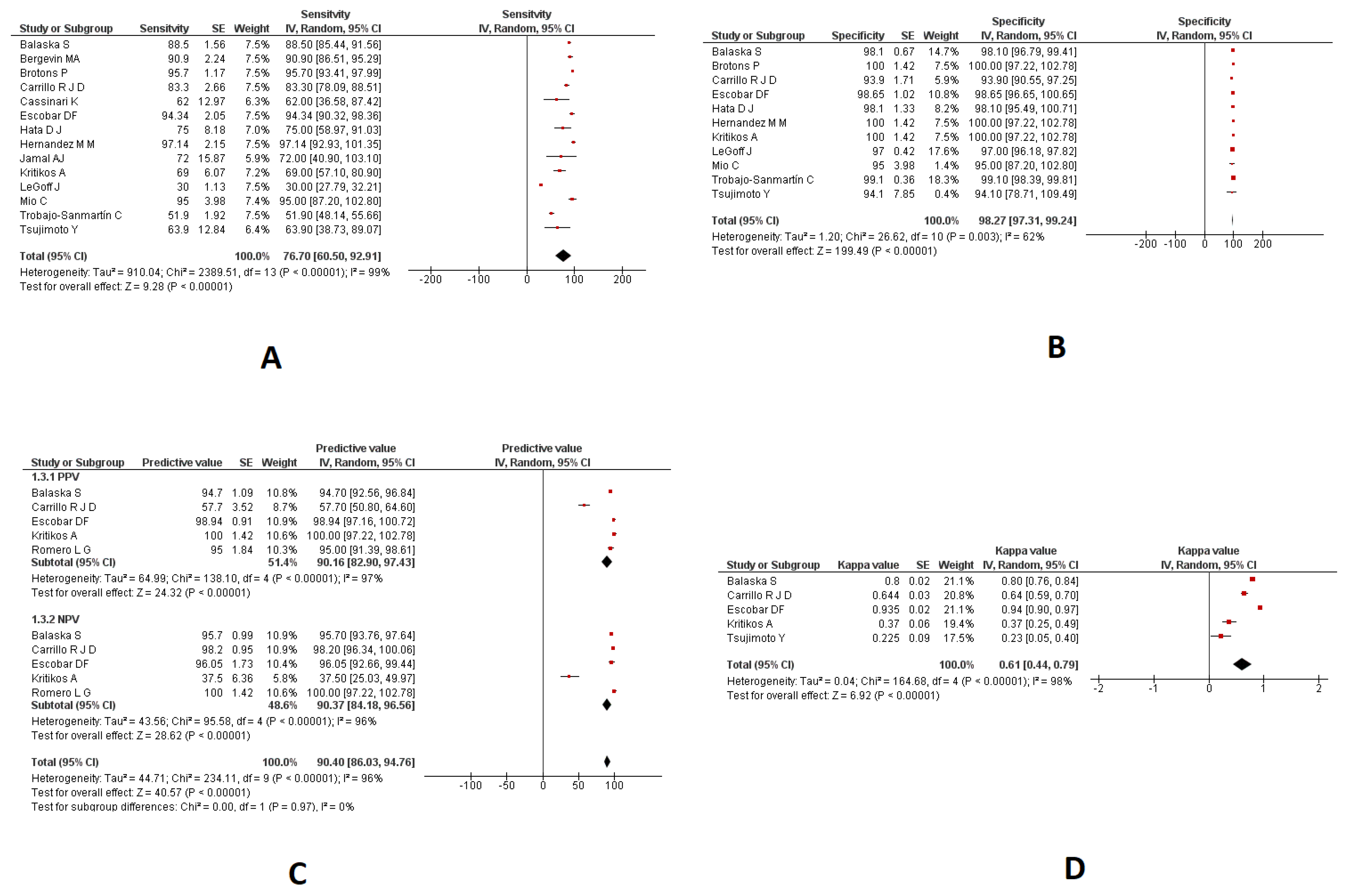
3.3. Saliva Samples
3.3.1. Sensitivity and Specificity
3.3.2. PPV and NPV
3.3.3. Kappa Coefficient
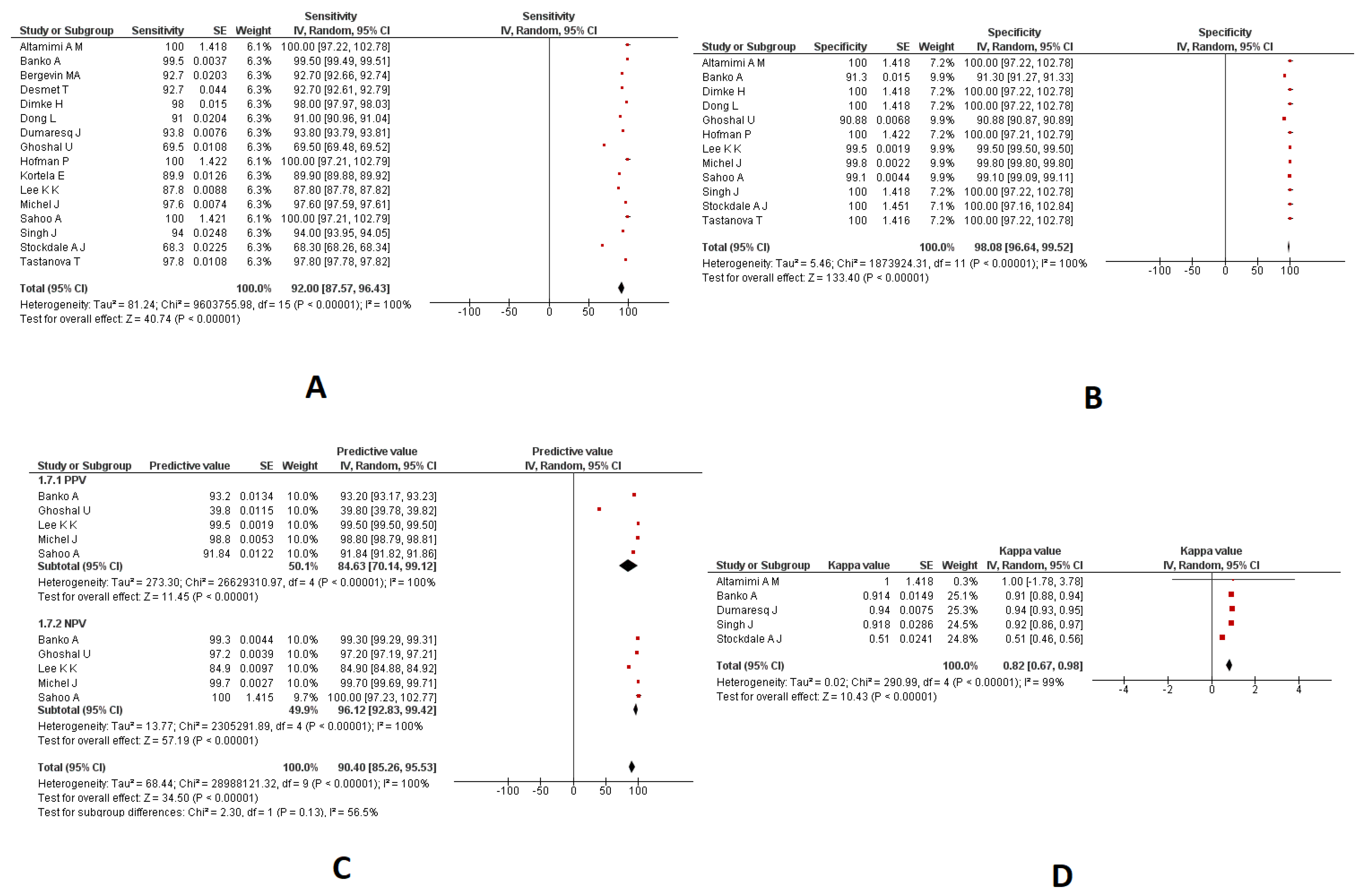
3.4. Combined Nasopharyngeal/Oropharyngeal Samples
3.4.1. Sensitivity and Specificity
3.4.2. PPV and NPV
3.4.3. Kappa Coefficient
3.5. Respiratory Samples
3.6. Sputum Samples
3.7. Broncho Aspirate Samples
3.8. Throat Swab Samples
3.9. Gargle Samples
3.10. Serum Samples
3.11. Mixed Samples
3.12. Publication Bias
3.13. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Gionfriddo, M.R.; Cortes-Penfield, N.; Thunga, G.; Rashid, M. The trade-off dilemma in pharmacotherapy of COVID-19: Systematic review, meta-analysis, and implications. Expert Opin. Pharmacother. 2020, 21, 1821–1849. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Foundation of Innovative New Diagnostics Sars-Cov-2 Molecular Assay Evaluation: Results. 18 March 2022. Available online: https://www.finddx.org/covid-19/sarscov2-eval-molecular/molecular-eval-results/ (accessed on 2 February 2023).
