A Functional Assay for the Determination of Heparin-Induced Thrombocytopenia via Flow Cytometry
Abstract
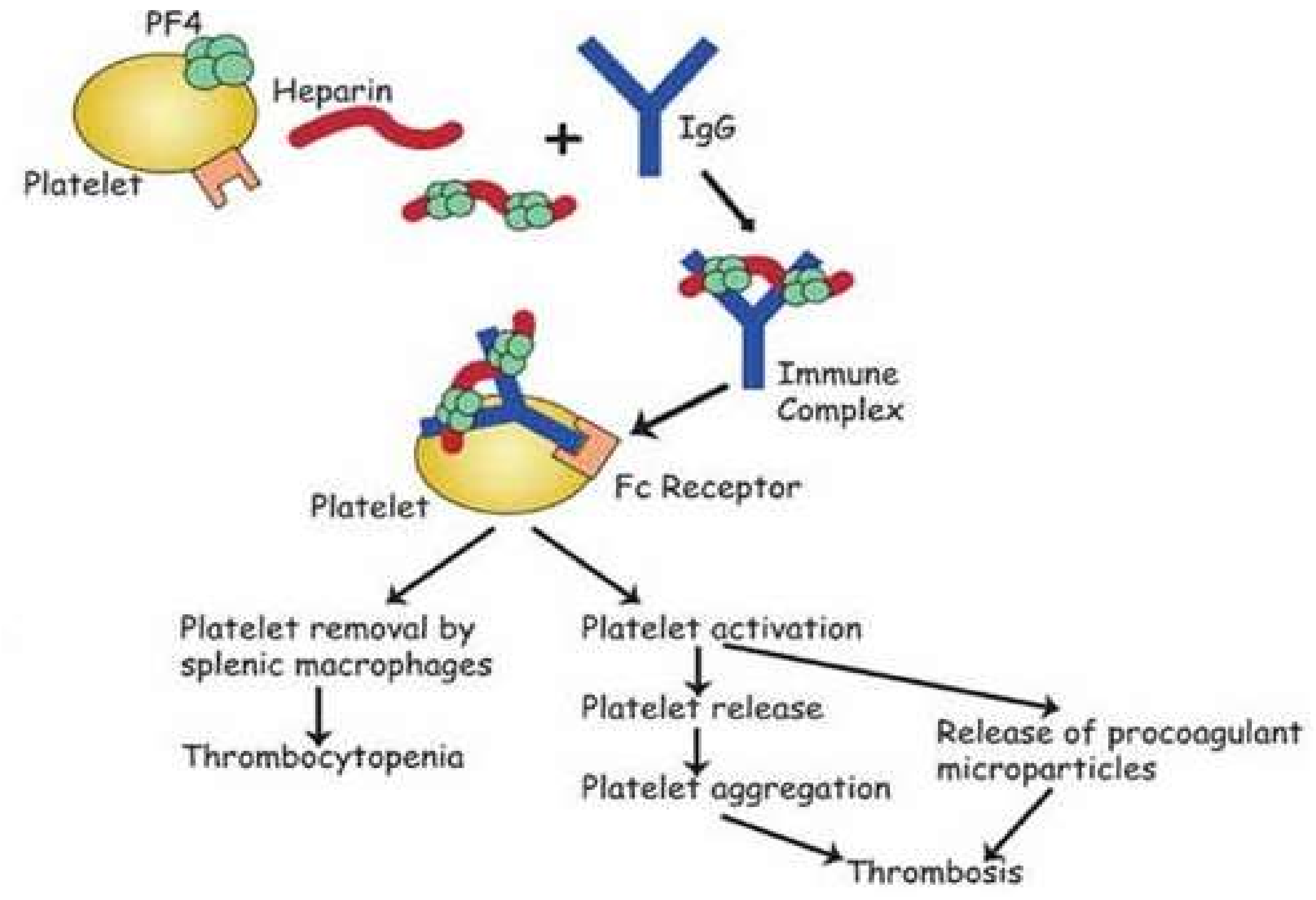
:1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. Donor Samples
2.1.2. Patient Samples
2.2. Immunological Assays
2.2.1. Particle Gel Immunoassay
2.2.2. Chemiluminescence Assay
2.2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Functional Assays
2.3.1. Serotonin Release Assay (SRA) using HPLC and Fluorometric Detection
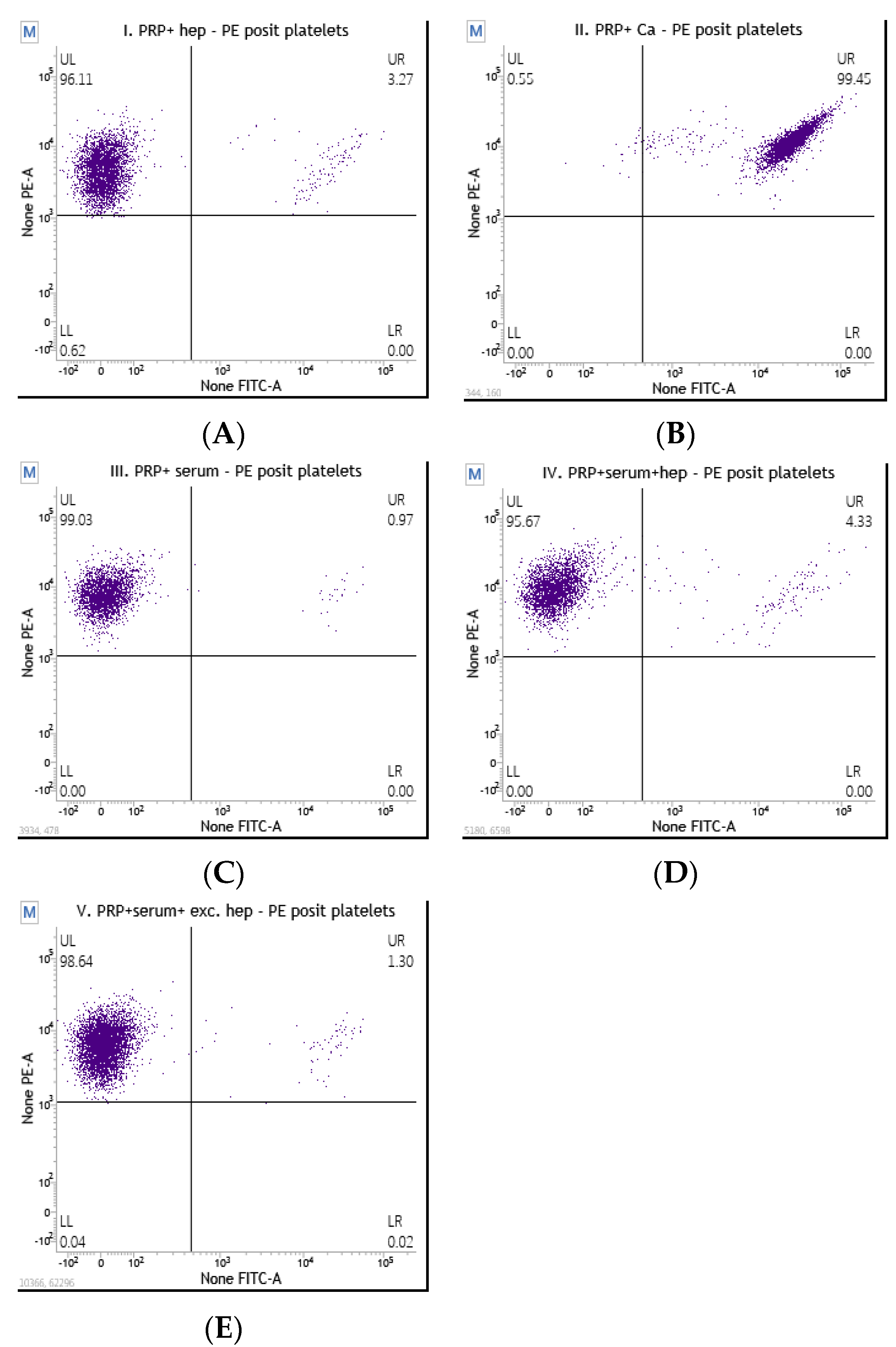
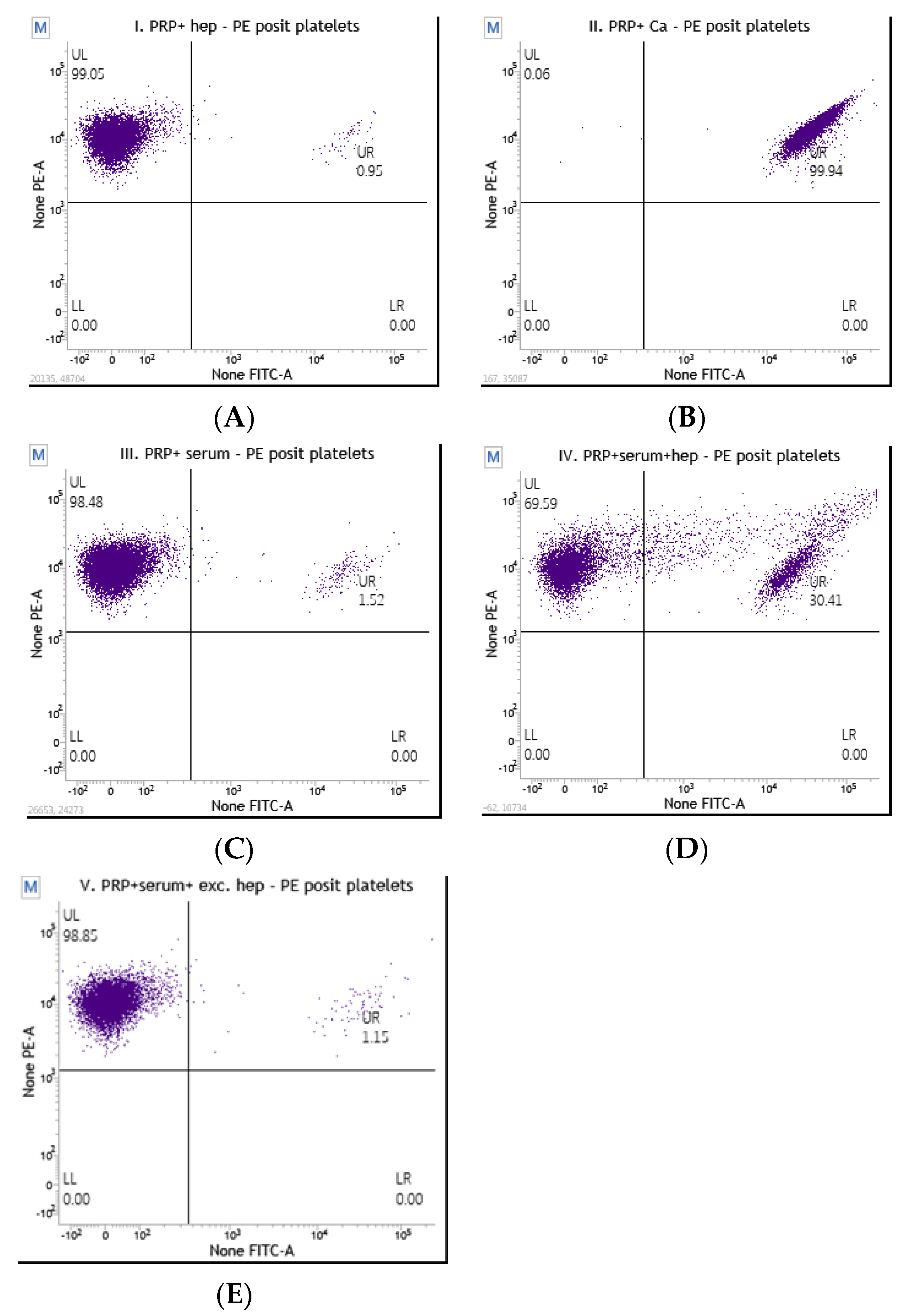
2.3.2. Flow Cytometry Platelet Activation Assay
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.1.1. Enrolled Patients
3.1.2. The Selected Number of Patients with a Detailed Clinical Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salter, B.S.; Weiner, M.M.; Trinh, M.A.; Heller, J.; Evans, A.S.; Adams, D.H.; Fischer, G.W. Heparin-induced Thrombocytopenia: A Comprehensive Clinical Review. J. Am. Coll. Cardiol. 2016, 67, 2519–2532. [Google Scholar] [CrossRef]
- Cuker, A.; Mark ACrowther, M.A. Clinical Practice Guideline on the Evaluation and Management of Adults with Suspected HeparinInduced Thrombocytopenia (HIT). Am. Soc. Hematol. 2013, 1, 1–4. [Google Scholar]
- Warkentin, T.E.; Greinacher, A. Heparin-induced thrombocytopenia: Recognition, treatment, and prevention: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 337. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Clinical practice. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Roberts, R.S.; Hirsh, J.; Kelton, J.G. An improved definition of immune heparin-induced thrombocytopenia in postoper-ative orthopedic patients. Arch. Intern. Med. 2003, 163, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced throm-bocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef]
- Cuker, A.; Gimotty, P.A.; Crowther, M.A.; Warkentin, T.E. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2012, 120, 4160–4167. [Google Scholar] [CrossRef]