- Lippi, G.; Simundic, A.M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhong, Z.; Zhao, W.; Zheng, C.; Wang, F.; Liu, J. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology 2020, 296, E41–E45. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef]
- Xiao, A.T.; Tong, Y.X.; Zhang, S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J. Med. Virol. 2020, 92, 1755–1756. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Geng, B.; Muenker, M.C.; Moore, A.J.; Vogels, C.B.; et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. MedRxiv 2020, 22, 2020-04. [Google Scholar] [CrossRef]
- Beyene, G.T.; Alemu, F.; Kebede, E.S.; Alemayehu, D.H.; Seyoum, T.; Tefera, D.A.; Assefa, G.; Tesfaye, A.; Habte, A.; Bedada, G.; et al. Saliva is superior over nasopharyngeal swab for detecting SARS-CoV2 in COVID-19 patients. Sci. Rep. 2021, 11, 22640. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Wang, X.; Liu, W.; Lu, Y.; Cheng, L.; Sun, Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infect. Dis. 2020, 94, 107–109. [Google Scholar] [CrossRef]
- Manzoor, S. Comparison of oropharyngeal and nasopharyngeal swabs for detection of SARS-COV-2 in patients with COVID-19. Chest 2020, 158, A2473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2020, 372, n71. [Google Scholar] [CrossRef]
- RevMan. Review Manager (RevMan). [Computer Program], 5.4 ed.; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2020. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Sterne, J.; Egger, M.; Moher, D.J. Chapter 10: Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Escobar, D.F.; Díaz, P.; Díaz-Dinamarca, D.; Puentes, R.; Alarcón, P.; Alarcón, B.; Rodríguez, I.; Manzo, R.A.; Soto, D.A.; Lamperti, L.; et al. Validation of a Methodology for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in Saliva by Real-Time Reverse Transcriptase-PCR. Front. Public Health 2021, 9, 743300. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yadav, A.K.; Pakhare, A.; Kulkarni, P.; Lokhande, L.; Soni, P.; Dadheech, M.; Gupta, P.; Masarkar, N.; Maurya, A.K. Comparative analysis of the diagnostic performance of five commercial COVID-19 qRT PCR kits used in India. Sci. Rep. 2021, 11, 22013. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.; Freire-Paspuel, B.; Vega-Mariño, P.; Velez, A.; Cruz, M.; Cardenas, W.B.; Garcia-Bereguiain, M.A. High sensitivity-low cost detection of SARS-CoV-2 by two steps end point RT-PCR with agarose gel electrophoresis visualization. Sci. Rep. 2021, 11, 21658. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, J.; Kernéis, S.; Elie, C.; Mercier-Delarue, S.; Gastli, N.; Choupeaux, L.; Fourgeaud, J.; Alby, M.L.; Quentin, P.; Pavie, J.; et al. Evaluation of a saliva molecular point of care for the detection of SARS-CoV-2 in ambulatory care. Sci. Rep. 2021, 11, 21126. [Google Scholar] [CrossRef]
- Villota, S.D.; Nipaz, V.E.; Carrazco-Montalvo, A.; Hernandez, S.; Waggoner, J.J.; Ponce, P.; Coloma, J.; Orlando, A.; Cevallos, V. Alternative RNA extraction-free techniques for the real-time RT-PCR detection of SARS-CoV-2 in nasopharyngeal swab and sputum samples. J. Virol. Methods 2021, 298, 114302. [Google Scholar] [CrossRef]
- De Pace, V.; Caligiuri, P.; Ricucci, V.; Nigro, N.; Galano, B.; Visconti, V.; Da Rin, G.; Bruzzone, B. Rapid diagnosis of SARS-CoV-2 pneumonia on lower respiratory tract specimens. BMC Infect. Dis. 2021, 21, 926. [Google Scholar] [CrossRef]
- Kanwar, N.; Banerjee, D.; Sasidharan, A.; Abdulhamid, A.; Larson, M.; Lee, B.; Selvarangan, R.; Liesman, R.M. Comparison of diagnostic performance of five molecular assays for detection of SARS-CoV-2. Diagn. Microbiol. Infect. Dis. 2021, 101, 115518. [Google Scholar] [CrossRef]
- Michel, J.; Neumann, M.; Krause, E.; Rinner, T.; Muzeniek, T.; Grossegesse, M.; Hille, G.; Schwarz, F.; Puyskens, A.; Förster, S.; et al. Resource-efficient internally controlled in-house real-time PCR detection of SARS-CoV-2. Virol. J. 2021, 18, 110. [Google Scholar] [CrossRef]
- Wu, S.; Shi, X.; Chen, Q.; Jiang, Y.; Zuo, L.; Wang, L.; Jiang, M.; Lin, Y.; Fang, S.; Peng, B.; et al. Comparative evaluation of six nucleic acid amplification kits for SARS-CoV-2 RNA detection. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Doudesis, D.; Ross, D.A.; Bularga, A.; MacKintosh, C.L.; Koch, O.; Johannessen, I.; Templeton, K.; Jenks, S.; Chapman, A.R.; et al. Diagnostic performance of the combined nasal and throat swab in patients admitted to hospital with suspected COVID-19. BMC Infect. Dis. 2021, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Borkakoty, B.; Bali, N.K. TSP-based PCR for rapid identification of L and S type strains of SARS-CoV-2. Indian J. Med. Microbiol. 2021, 39, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hata, D.J.; White, E.L., Jr.; Bridgeman, M.M.; Gasson, S.L.; Jones, D.S.; Vicari, B.R.; Van Siclen, C.P.; Palmucci, C.; Marquez, C.P.; Parkulo, M.A.; et al. Performance Accuracy of a Laboratory-Developed Real-Time RT-PCR Method for Detection of SARS-CoV-2 in Self-Collected Saliva Specimens. Ann. Clin. Lab. Sci. 2021, 51, 741–749. [Google Scholar]
- Wang, B.; Hu, M.; Ren, Y.; Xu, X.; Wang, Z.; Lyu, X.; Wu, W.; Li, Z.; Gong, X.; Xiang, Z.; et al. Evaluation of seven commercial SARS-CoV-2 RNA detection kits based on real-time polymerase chain reaction (PCR) in China. Clin. Chem. Lab. Med. 2020, 58, e149–e153. [Google Scholar] [CrossRef]
- Mollaei, H.R.; Afshar, A.A.; Kalantar-Neyestanaki, D.; Fazlalipour, M.; Aflatoonian, B. Comparison five primer sets from different genome region of COVID-19 for detection of virus infection by conventional RT-PCR. Iran. J. Microbiol. 2020, 12, 185–193. [Google Scholar] [CrossRef]
- Pierri, B.; Mancusi, A.; Proroga, Y.T.R.; Capuano, F.; Cerino, P.; Girardi, S.; Vassallo, L.; Lo Conte, G.; Tafuro, M.; Cuomo, M.C.; et al. SARS-CoV-2 detection in nasopharyngeal swabs: Performance characteristics of a real-time RT-qPCR and a droplet digital RT-PCR assay based on the exonuclease region (ORF1b, nsp 14). J. Virol. Methods 2020, 300, 114420. [Google Scholar] [CrossRef]
- Torres, A.; Fors, M.; Rivero, T.; Pantoja, K.; Ballaz, S. Comparison between RT-qPCR for SARS-CoV-2 and expanded triage in sputum of symptomatic and asymptomatic COVID-19 subjects in Ecuador. BMC Infect. Dis. 2021, 21, 558. [Google Scholar] [CrossRef]
- Pearson, J.D.; Trcka, D.; Lu, S.; Hyduk, S.J.; Jen, M.; Aynaud, M.M.; Hernández, J.J.; Peidis, P.; Barrios-Rodiles, M.; Chan, K.; et al. Comparison of SARS-CoV-2 indirect and direct RT-qPCR detection methods. Virol J. 2021, 18, 99. [Google Scholar] [CrossRef]
- Kriegova, E.; Fillerova, R.; Raska, M.; Manakova, J.; Dihel, M.; Janca, O.; Sauer, P.; Klimkova, M.; Strakova, P.; Kvapil, P. Excellent option for mass testing during the SARS-CoV-2 pandemic: Painless self-collection and direct RT-qPCR. Virol. J. 2021, 18, 95. [Google Scholar] [CrossRef]
- Onyilagha, C.; Mistry, H.; Marszal, P.; Pinette, M.; Kobasa, D.; Tailor, N.; Berhane, Y.; Nfon, C.; Pickering, B.; Mubareka, S.; et al. Evaluation of mobile real-time polymerase chain reaction tests for the detection of severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 9387. [Google Scholar] [CrossRef]
- Desmet, T.; Paepe, P.; Boelens, J.; Coorevits, L.; Padalko, E.; Vandendriessche, S.; Leroux-Roels, I.; Aerssens, A.; Callens, S.; Braeckel, E.V.; et al. Combined oropharyngeal/nasal swab is equivalent to nasopharyngeal sampling for SARS-CoV-2 diagnostic PCR. BMC Microbiol. 2021, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Zelyas, N.; MacDonald, C.; Pabbaraju, K.; Khan, M.N.; Prasad, A.; Hu, J.; Diggle, M.; Berenger, B.M.; Tipples, G. False negative rate of COVID-19 PCR testing: A discordant testing analysis. Virol. J. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, L.; Tovar, H.; Moreno-Contreras, J.; Espinoza, M.A.; de-Anda-Jáuregui, G. Automated Reverse Transcription Polymerase Chain Reaction Data Analysis for Sars-CoV-2 Detection. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2021, 73, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, J.; Lu, M.; Greene, W.; He, H.Z.; Zheng, S.Y. An Ultrafast One-Step Quantitative Reverse Transcription-Polymerase Chain Reaction Assay for Detection of SARS-CoV-2. Front. Microbiol. 2021, 12, 749783. [Google Scholar] [CrossRef] [PubMed]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-Based Testing but Not Real-Time Polymerase Chain Reaction Correlates with Severe Acute Respiratory Syndrome Coronavirus 2 Viral Culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef]
- Ferreira, B.I.D.S.; da Silva-Gomes, N.L.; Coelho, W.L.D.C.N.P.; da Costa, V.D.; Carneiro, V.C.S.; Kader, R.L.; Amaro, M.P.; Villar, L.M.; Miyajima, F.; Alves-Leon, S.V.; et al. Validation of a novel molecular assay to the diagnostic of COVID-19 based on real time PCR with high resolution melting. PLoS ONE 2021, 16, e0260087. [Google Scholar] [CrossRef]
- Dumaresq, J.; Coutlée, F.; Dufresne, P.J.; Longtin, J.; Fafard, J.; Bestman-Smith, J.; Bergevin, M.; Vallières, E.; Desforges, M.; Labbé, A.C. Natural spring water gargle and direct RT-PCR for the diagnosis of COVID-19 (COVID-SPRING study). J. Clin. Virol. 2021, 144, 104995. [Google Scholar] [CrossRef]
- Morecchiato, F.; Coppi, M.; Baccani, I.; Maggini, N.; Ciccone, N.; Antonelli, A.; Rossolini, G.M. Evaluation of extraction-free RT-PCR methods for faster and cheaper detection of SARS-CoV-2 using two commercial systems. Int. J. Infect. Dis. 2021, 112, 264–268. [Google Scholar] [CrossRef]
- Olearo, F.; Nörz, D.; Hoffman, A.; Grunwald, M.; Gatzemeyer, K.; Christner, M.; Both, A.; Campos, C.E.B.; Braun, P.; Andersen, G.; et al. Clinical performance and accuracy of a qPCR-based SARS-CoV-2 mass-screening workflow for healthcare-worker surveillance using pooled self-sampled gargling solutions: A cross-sectional study. J. Infect. 2021, 83, 589–593. [Google Scholar] [CrossRef]
- Ghoshal, U.; Garg, A.; Vasanth, S.; Arya, A.K.; Pandey, A.; Tejan, N.; Patel, V.; Singh, V.P. Assessing a chip-based rapid RTPCR test for SARS CoV-2 detection (TrueNat assay): A diagnostic accuracy study. PLoS ONE 2021, 16, e0257834. [Google Scholar] [CrossRef] [PubMed]
- Balaska, S.; Pilalas, D.; Takardaki, A.; Koutra, P.; Parasidou, E.; Gkeka, I.; Tychala, A.; Meletis, G.; Fyntanidou, B.; Metallidis, S.; et al. Evaluation of the Advanta Dx SARS-CoV-2 RT-PCR Assay, a High-Throughput Extraction-Free Diagnostic Test for the Detection of SARS-CoV-2 in Saliva: A Diagnostic Accuracy Study. Diagnostics 2021, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Oikawa, R.; Suzuki, T.; Funabashi, H.; Asai, D.; Hatori, Y.; Takemura, H.; Yamamoto, H.; Itoh, F. Evaluation of a new point-of-care quantitative reverse transcription polymerase chain test for detecting severe acute respiratory syndrome coronavirus 2. J. Clin. Lab. Anal. 2021, 35, e23992. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; De Pace, V.; Pennati, B.M.; Caligiuri, P.; Varesano, S.; Bruzzone, B.; Orsi, A. Evaluation of extraction-free RT-qPCR methods for SARS-CoV-2 diagnostics. Arch. Virol. 2021, 166, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chang, S.H. Clinical usefulness of extraction-free PCR assay to detect SARS-CoV-2. J. Virol. Methods 2021, 296, 114217. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Oliveira, M.D.S.; Ribeiro, J.; Dos Santos, I.G.C.; Almeida, K.S.; Conti, A.C.M.; Alexandrino, B.; Campos, F.S.; Soares, C.M.A.; Ribeiro Júnior, J.C. Validation of conventional PCR-like alternative to SARS-CoV-2 detection with target nucleocapsid protein gene in naso-oropharyngeal samples. PLoS ONE 2021, 16, e0257350. [Google Scholar] [CrossRef]
- Kritikos, A.; Caruana, G.; Brouillet, R.; Miroz, J.P.; Abed-Maillard, S.; Stieger, G.; Opota, O.; Croxatto, A.; Vollenweider, P.; Bart, P.A.; et al. Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART). Microorganisms 2021, 9, 1910. [Google Scholar] [CrossRef]
- Brotons, P.; Perez-Argüello, A.; Launes, C.; Torrents, F.; Subirats, M.P.; Saucedo, J.; Claverol, J.; Garcia-Garcia, J.J.; Rodas, G.; Fumado, V.; et al. Validation and implementation of a direct RT-qPCR method for rapid screening of SARS-CoV-2 infection by using non-invasive saliva samples. Int. J. Infect. Dis. 2021, 110, 363–370. [Google Scholar] [CrossRef]
- Laverack, M.; Tallmadge, R.L.; Venugopalan, R.; Cronk, B.; Zhang, X.; Rauh, R.; Saunders, A.; Nelson, W.M.; Plocharczyk, E.; Diel, D.G. Clinical evaluation of a multiplex real-time RT-PCR assay for detection of SARS-CoV-2 in individual and pooled upper respiratory tract samples. Arch. Virol. 2021, 166, 2551–2561. [Google Scholar] [CrossRef]
- Avetyan, D.; Chavushyan, A.; Ghazaryan, H.; Melkonyan, A.; Stepanyan, A.; Zakharyan, R.; Hayrapetyan, V.; Atshemyan, S.; Khachatryan, G.; Sirunyan, T.; et al. SARS-CoV-2 detection by extraction-free qRT-PCR for massive and rapid COVID-19 diagnosis during a pandemic in Armenia. J. Virol. Methods 2021, 295, 114199. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Banu, R.; Shrestha, P.; Patel, A.; Chen, F.; Cao, L.; Fabre, S.; Tan, J.; Lopez, H.; Chiu, N.; et al. RT-PCR/MALDI-TOF mass spectrometry-based detection of SARS-CoV-2 in saliva specimens. J. Med. Virol. 2021, 93, 5481–5486. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Florez, C.; Castañeda, S.; Ballesteros, N.; Martínez, D.; Castillo, A.; Muñoz, M.; Gomez, S.; Rico, A.; Pardo, L.; et al. Evaluation of the diagnostic performance of nine commercial RT-PCR kits for the detection of SARS-CoV-2 in Colombia. J. Med. Virol. 2021, 93, 5618–5622. [Google Scholar] [CrossRef] [PubMed]
- Leber, W.; Lammel, O.; Redlberger-Fritz, M.; Mustafa-Korninger, M.E.; Glehr, R.C.; Camp, J.; Agerer, B.; Lercher, A.; Popa, A.; Genger, J.W.; et al. Rapid, early and accurate SARS-CoV-2 detection using RT-qPCR in primary care: A prospective cohort study (REAP-1). BMJ Open 2021, 11, e045225. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, V.J.; Goldfarb, D.M.; Young, V.; Watson, N.; Al-Rawahi, G.N.; Srigley, J.A.; Tilley, P. Development and validation of a new triplex real-time quantitative reverse Transcriptase-PCR assay for the clinical detection of SARS-CoV-2. Mol. Cell. Probes 2021, 58, 101744. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; de Mora, D.; Freire-Paspuel, B.; Rodriguez, A.S.; Paredes-Espinosa, M.B.; Olmedo, M.; Sanchez, M.; Romero, J.; Paez, M.; Gonzalez, M.; et al. Analytical and clinical evaluation of a heat shock SARS-CoV-2 detection method without RNA extraction for N and E genes RT-qPCR. Int. J. Infect. Dis. 2021, 109, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Achkar, S.; Ammari, S.; Bockel, S.; Gallois, E.; Bayle, A.; Battistella, E.; Salviat, F.; Merad, M.; Laville, A.; et al. Systematic Screening of COVID-19 Disease Based on Chest CT and RT-PCR for Cancer Patients Undergoing Radiation Therapy in a Coronavirus French Hotspot. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 947–956. [Google Scholar] [CrossRef]