- Cuker, A.; Cines, D.B. How I treat heparin-induced thrombocytopenia. Blood 2012, 119, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.M.; Chong, B.H.; Cines, D.B.; Greinacher, A.; Gruel, Y.; Linkins, L.A.; Rodner, S.B.; Selleng, S.; Warkentin, T.E.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018, 2, 3360–3392. [Google Scholar] [CrossRef]
- Lee, J.; Spidlen, J.; Scheuermann, R.; Brinkman, R. MIFlowCyt: The minimum information about a Flow Cytometry Experiment. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A. A sensitive and specific functional flow cytometric assay for the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 1997, 98, 648–656. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Heddle, N.M. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr. Hematol. Rep. 2003, 2, 148–157. [Google Scholar]
- Tomer, A.; Masalunga, C.; Abshire, T.C. Determination of heparin-induced thrombocytopenia: A rapid flow cytometric assay for direct demonstration of antibodymediated platelet activation. Am. J. Hematol. 1999, 61, 53–61. [Google Scholar] [CrossRef]
- Greinacher, A.; Juhl, D.; Strobel, U.; Wessel, A.; Lubenow, N.; Selleng, K.; Eichler, P.; Warkentin, T.E. Heparin-induced thrombocytopenia: A prospective study on the incidence, plate-let-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J. Thromb. Haemost. 2007, 5, 1666. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A. Sensitivity and specificity of laboratory tests for the diagnosis of heparin- induced thrombocytopenia. Lab. Hematol. 1997, 3, 174–175. [Google Scholar]
- Warkentin, T.E.; Sheppard, J.I.; Raschke, R.; Greinacher, A. Performance characteristics of a rapid assay for antiPF4/heparin antibodies: The particle immunofiltration assay. J. Thromb. Haemost. 2007, 5, 2308–2310. [Google Scholar] [CrossRef] [PubMed]
- Runser, A.; Schaning, C.; Allemand, F.; Amiral, J. An Optimized and Standardized Rapid Flow Cytometry Functional Method for Heparin-Induced Thrombocytopenia. Biomedicines 2021, 9, 296. [Google Scholar] [CrossRef]
- Kuhn, M.; Vaughan, D. Yardstick: Tidy Characterizations of Model Performance. R Package Version 0.0.8. 2021. Available online: https://CRAN.R-project.org/package=yardstick (accessed on 15 June 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 June 2023).
- Arepally, G.M. Heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Mutsando, H.; Self, M.; Morel-Kopp, M.-C.; Mollee, P. Using HitAlert flow cytometry to detect heparin-induced thrombocytopenia antibodies in a tertiary care hospital. Blood Coagul. Fibrinolysis 2013, 24, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Laboratory tests for identification or exclusion of heparin induced thrombocytopenia: HIT or miss? Am. J. Hematol. 2018, 93, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.; Cook, D.; Guyatt, G.; Zytaruk, N.; McDonald, E.; Williamson, D.; Albert, M.; Dodek, P.; Finfer, S.; Vallance, S.; et al. Heparin-induced thrombocytopenia in the critically ill: Interpreting the 4Ts test in a randomized trial. J. Crit. Care 2014, 29, 470.e7–470.e15. [Google Scholar] [CrossRef]
- Linkins, L.A.; Bates, S.M.; Lee, A.Y.; Heddle, N.M.; Wang, G.; Warkentin, T.E. Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of hepa-rin-induced thrombocytopenia: Prospective cohort study. Blood 2015, 126, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Chittal, A.R.; Kumar, A.; Rao, S.J.; Lakra, P.; Nacu, N. Arterial and Venous Thrombosis From Delayed-Onset Heparin-Induced Thrombocytopenia. J. Community Hosp. Intern. Med. Perspect. 2022, 12, 102–106. [Google Scholar] [CrossRef]
- Hogan, M.; Berger, J.S. Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc. Med. 2020, 25, 160–173. [Google Scholar] [CrossRef]
- Hvas, A.-M.; Favaloro, E.J.; Hellfritzsch, M. Heparin-induced thrombocytopenia: Pathophysiology, diagnosis and treatment. Expert Rev. Hematol. 2021, 14, 335–346. [Google Scholar] [CrossRef]
- Sartori, M.; Cosmi, B. Heparin-Induced Thrombocytopenia and COVID-19. Hematol. Rep. 2021, 13, 8857. [Google Scholar] [CrossRef]