- Rigo, S.; Durigon, S.; Basaglia, G.; Avolio, M.; De Rosa, R. Molecular diagnosis of SARS-Cov-2, performances and throughput by Direct RT-PCR. New Microbiol. 2021, 44, 173–176. [Google Scholar]
- Banko, A.; Petrovic, G.; Miljanovic, D.; Loncar, A.; Vukcevic, M.; Despot, D.; Cirkovic, A. Comparison and Sensitivity Evaluation of Three Different Commercial Real-Time Quantitative PCR Kits for SARS-CoV-2 Detection. Viruses 2021, 13, 1321. [Google Scholar] [CrossRef]
- Tastanova, A.; Stoffel, C.I.; Dzung, A.; Cheng, P.F.; Bellini, E.; Johansen, P.; Duda, A.; Nobbe, S.; Lienhard, R.; Bosshard, P.P.; et al. A Comparative Study of Real-Time RT-PCR-Based SARS-CoV-2 Detection Methods and Its Application to Human-Derived and Surface Swabbed Material. J. Mol. Diagn. 2021, 23, 796–804. [Google Scholar] [CrossRef]
- Noor, F.A.; Safain, K.S.; Hossain, M.W.; Arafath, K.; Mannoor, K.; Kabir, M. Development and performance evaluation of the first in-house multiplex rRT-PCR assay in Bangladesh for highly sensitive detection of SARS-CoV-2. J. Virol. Methods 2021, 293, 114147. [Google Scholar] [CrossRef]
- Fitoussi, F.; Dupont, R.; Tonen-Wolyec, S.; Bélec, L. Performances of the VitaPCR™ SARS-CoV-2 Assay during the second wave of the COVID-19 epidemic in France. J. Med. Virol. 2021, 93, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Analytical and Clinical Evaluation of “AccuPower SARS-CoV-2 Multiplex RT-PCR kit (Bioneer, South Korea)” and “Allplex 2019-nCoV Assay (Seegene, South Korea)” for SARS-CoV-2 RT-PCR Diagnosis: Korean CDC EUA as a Quality Control Proxy for Developing Countries. Front. Cell. Infect. Microbiol. 2021, 11, 630552. [Google Scholar] [CrossRef]
- Dierks, S.; Bader, O.; Schwanbeck, J.; Groß, U.; Weig, M.S.; Mese, K.; Lugert, R.; Bohne, W.; Hahn, A.; Feltgen, N.; et al. Diagnosing SARS-CoV-2 with Antigen Testing, Transcription-Mediated Amplification and Real-Time PCR. J. Clin. Med. 2021, 10, 2404. [Google Scholar] [CrossRef]
- Nakura, Y.; Wu, H.N.; Okamoto, Y.; Takeuchi, M.; Suzuki, K.; Tamura, Y.; Oba, Y.; Nishiumi, F.; Hatori, N.; Fujiwara, S.; et al. Development of an efficient one-step real-time reverse transcription polymerase chain reaction method for severe acute respiratory syndrome-coronavirus-2 detection. PLoS ONE 2021, 16, e0252789. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, A.J.; Fyles, F.; Farrell, C.; Lewis, J.; Barr, D.; Haigh, K.; Abouyannis, M.; Hankinson, B.; Penha, D.; Fernando, R.; et al. Sensitivity of SARS-CoV-2 RNA polymerase chain reaction using a clinical and radiological reference standard. J. Infect. 2021, 82, 260–268. [Google Scholar] [CrossRef]
- Kortela, E.; Kirjavainen, V.; Ahava, M.J.; Jokiranta, S.T.; But, A.; Lindahl, A.; Jääskeläinen, A.E.; Jääskeläinen, A.J.; Järvinen, A.; Jokela, P.; et al. Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients. PLoS ONE 2021, 16, e0251661. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, A.M.; Obeid, D.A.; Alaifan, T.A.; Taha, M.T.; Alhothali, M.T.; Alzahrani, F.A.; Albarrag, A.M. Assessment of 12 qualitative RT-PCR commercial kits for the detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 3219–3226. [Google Scholar] [CrossRef]
- Visseaux, B.; Collin, G.; Houhou-Fidouh, N.; Le Hingrat, Q.; Ferré, V.M.; Damond, F.; Ichou, H.; Descamps, D.; Charpentier, C. Evaluation of three extraction-free SARS-CoV-2 RT-PCR assays: A feasible alternative approach with low technical requirements. J. Virol. Methods 2021, 291, 114086. [Google Scholar] [CrossRef]
- Cassinari, K.; Alessandri-Gradt, E.; Chambon, P.; Charbonnier, F.; Gracias, S.; Beaussire, L.; Alexandre, K.; Sarafan-Vasseur, N.; Houdayer, C.; Etienne, M.; et al. Assessment of Multiplex Digital Droplet RT-PCR as a Diagnostic Tool for SARS-CoV-2 Detection in Nasopharyngeal Swabs and Saliva Samples. Clin. Chem. 2021, 67, 736–741. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Sarmiento, A.D.; Ang, M.A.; Diwa, M.H.; Dungog, C.C.; Tan, D.I.; Lacuata, J.A.; Salud, J.E.; Lopa, R.A.; Velasco, J.M.; et al. Validation of snort-spit saliva in detecting COVID-19 using RT-PCR and rapid antigen detection test. Acta Medica Philipp. 2021, 55. [Google Scholar] [CrossRef]
- Girish, P.; Jayasankar, P.; Abhishek, P.; Sumeeta, S.; Gunvant, P.; Shalin, P. Comparative analysis of the naso/oropharyngeal swab and oral bio-fluid (whole saliva) samples for the detection of SARS-CoV-2 using RT-qPCR. Indian J. Dent. Res. 2021, 32, 206–210. [Google Scholar] [CrossRef]
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Clinical Performance and Analytical Sensitivity of Three SARS-CoV-2 Nucleic Acid Diagnostic Tests. Am. J. Trop. Med. Hyg. 2021, 104, 1516–1518. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhou, J.; Niu, C.; Wang, Q.; Pan, Y.; Sheng, S.; Wang, X.; Zhang, Y.; Yang, J.; Liu, M.; et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta 2021, 224, 121726. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Wright, A.; Macleod, C.K.; Barrett, J.; Filson, S.A.; Corrah, T.; Parris, V.; Sandhu, G.; Harris, M.; Tennant, R.; Vaid, N.; et al. False-negative RT-PCR for COVID-19 and a diagnostic risk score: A retrospective cohort study among patients admitted to hospital. BMJ Open 2021, 11, e047110. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Larsen, S.L.; Skov, M.N.; Larsen, H.; Hartmeyer, G.N.; Moeller, J.B. Phenol-chloroform-based RNA purification for detection of SARS-CoV-2 by RT-qPCR: Comparison with automated systems. PLoS ONE 2021, 16, e0247524. [Google Scholar] [CrossRef]
- Alaifan, T.; Altamimi, A.; Obeid, D.; Alshehri, T.; Almatrrouk, S.; Albarrag, A. SARS-CoV-2 direct real-time polymerase chain reaction testing in laboratories with shortage challenges. Future Virol. 2021, 16, 133–139. [Google Scholar] [CrossRef]
- Onwuamah, C.K.; Okwuraiwe, A.P.; Salu, O.B.; Shaibu, J.O.; Ndodo, N.; Amoo, S.O.; Okoli, L.C.; Ige, F.A.; Ahmed, R.A.; Bankole, M.A.; et al. Comparative performance of SARS-CoV-2 real-time PCR diagnostic assays on samples from Lagos, Nigeria. PLoS ONE 2021, 16, e0246637. [Google Scholar] [CrossRef]
- Price, T.K.; Bowland, B.C.; Chandrasekaran, S.; Garner, O.B.; Yang, S. Performance Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 RT-PCR Tests in a Single Health System: Analysis of >10,000 Results from Three Different Assays. J. Mol. Diagn. 2021, 23, 159–163. [Google Scholar] [CrossRef]
- Trobajo-Sanmartín, C.; Adelantado, M.; Navascués, A.; Guembe, M.J.; Rodrigo-Rincón, I.; Castilla, J.; Ezpeleta, C. Self-Collection of Saliva Specimens as a Suitable Alternative to Nasopharyngeal Swabs for the Diagnosis of SARS-CoV-2 by RT-qPCR. J. Clin. Med. 2021, 10, 299. [Google Scholar] [CrossRef]
- Omar, S.; Brown, J.M.; Mathivha, R.L.; Bahemia, I.; Nabeemeeah, F.; Martinson, N. The impact of a mobile COVID-19 polymerase chain reaction laboratory at a large tertiary hospital during the first wave of the pandemic: A retrospective analysis. S. Afr. Med. J. 2021, 111, 957–960. [Google Scholar] [CrossRef]
- Bergevin, M.A.; Freppel, W.; Robert, G.; Ambaraghassi, G.; Aubry, D.; Haeck, O.; Saint-Jean, M.; Carignan, A. Validation of saliva sampling as an alternative to oro-nasopharyngeal swab for detection of SARS-CoV-2 using unextracted rRT-PCR with the Allplex 2019-nCoV assay. J. Med. Microbiol. 2021, 70, 001404. [Google Scholar] [CrossRef]
- Yip, C.C.Y.; Leung, K.H.; Ng, A.C.K.; Chan, K.H.; To, K.K.W.; Chan, J.F.W.; Hung, I.F.N.; Cheng, V.C.C.; Sridhar, S. Comparative evaluation of a dual-target real-time RT-PCR assay for COVID-19 diagnosis and assessment of performance in pooled saliva and nasopharyngeal swab samples. Expert Rev. Mol. Diagn. 2021, 21, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Terada, J.; Kimura, M.; Moriya, A.; Motohashi, A.; Izumi, S.; Kawajiri, K.; Hakkaku, K.; Morishita, M.; Saito, S.; et al. Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect. Dis. 2021, 53, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mio, C.; Cifù, A.; Marzinotto, S.; Marcon, B.; Pipan, C.; Damante, G.; Curcio, F. Validation of a One-Step Reverse Transcription-Droplet Digital PCR (RT-ddPCR) Approach to Detect and Quantify SARS-CoV-2 RNA in Nasopharyngeal Swabs. Dis. Markers 2021, 2021, 8890221. [Google Scholar] [CrossRef]
- Lau, Y.L.; Ismail, I.B.; Mustapa, N.I.B.; Lai, M.Y.; Tuan Soh, T.S.; Haji Hassan, A.; Peariasamy, K.M.; Lee, Y.L.; Abdul Kahar, M.K.B.; Chong, J.; et al. Development of a reverse transcription recombinase polymerase amplification assay for rapid and direct visual detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). PLoS ONE 2021, 16, e0245164. [Google Scholar] [CrossRef]
- Shen, L.; Cui, S.; Zhang, D.; Lin, C.; Chen, L.; Wang, Q. Comparison of four commercial RT-PCR diagnostic kits for COVID-19 in China. J. Clin. Lab. Anal. 2021, 35, e23605. [Google Scholar] [CrossRef]
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Poor sensitivity of “AccuPower SARS-CoV-2 real time RT-PCR kit (Bioneer, South Korea)”. Virol. J. 2020, 17, 178. [Google Scholar] [CrossRef]
- Guo, J.J.; Yu, Y.H.; Ma, X.Y.; Liu, Y.N.; Fang, Q.; Qu, P.; Guo, J.; Lou, J.L.; Wang, Y.J. A multiple-center clinical evaluation of a new real-time reverse transcriptase PCR diagnostic kit for SARS-CoV-2. Future Virol. 2020, 15, 673–681. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Ren, S.; Liu, X.; Zhang, L.; Li, W.; Yu, H. Comparison of the diagnostic efficacy between two PCR test kits for SARS-CoV-2 nucleic acid detection. J. Clin. Lab. Anal. 2020, 34, e23554. [Google Scholar] [CrossRef]
- Martín Ramírez, A.; Zurita Cruz, N.D.; Gutiérrez-Cobos, A.; Rodríguez Serrano, D.A.; González Álvaro, I.; Roy Vallejo, E.; Gómez de Frutos, S.; Fontán García-Rodrigo, L.; Cardeñoso Domingo, L. Evaluation of two RT-PCR techniques for SARS-CoV-2 RNA detection in serum for microbiological diagnosis. J. Virol. Methods 2022, 300, 114411. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cao, S.; Liu, Y.; Zhang, Z.; Zheng, R.; Li, Y.; Zhou, J.; Zong, C.; Cao, D.; Qin, X. Performance verification of five commercial RT-qPCR diagnostic kits for SARS-CoV-2. Clin. Chim. Acta Int. J. Clin. Chem. 2022, 525, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Shulania, A.; Chhabra, M.; Kansra, S.; Achra, A.; Nirmal, K.; Katiyar, S.; Duggal, N. Performance of Chip Based Real Time RTPCR (TrueNat) and Conventional Real Time RT-PCR for Detection of SARS-CoV-2. J. Clin. Diagn. Res. 2021, 15, DC25–DC28. [Google Scholar] [CrossRef]
- Hofman, P.; Boutros, J.; Benchetrit, D.; Benzaquen, J.; Leroy, S.; Tanga, V.; Bordone, O.; Allégra, M.; Lespinet, V.; Fayada, J.; et al. A rapid near-patient RT-PCR test for suspected COVID-19: A study of the diagnostic accuracy. Ann. Transl. Med. 2021, 9, 921. [Google Scholar] [CrossRef]
- Jamal, A.J.; Mozafarihashjin, M.; Coomes, E.; Powis, J.; Li, A.X.; Paterson, A.; Anceva-Sami, S.; Barati, S.; Crowl, G.; Faheem, A.; et al. Sensitivity of Nasopharyngeal Swabs and Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2021, 72, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Ngaba, G.P.; Kalla, G.C.M.; Assob, J.C.N.; Njouendou, A.J.; Jembe, C.N.; Mboudou, E.T.; Mbopi-Keou, F.X. Comparative analysis of two molecular tests for the detection of COVID-19 in Cameroon. Pan. Afr. Med. J. 2021, 39, 214. [Google Scholar] [CrossRef]
- Procop, G.W.; Brock, J.E.; Reineks, E.Z.; Shrestha, N.K.; Demkowicz, R.; Cook, E.; Ababneh, E.; Harrington, S.M. A Comparison of Five SARS-CoV-2 Molecular Assays with Clinical Correlations. Am. J. Clin. Pathol. 2021, 155, 69–78. [Google Scholar] [CrossRef]
- Rashid, M.; Rajan, A.K.; Thunga, G.; Shanbhag, V.; Nair, S. Impact of Diabetes in COVID-19 Associated Mucormycosis and its Management: A Non-Systematic Literature Review. Curr. Diabetes Rev. 2023, 19, e240222201411. [Google Scholar] [CrossRef]
- Tsang, N.N.Y.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ip, D.K.M. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1233–1245. [Google Scholar] [CrossRef]
- Becker, D.; Sandoval, E.; Amin, A.; De Hoff, P.; Diets, A.; Leonetti, N.; Lim, Y.W.; Elliott, C.; Laurent, L.; Grzymski, J.; et al. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. MedRxiv 2020. [Google Scholar] [CrossRef]
- Lee, R.A.; Herigon, J.C.; Benedetti, A.; Pollock, N.R.; Denkinger, C.M. Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: A Systematic Review and Meta-analysis. J. Clin. Microbiol. 2021, 59, e02881-20. [Google Scholar] [CrossRef]
- Torres, M.; Collins, K.; Corbit, M.; Ramirez, M.; Winters, C.R.; Katz, L.; Ross, M.; Relkin, N.; Zhou, W. Comparison of saliva and nasopharyngeal swab SARS-CoV-2 RT-qPCR testing in a community setting. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef] [PubMed]
- Altawalah, H.; AlHuraish, F.; Alkandari, W.A.; Ezzikouri, S. Saliva specimens for detection of severe acute respiratory syndrome coronavirus 2 in Kuwait: A cross-sectional study. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 132, 104652. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Guirguis, M.; Alsabeeha, N.H.M.; Cannon, R.D. The diagnostic accuracy of saliva testing for SARS-CoV-2: A systematic review and meta-analysis. Oral Dis. 2022, 28 (Suppl. S2), 2347–2361. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Schreiber, P.W.; Scheier, T.; Audigé, A.; Buonomano, R.; Rudiger, A.; Braun, D.L.; Eich, G.; Keller, D.I.; Hasse, B.; et al. High Efficacy of Saliva in Detecting SARS-CoV-2 by RT-PCR in Adults and Children. Microorganisms 2021, 9, 642. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Hu, J.; Zhou, M.; Yu, M.Q.; Li, K.Y.; Xu, D.; Xiao, Y.; Yang, J.Y.; Lu, Y.J.; et al. Nasopharyngeal Swabs Are More Sensitive than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load. Front. Med. 2020, 7, 334. [Google Scholar] [CrossRef]
- Sasikala, M.; Sadhana, Y.; Vijayasarathy, K.; Gupta, A.; Daram, S.K.; Podduturi, N.C.R.; Reddy, D.N. Comparison of saliva with healthcare workers- and patient-collected swabs in the diagnosis of COVID-19 in a large cohort. BMC Infect. Dis. 2021, 21, 648. [Google Scholar] [CrossRef]
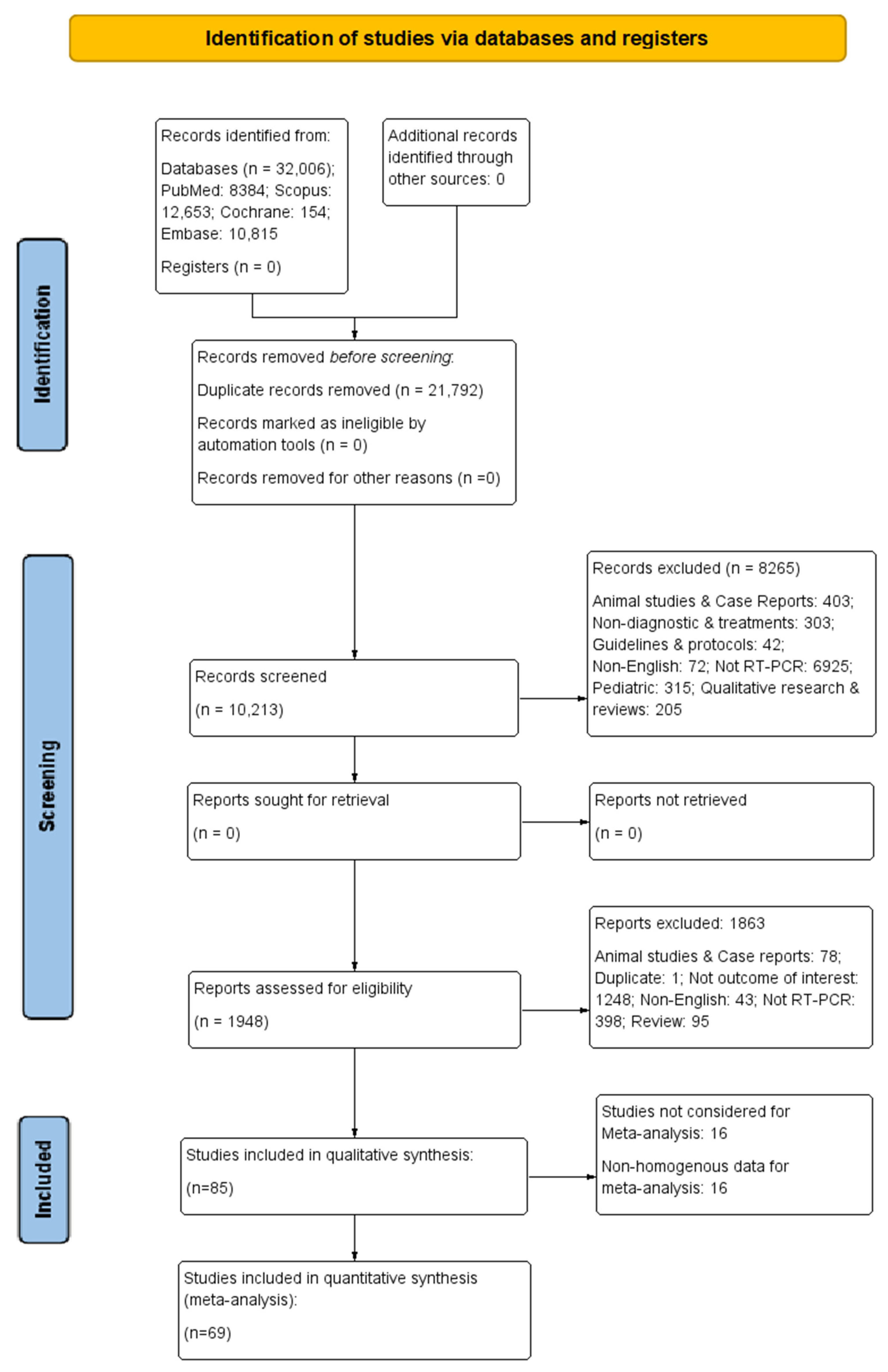