- Tardy-Poncet, B.; Montmartin, A.; Piot, M.; Alhenc-Gelas, M.; Nguyen, P.; Elalamy, I.; Greinacher, A.; De Maistre, E.; Lasne, D.; Horellou, M.-H.; et al. Functional Flow Cytometric Assay for Reliable and Convenient Heparin-Induced Thrombocytopenia Diagnosis in Daily Practice. Biomedicines 2021, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Bauer, S.; Immohr, M.B.; Hermsen, D.F.; Westenfeld, R.; Boeken, U.; Aubin, H.; Tudorache, I.; Lichtenberg, A.; Akhyari, P. Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock. J. Cardiovasc. Dev. Dis. 2021, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Minet, V.; Dogné, J.-M.; Mullier, F. Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules 2017, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Smythe, M.A.; Ali, M.A.; Aslam, N.; Sheppard, J.A.; Smith, J.W.; Moore, J.C.; Arnold, D.M.; Nazy, I. Serotonin-release assay-positive but platelet factor 4-dependent en-zyme-immunoassay negative: HIT or not HIT? Am. J. Hematol. 2021, 96, 320–329. [Google Scholar] [CrossRef]
- Nicolas, D.; Nicolas, S.; Hodgens, A.; Reed, M. Heparin-Induced Thrombocytopenia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marchetti, M.; Barelli, S.; Zermatten, M.G.; Monnin-Respen, F.; Matthey-Guirao, E.; Nicolas, N.; Gomez, F.; Goodyer, M.; Gerschheimer, C.; Alberio, L. Rapid and accurate Bayesian diagnosis of heparin-induced thrombocytopenia. Blood 2020, 135, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Sex | Year of Birth | Thrombosis | 4Ts Score | ID-PaGIA | HemosIL Acustar IgG (Cut Off =1 U/mL) | ELISA | SRA | HITAlert |
| 1 | M | 1979 | VT | 8 | POSITIVE | 41.86 | POSITIVE | POSITIVE | POSITIVE |
| 2 | F | 1942 | no | 7–8 | POSITIVE | 8.81 | POSITIVE | POSITIVE | POSITIVE |
| 3 | M | 1975 | AT | 7–8 | POSITIVE | 21.6 | POSITIVE | POSITIVE | POSITIVE |
| 4 | M | 1949 | 7–8 | POSITIVE | 28.57 | POSITIVE | POSITIVE | POSITIVE | |
| 5 | F | 1950 | VT | 7 | POSITIVE | 1.13 | POSITIVE | POSITIVE | NEGATIVE |
| 6 | M | 1953 | no | 4–6 | POSITIVE | 0.76 | POSITIVE | POSITIVE | POSITIVE |