| Study ID, Year, Country | Study Design | Study Settings | Total Number of Samples/ Participants | Age of Cohort | Male/ Female | Clinical Presentation/ Characteristics |
|---|---|---|---|---|---|---|
| Escobar et al., 2021; Chile [18] | Cohort selection of a cross-sectional study | Multi-specialized Guillermo Grant Benavente Hospital (HGGB) and three Family Health Centers (FHCs) in the Chilean city of Concepción | 127 saliva and 127 NPS | <18: 5; 18–34: 69; 35–50: 29; 51–65: 20; >65: 4 | 68/59 | Symptomatic: 111; Asymptomatic:15; No information: 1; RT-PCR positive: 104; RT-PCR negative: 150 |
| Singh et al., 2021; India [19] | Quality audit study | Medical college institution | 92 samples and 60 controls | NR | NR | RT-PCR positive: 92; Healthy individuals: 30; Other respiratory disease: 30 |
| Figueroa et al., 2021; Ecuador [20] | Case–control study | NR | 242 clinical specimens and 11 negative controls | NR | NR | 122 SARS-CoV-2-positive and 120 SARS-CoV-2-negative |
| LeGof et al., 2021; France [21] | Prospective observational study | Two community COVID-19 screening centers | 1718 | 37 (26–52) a | 774/944 | +NPS RT-PCR: 117; Symptomatic: 530 |
| Villota et al., 2021; Ecuador [22] | Cross-sectional study | Two centers from Ecuador and USA | 192 clinical samples (NPS: 132; sputum: 60) | NR | NR | Positive: 142; Negative: 50 |
| De Pace et al., 2021; Italy [23] | Consecutive prospective observational study | Intensive Care Units of San Martino Hospital (Genoa, Italy | 75 patients | 65 (31–81) b | 56/19 | BAS: 43 (57.3%); Negative: 30.2%; Positive: 69.8%; BAL: 32 (42.7%); Negative: 37.5%; Positive: 62.5% |
| Kanwar et al., 2021; USA [24] | Prospective salvage sample study | University of Kansas Health System (TUKHS) | 201 samples | 57 (15–92) a | 103/98 | Positive: 99; Negative: 102 |
| Michel et al., 2021; Germany [25] | NR | Robert Koch Institute | 424 specimens | NR | NR | Positive: 424 |
| Wu et al., 2021; China [26] | Cross-sectional study | Shenzhen Third People’s Hospital and a compulsory quarantine facility | 52 (throat: 30; nasal: 7; NPS: 7; sputum: 8 | NR | NR | Positive: 26; Negative: 26 |
| Lee et al., 2021; UK [27] | Prospective, multi-center, cohort study | Secondary and tertiary care hospitals in Scotland | 1368 patients with 3822 tests | 68 (53–80) b | 731/637 | Confirmed positive: 496 |
| Borkakoty et al., 2021; India [28] | NR | State of Assam | 240 random samples | NR | NR | Positive: 120; Negative: 120 |
| Hata et al., 2021; USA [29] | NR | Mayo Clinic | 135 participants | 20–83 c | NR | Positive: 28; Negative: 106 |
| Wang et al., 2020; China [30] | NR | The Second Xiangya Hospital | 242 samples | NR | NR | Positive: 42 (34 throat swabs and 8 fecal samples); Negative: 200 |
| Mollaei et al., 2020; Iran [31] | NR | Kerman Reference Laboratory | 30 infected patients | NR | NR | Varies based on the gene chosen |
| Pierri et al., 2022; Italy [32] | Post-analysis of a GENCOVID study | GENCOVID people in direct contact with positive patients from the Campania region, Italy | 258 samples | NR | NR | Positive: 164 |
| Torres et al., 2021; Ecuador [33] | Descriptive-correlating, retrospective, cross-sectional study | Santo Domingo General Hospital (Santo Domingo de los Tsáchilas, Ecuador) | 773 samples | 1–14 years: 38; 15–19 years: 33; 20–49 years: 461; 50–64 years: 127; >65 years: 74 | 344/389 | Symptomatic: 515; Asymptomatic: 218 |
| Pearson et al., 2021; Canada [34] | NR | MSH/UHN clinical diagnostics lab | 59 samples | NR | NR | Positive: 29; Negative: 30 |
| Kriegova et al., 2021; Czech Republic [35] | Large prospective cohort | University Hospital Olomouc and Sumperk Hospital, Czechia | 1038 subjects | NR | NR | Positive: 297; Negative 741 |
| Onyilagha et al., 2021; Canada [36] | Cross-sectional study | NR | 90 samples | NR | NR | Negative: 40; Positive: 50 |
| Desmet et al., 2021; Belgium [37] | Prospective observational study | Ghent University Hospital | 36 patients | 61 (22–90) b | 21/25 | NP or OP/N positive: 35; Combined positive: 31; Mild: 7; Moderate: 10; Severe: 13; Critical care: 5; Pre-symptomatic: 1 |
| Kanji et al., 2021; Canada [38] | Prospective cross-sectional study | Province of Alberta, Canada | 49 patients | 72 (25–97) b | 15/34 | Positive: 49; Negative: 52 |
| Gómez-Romero et al., 2021; Mexico [39] | Prospective database study | Epidemiology department of the Health Ministry of the State of Morelos (Secretaría de Salud Morelos, SSM) | 140 healthcare workers/sample | NR | NR | Positive: 36; Negative: 104 |
| Milosevic et al., 2021; United States [40] | Prospective cohort study | Penn State Health Milton S. Hershey Medical Center | 60 samples | NR | NR | Positive: 30; Negative: 30 |
| Pekosz et al., 2021; United States [41] | Prospective cohort study | FDA EUA study samples which occurred across 21 geographically diverse study sites | 251 sample | Symptomatic: 251 | ||
| Ferreira et al., 2021; Brazil [42] | Prospective cohort study | State of Rio de Janeiro and the state of Ceará | 65 patients | NR | NR | NPS: 42; Serum: 12; Saliva: 11; Positive: 51; Negative: 14 |
| Dumaresq et al., 2021; Canada [43] | Prospective cohort study (SPRING study) | Département de microbiologie et d’infectiologie du centre hospitalier universitaire | 2010 sample from 987 patients | 40 (6–91) a | NR | 1005 ONPS and 1005 gargles; Symptomatic: 987; Asymptomatic: 987 |
| Morecchiato et al., 2021; Italy [44] | Prospective cohort study (SPRING study) | Microbiology and Virology Unit of Florence Careggi University Hospital (Florence, Italy) | 139 samples | NR | NR | Positive: 96; Negative: 43 |
| Olearo et al., 2021; Germany [45] | Cross-sectional retrospective study | University Hospital Hamburg. | 7513 HCWs (55,122 samples); 11,192 sample pools | NR | NR | Negative: 11,041; Invalid: 82; Positive: 69 |
| Ghoshal et al., 2021; India [46] | Retrospective observational study | Triage of a dedicated COVID-19 tertiary care center with 180 beds including 30 ICU ventilator beds | 1807 patients | NR | NR | RT-PCR positive; 174; TrueNat: 174 |
| Balaska et al., 2021; Greece [47] | Prospective observational study | AHEPA University Hospital, Thessaloniki | 420 pairs of samples | 44.7 (13) a | 161/259 | Positive diagnostic sample: 27.7%; Screening sample: 5% |
| Watanabe et al., 2021; Japan [48] | NR | Kawasaki Rinko General Hospital and the Matsudo City General Hospital | 96 patients | 49.3 (27.8) a | 45/51 | Positive: 20; Negative: 76 |
| Domnich et al., 2021; Italy [49] | Prospective observational study | San Martino Policlinico Hospital (Genoa, Liguria, Northwest Italy) | 98 samples | NR | NR | Positive: 98 |
| Kim et al., 2021; South Korea [50] | NR | Kyungpook National University | 300 samples | NR | NR | Positive: 260; Negative: 40 |
| Carvalho et al., 2021; Brazil [51] | NR | Municipal medical service | 346 samples | NR | NR | Detectable: 194; Undetectable: 152 |
| Kritikos et al., 2021; Switzerland [52] | Prospective observational study | Tertiary university hospital in Lausanne, Switzerland | 58 patients | 70 (61–77) b | 45/13 | Symptomatic: 49 |
| Brotons et al., 2021; Spain [53] | Three-phase cross-sectional study | Molecular Microbiology Department of Sant Joan de Déu Hospital | 183 samples | NR | NR | Positive: 10; Negative: 173 |
| Laverack et al., 2021; USA [54] | NR | Cornell COVID-19 Testing Laboratory by three other COVID-19 testing laboratories in the United States | 225 samples | NR | NR | NPS: 201; AN: 24; NPS positive: 100; Negative: 101; AN positive: 12; AN negative: 12 |
| Avetyan et al., 2021; Armenia [55] | Cross-sectional study | Institute of Molecular Biology, National Academy of Sciences | NPS: 74; RNA sample: 196 | NR | NR | NPS: Positive: 44; Negative: 30; RNA sample positive: 196 |
| Hernandez et al., USA; 2021 [56] | NR | Clinical Microbiology Laboratory at the Mount Sinai Health System | 60 patients | NR | NR | NR |
| Hernández et al., 2021; Colombia [57] | NR | Not reported | 94 samples | Positive: 49; Negative: 45 | ||
| Leber et al., 2021; UK [58] | Prospective cohort study | GP participating in the National Influenza Surveillance Network in the ski resort of Schladming-Dachstein | 66 patients | NR | NR | Positive: 22; Negative: 44 |
| Gadkar et al., 2021; Canada [59] | NR | Microbiology and virology laboratories of BC Children’s Hospital | 372 samples | NR | NR | Positive: 142 |
| Bruno et al., 2021; Ecuador [60] | NR | INSPI and UDLA | 1036 samples | NR | NR | Positive: 543; Negative: 493 |
| Sun et al., 2021; France [61] | Single center, retrospective, observational study | Radiation therapy department, Gustave Roussy, Paris-Saclay University | 480 patients | 62 (50–70) b | 228/252 | Positive: 26; Negative: 446 |
| Rigo et al., 2021; Pordenone [62] | NR | Microbiology and Virology Department Laboratory | 180 samples | NR | NR | Positive: 93; Negative: 88 |
| Banko et al., 2021; Serbia [63] | NR | Laboratory of Molecular Microbiology, Institute for Biocides and Medical Ecology, Belgrade | 354 samples | NR | NR | Sansure Biotech: Positive: 190; Negative: 164 GeneFinderTM: Positive: 176; Negative: 178 TaqPathTM: Positive: 178; Negative: 176 |
| Tastanova et al., 2021; Switzerland [64] | NR | University Hospital Zurich and at ADMed Laboratory in La Chaux-de-Fonds, Switzerland | 184 samples | NR | NR | Positive: 92; Negative: 92 |
| Noor et al., 2021; Bangladesh [65] | Case–control sample study | Department of Biochemistry and Molecular Biology | 240 samples | NR | NR | Positive: 120; Negative: 120 |
| Fitoussi et al., 2021; France [66] | Prospective observational study | entre Cardiologique du Nord-CCN, Saint-Denis, France | 239 patients | NR | NR | Positive: 140; Negative: 99 |
| Freire-Paspuel et al., 2020; Ecuador [67] | Prospective observational study | Laboratory of “Universidad de Las Américas” in Quito (Ecuador) | 89 samples | NR | NR | Positive: 57; Negative: 32 |
| Dierks et al., 2021; Germany [68] | NR | University Medical Center Göttingen | 322 samples | NR | NR | Positive: 21; Negative: 301 |
| Nakura et al., 2021; Japan [69] | NR | Osaka Women’s and Children’s Hospital, Osaka Habikino Medical Center, and Osaka General Medical Center of the Osaka Prefectural Hospital | 213 samples | NR | NR | Sputum: 35; NPS: 124; Saliva: 7 |
| Stockdale et al., 2021; UK [70] | NR | Liverpool University Hospitals NHS Foundation Trust | 429 patients | 67 (55–78) b | 257/172 | Positive: 293; Negative: 136 |
| Kortela et al., 2021; Finland [71] | Population-based retrospective study | Helsinki Capital Region, Finland | 3008 patients | 52.5 (19.7) a; 51 (36–69) b | 1215/1794 | Not suspected: 514; Not excluded: 1318; High suspicion: 516; Laboratory confirmed: 574; Not known: 86; Positive: 585; Negative: 2246 |
| Altamimi et al., 2021; Saudi Arabia [72] | NR | Saudi Center for Disease Prevention and Control (SCDC) Laboratories | 94 samples | NR | NR | Positive: 63; Negative: 31 |
| Visseaux et al., 2021; France [73] | NR | Virology Laboratory of Bichat-Claude Bernard University Hospital, Paris, France | 94 samples | NR | NR | Positive: 69; Negative: 25 |
| Cassinari et al., 2021; France [74] | Prospective observational study | Rouen University Hospital | 130 patients | NR | NR | Positive: 13; Negative: 117 |
| Carrillo et al., 2021; Manila [75] | Prospective cross-sectional diagnostic accuracy study | Philippine General Hospital | 197 patients | 32 (22–64) | 74/123 | Positive: 18; Negative: 179 |
| Girish et al., 2021; India [76] | Cross-sectional, analytical study | BJ Medical College and Civil Hospital | 309 patients | NR | NR | Positive: 55; Negative: 254 |
| Freire-Paspuel et al., 2021; Ecuador [77] | NR | NR | 97 samples | NR | NR | Positive: 43; Negative: 54 |
| Dong et al., 2021; China [78] | NR | Hospitalized patients or close contacts of hospitalized patients tested by Beijing CDC (BJCDC), Wuhan CDC (WHCDC), and a government-designated clinical test laboratory | 196 samples | NR | NR | Febrile suspected patients: 103; Close contacts: 77; Convalescents: 16; Positive: 132; Negative: 64 |
| Gupta-Wright et al., 2021; UK [79] | Retrospective cohort study | Two hospitals within an acute NHS Trust in London, UK | 4008 patients | 69 (56–81) b* | 1142/651 * | Non-COVID-19: 2215; COVID-19 diagnosis: 1793; Positive: 1391; Negative: 283 |
| Dimke et al., 2021; Denmark [80] | NR | Department of Clinical Microbiology, Odense University Hospital | 87 samples | NR | NR | Positive: 57; Negative: 30 |
| Alaifan et al., 2021; Saudi Arabia [81] | NR | Diagnostic laboratories at the Saudi Center for Diseases Control and Prevention | 185 samples | NR | NR | Positive: 121; Negative: 64 |
| Onwuamah et al., 2021; Nigeria [82] | Retrospective study | The Nigerian Institute of Medical Research from people living in Lagos, Nigeria | 63 samples | NR | NR | Positive: 48; Negative: 15 |
| Price et al., 2021; USA [83] | Prospective observational study | University of California, Los Angeles Health System | 10,165 samples from 8948 patients | NR | NR | NPS: 10,215; Bronchoalveolar lavage: 121; Expectorated sputum: 22; Miscellaneous sample types: 35; Positive: 630; Negative: 9535 |
| Trobajo-Sanmartín et al., 2021; Spain [84] | Prospective study | Clinical microbiology department of the Navarra Hospital Complex | 674 pairs of samples (NP and saliva) | 36 (19) b | 300/374 | Positive: 337; Negative: 337; Symptomatic: 333; Non-symptomatic: 341 |
| Omar et al., 2021; South Africa [85] | Retrospective descriptive cross-sectional study | Data from the mobile COVID-19 PCR testing laboratory database and the non-COVID-19 ICU database | 315 samples from 1032 patients | 40 (20.4) a | 551/481 | NPS: 281 Nasal swab: 17; OPS: 1; Tracheal respirate: 7; Not specified: 13; Positive: 51; Negative: 264 |
| Bergevin et al., 2021; Canada [86] | Prospective evaluation | Laval region of Quebec, Canada | 773 pairs | Positive: 44 (31–58) b | Positive: 80/85 | Positive: 165 (symptomatic: 148; asymptomatic: 17) |
| Yip et al., 2021; China [87] | NR | The University of Hong Kong-Shenzhen Hospital | 296 samples | NR | NR | Positive: 105; Negative: 191 |
| Renzoni et al., 2021; Switzerland [88] | Retrospective analysis | Geneva University Hospitals | 61 samples | NR | NR | Positive: 61; Control: 16 |
| Tsujimoto et al., 2021; Japan [89] | Single-center, prospective study | National Centre for Global Health and Medicine (Tokyo, Japan) | 10 patients (57 sets of NPS, NS, and SS samples) | 47 (30–70) b | 2/8 | Positive: 48; Negative: 9 |
| Mio et al., 2021; Italy [90] | NR | Department of Laboratory Medicine, University Hospital of Udine, Italy | 30 patient samples | NR | NR | Positive: 19; Negative: 11 |