| 7 | F | 1938 | no | 5 | POSITIVE | 5.83 | POSITIVE | ||
| 8 | F | 1954 | no | 5 | POSITIVE | 33.97 | POSITIVE | POSITIVE | POSITIVE |
| 9 | M | 1949 | no | 5 | POSITIVE | 5.5 | POSITIVE | POSITIVE | POSITIVE |
| 10 | M | 1984 | AT | 5 | POSITIVE | 1.94 | POSITIVE | POSITIVE | NEGATIVE |
| 11 | M | 1963 | no | 5 | POSITIVE | 2.77 | POSITIVE | POSITIVE | NEGATIVE |
| 12 | M | 1959 | no | 5 | POSITIVE | 2.4 | POSITIVE | POSITIVE | NEGATIVE |
| 13 | M | 1973 | 5 | POSITIVE | 2.66 | NEGATIVE | |||
| 14 | M | 1962 | VT | 4–5 | POSITIVE | 22.3 | POSITIVE | POSITIVE | NEGATIVE |
| 15 | M | 2002 | no | 4–5 | POSITIVE | 0.99 | POSITIVE | NEGATIVE | NEGATIVE |
| 16 | F | 1953 | no | 4 | POSITIVE | 53.67 | POSITIVE | POSITIVE | POSITIVE |
| 17 | M | 1931 | no | 4 | POSITIVE | 1.9 | POSITIVE | POSITIVE | POSITIVE |
| 18 | F | 1969 | no | 4 | POSITIVE | 1.36 | POSITIVE | POSITIVE | POSITIVE |
| 19 | F | 1950 | no | 4 | POSITIVE | 25.99 | POSITIVE | POSITIVE | POSITIVE |
| 20 | F | 1941 | no | 3 | POSITIVE | 2.66 | POSITIVE | POSITIVE | POSITIVE |
| 21 | F | 1940 | no | 4 | POSITIVE | 22.6 | POSITIVE | POSITIVE | POSITIVE |
| 22 | M | 1953 | no | 4 | POSITIVE | 16.4 | POSITIVE | POSITIVE | POSITIVE |
| 23 | M | 1957 | 4 | POSITIVE | 31.67 | POSITIVE | POSITIVE | POSITIVE | |
| 24 | M | 1943 | no | 3–4 | weak positive | 16.86 | POSITIVE | POSITIVE | POSITIVE |
| 25 | M | 1964 | no | 3–4 | POSITIVE | 2.51 | POSITIVE | POSITIVE | NEGATIVE |
| 26 | M | 1953 | no | 2 | POSITIVE | 3.15 | POSITIVE | NEGATIVE | NEGATIVE |
| 27 | M | 1937 | POSITIVE | POSITIVE | POSITIVE | ||||
| 28 | M | 1990 | POSITIVE | POSITIVE | POSITIVE | ||||
| 29 | F | 1942 | no | POSITIVE | 0.95 | POSITIVE | POSITIVE | NEGATIVE | |
| 30 | F | 1954 | POSITIVE | POSITIVE | NEGATIVE | ||||
| (B) | |||||||||
| No. | Sex M/F | Count | Year of Birth Median (SD) | ID-PaGIA | HITAlert | ||||
| 31–50 | M | 8/20 | 1951 (10.5) | POSITIVE | POSITIVE | ||||
| F | 12/20 | 1951 (11.7) | |||||||
| 52–99 | M | 29/49 | 1958 (14.3) | POSITIVE | NEGATIVE | ||||
| F | 20/49 | 1946 (11.0) | |||||||
| 101–114 | M | 10/15 | 1952 (18.7) | weak positive | NEGATIVE | ||||
| F | 5/15 | 1953 (14.0) | |||||||
| 116–122 | M | 4/8 | 1951 (9.2) | NEGATIVE | NEGATIVE | ||||
| F | 4/8 | 1956 (26.4) | |||||||
| Patient Category | PaGIA | HemosIL Acustar IgG (Cut Off = 1 U/mL) | ELISA | SRA | HITAlert |
|---|---|---|---|---|---|
| Total number tested (n) | 122 | 30 | 25 | 25 | 122 |
| Positive | 98 | 27 | 25 | 23 | 39 |
| Negative | 8 | 3 | 0 | 2 | 83 |
| Equivocal (or weak positive) | 16 | 0 | 0 | 0 | 0 |
| Not tested from the total number 122 patients | 0 | 92 | 97 | 97 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skornova, I.; Simurda, T.; Stanciakova, L.; Lauko, V.; Holly, P.; Samos, M.; Bolek, T.; Schnierer, M.; Drotarova, M.; Belakova, K.M.; et al. A Functional Assay for the Determination of Heparin-Induced Thrombocytopenia via Flow Cytometry. Diagnostics 2023, 13, 3019. https://doi.org/10.3390/diagnostics13183019
Skornova I, Simurda T, Stanciakova L, Lauko V, Holly P, Samos M, Bolek T, Schnierer M, Drotarova M, Belakova KM, et al. A Functional Assay for the Determination of Heparin-Induced Thrombocytopenia via Flow Cytometry. Diagnostics. 2023; 13(18):3019. https://doi.org/10.3390/diagnostics13183019
Chicago/Turabian StyleSkornova, Ingrid, Tomas Simurda, Lucia Stanciakova, Viliam Lauko, Pavol Holly, Matej Samos, Tomas Bolek, Martin Schnierer, Miroslava Drotarova, Kristina Maria Belakova, and et al. 2023. "A Functional Assay for the Determination of Heparin-Induced Thrombocytopenia via Flow Cytometry" Diagnostics 13, no. 18: 3019. https://doi.org/10.3390/diagnostics13183019
APA StyleSkornova, I., Simurda, T., Stanciakova, L., Lauko, V., Holly, P., Samos, M., Bolek, T., Schnierer, M., Drotarova, M., Belakova, K. M., Sokol, J., Stasko, J., Mokan, M., Gumulec, J., & Chrastinova, L. (2023). A Functional Assay for the Determination of Heparin-Induced Thrombocytopenia via Flow Cytometry. Diagnostics, 13(18), 3019. https://doi.org/10.3390/diagnostics13183019