| Lau et al., 2021; Malaysia [91] | NR | Hospital Sungai Buloh, Malaysia | 113 samples | NR | NR | Positive: 78; Negative: 35 |
| Shen et al., 2021; China [92] | NR | Beijing Center for Disease Prevention and Control (BJCDC) | 142 samples | NR | NR | Kit I: Positive: 130; Negative: 12; Kit II: Positive: 116; Negative: 26; Kit III: Positive: 114; Negative: 28; Kit IV: Positive: 129; Negative: 13 |
| Freire-Paspuel et al., (B) 2020; Ecuador [93] | NR | Laboratory of “Universidad de Las Américas” in Quito (Ecuador) | 48 samples | NR | NR | Positive: 30; Negative: 18 |
| Guo et al., 2020; China [94] | NR | Three centers in China | 500 subjects | 0.75–93c | 258/242 | Positive: 242; Negative: 258; OPS: 395; Sputum: 167 |
| Lu et al., 2020; China [95] | NR | Liuzhou People’s Hospital | 118 patients | Cases: 35.94 (16.32); Control: 36.50 (19.93) a | 72/46 | COVD-19: 18; Control: 100 |
| Martín Ramírez et al., 2022; Spain [96] | Retrospective cohort study | Princesa University Hospital | 303 patients | Pre-pandemic control: 73.5 (62.5–85.5); Pandemic control: 69 (62–83); Positive: 64 (56–72) b | Pre-pandemic control: 25/25; Pandemic control: 32/18; positive: 139/64 | Positive: 203; Pre-pandemic control: 50; Pandemic control: 50; |
| Yang et al., 2022; China [97] | NR | Department of Laboratory Medicine, Shengjing Hospital of China Medical University, | 63 samples | NR | NR | Positive: 28; Negative: 35 |
| Sahoo et al., 2021; India [98] | Cross-sectional observational study | Department of Microbiology, ABVIMS, and Dr. RML Hospital | 500 | NR | NR | Positive: 49; Negative: 451 |
| Hofman et al., 2021; France [99] | Prospective cohort study | Downtown free screening centers available to the population of the Nice metropolitan area and the outpatient clinic of the Department of Pulmonary Medicine of the University Hospital of Nice | 112 samples/subjects | 40 (15) b | 69/43 | Positive: 45; Negative: 67 |
| Jamal et al., 2020; Canada [100] | Population-based surveillance of consecutive patients | Six Toronto Invasive Bacterial Disease Network | 91 patients | 66 (23–106) b | 52/39 | Positive: 72; Negative: 19 |
| Ngaba et al., 2021; Cameroon [101] | Cross-sectional and comparative study | Douala Gynaeco-Obstetrics and Pediatric Hospital molecular biology laboratory | 1810 patients | 0–71 + c | 1226/559 | NPS: 1736; Saliva: 2; Throat swab: 1; Positive: 35; Negative: 1775 |
| Procop et al., 2020; USA [102] | NR | Cleveland Clinic | 239 samples | 49.28 (16.86) a | NR | Positive: 168; Negative: 71 |
| Parameter | Number of Studies | Pooled Effect Measure (95%CI) | Heterogeneity |
|---|---|---|---|
| Nasopharyngeal swabs | |||
| Sensitivity | 43 | 91.06% (95%CI: 88.91 to 93.21) | 100% |
| Specificity | 37 | 95.57% (95%CI: 95.19 to 95.96) | 100% |
| PPV | 15 | 95.88% (95%CI: 91.59 to 100.16) | 100% |
| NPV | 15 | 91.58% (95%CI: 88.03 to 95.13) | 100% |
| Kappa coefficient | 16 | 0.79 (95%CI: 0.71 to 0.87) | 98% |
| Saliva samples | |||
| Sensitivity | 14 | 76.70% (95%CI: 60.50 to 92.91) | 99% |
| Specificity | 11 | 98.27% (95%CI: 97.31 to 99.24) | 62% |
| PPV | 5 | 90.16% (95%CI: 82.90 to 97.43) | 97% |
| NPV | 5 | 90.37% (95%CI: 84.18 to 96.56) | 96% |
| Kappa coefficient | 5 | 0.61 (95%CI: 0.44 to 0.79) | 98% |
| Combined nasopharyngeal/oropharyngeal samples | |||
| Sensitivity | 16 | 92.00% (95%CI: 87.57 to 96.43) | 100% |
| Specificity | 12 | 98.08% (95%CI: 96.64 to 99.52) | 100% |
| PPV | 5 | 84.63% (95%CI: 70.14 to 99.12) | 100% |
| NPV | 5 | 96.12% (95%CI: 92.83 to 99.42) | 100% |
| Kappa coefficient | 5 | 0.82 (95%CI: 0.67 to 0.98) | 98% |
| Parameter | Study | Total Participants | Effect Measure |
|---|---|---|---|
| Respiratory samples | |||
| Sensitivity | Wu S et al. [26] | 52 | 100 |
| Nakura Y et al. [69] | 213 | 99.44 | |
| Specificity | Wu S et al. [26] | 52 | 100 |
| Nakura Y et al. [69] | 213 | 100 | |
| PPV | Wu S et al. [26] | 52 | 100 |
| Pekoz A et al. [41] | 251 | 73.7 | |
| NPV | Wu S et al. [26] | 52 | 100 |
| Pekoz A et al., [41] | 251 | 100 | |
| Price T K et al. [83] | 10,165 | 98 | |
| Kappa Coefficient | Wu S et al. [26] | 52 | 1 |
| Sputum samples | |||
| Sensitivity | Villota S D et al. [22] | 50 | 90 |
| Torres A et al. [33] | 229 | 86 | |
| Specificity | Villota S D et al. [22] | 50 | 100 |
| Torres A et al. [33] | 229 | 37 | |
| PPV | Torres A et al. [33] | 229 | 38 |
| NPV | Torres A et al. [33] | 229 | 85 |
| Kappa Coefficient | Torres A et al. [33] | 229 | 0.73 |
| Broncho aspirate samples | |||
| Sensitivity | Pace V D et al. [23] | 75 | 96 |
| Specificity | Pace V D et al. [23] | 75 | 100 |
| Kappa Coefficient | Pace V D et al. [23] | 75 | 0.94 |
| Throat swab samples | |||
| Sensitivity | Wang B et al. [30] | 42 | 97.62 |
| Lu Y et al. [95] | 18 | 94.4 | |
| Specificity | Wang B et al. [30] | 158 | 100 |
| Lu Y et al. [95] | 18 | 100 | |
| PPV | Wang B et al. [30] | 200 | 100 |
| Lu Y et al. [95] | 18 | 100 | |
| NPV | Wang B et al. [30] | 200 | 98.52 |
| Lu Y et al. [95] | 18 | 99 | |
| Kappa Coefficient | Wang B et al. [30] | 200 | 0.985 |
| Lu Y et al. [95] | 18 | 0.996 | |
| Gargle samples | |||
| Sensitivity | Dumaresq J et al. [43] | 1005 | 95.3 |
| Kappa Coefficient | Dumaresq J et al. [43] | 1005 | 0.94 |
| Serum samples | |||
| Sensitivity | Ramírez AM et al. [96] | 265 | 73.63 |
| Specificity | Ramírez AM et al. [96] | 265 | 97.6 |
| PPV | Ramírez AM et al. [96] | 265 | 96.73 |
| NPV | Ramírez AM et al. [96] | 265 | 75 |
| Kappa Coefficient | Ramírez AM et al. [96] | 265 | 0.69 |
| Mixed samples | |||
| Sensitivity | Silva ferreira B I et al. [42] | 65 | 98.04 |
| Omar S et al. [85] | 319 | 95 | |
| Yip CCY et al. [87] | 106 | 99.1 | |
| Yang M et al. [97] | 35 | 100 | |
| Specificity | Silva ferreira B I et al. [42] | 65 | 100 |
| Omar S et al. [85] | 319 | 97 | |
| Yip CCY et al. [87] | 106 | 100 | |
| Yang M et al. [97] | 28 | 100 | |
| PPV | Silva ferreira B I et al. [42] | 65 | 100 |
| Omar S et al. [85] | 319 | 82.4 | |
| Yang M et al. [97] | 63 | 100 | |
| NPV | Silva ferreira B I et al. [42] | 65 | 93.3 |
| Omar S et al. [85] | 319 | 99.9 | |
| Yang M et al. [97] | 63 | 100 | |
| Kappa Coefficient | Silva ferreira B I et al. [42] | 65 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shaibari, K.S.A.; Mousa, H.A.-L.; Alqumber, M.A.A.; Alqfail, K.A.; Mohammed, A.; Bzeizi, K. The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies. Diagnostics 2023, 13, 3057. https://doi.org/10.3390/diagnostics13193057
Al-Shaibari KSA, Mousa HA-L, Alqumber MAA, Alqfail KA, Mohammed A, Bzeizi K. The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies. Diagnostics. 2023; 13(19):3057. https://doi.org/10.3390/diagnostics13193057
Chicago/Turabian StyleAl-Shaibari, Khaled Sadeq Ali, Haider Abdul-Lateef Mousa, Mohammed Abdullah A. Alqumber, Khaled A. Alqfail, AbdulHakim Mohammed, and Khalid Bzeizi. 2023. "The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies" Diagnostics 13, no. 19: 3057. https://doi.org/10.3390/diagnostics13193057
APA StyleAl-Shaibari, K. S. A., Mousa, H. A.-L., Alqumber, M. A. A., Alqfail, K. A., Mohammed, A., & Bzeizi, K. (2023). The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies. Diagnostics, 13(19), 3057. https://doi.org/10.3390/diagnostics13193057







