Factors Influencing Visual Acuity in Patients with Active Subfoveal Circumscribed Polypoidal Choroidal Vasculopathy and Changes in Imaging Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria and Anti-VEGF Treatment
2.2. Clinical Measurements
2.3. Statistical Analyses
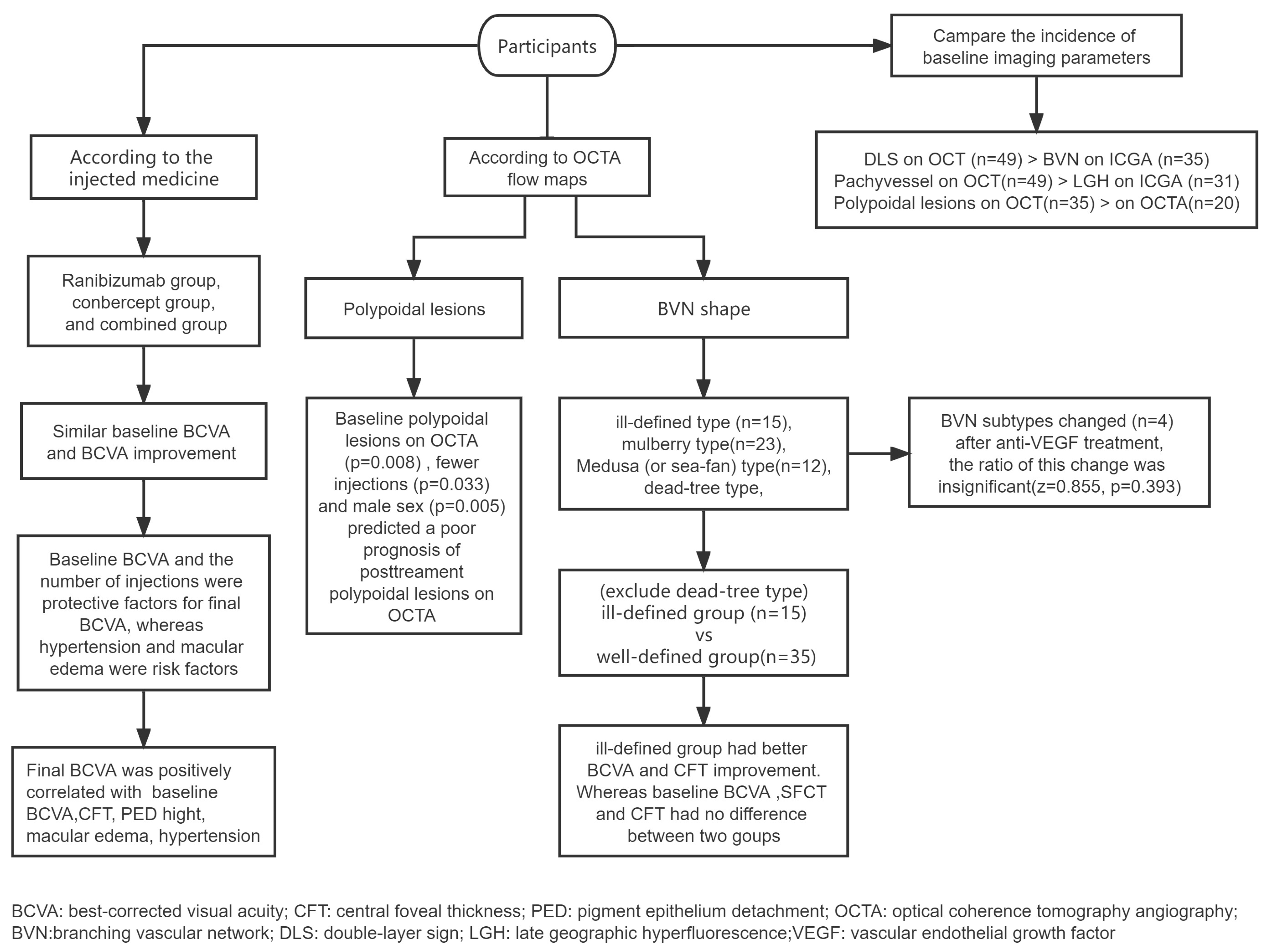
3. Results
3.1. General Patient Information
3.2. Variation and Correlation of Imaging Parameters
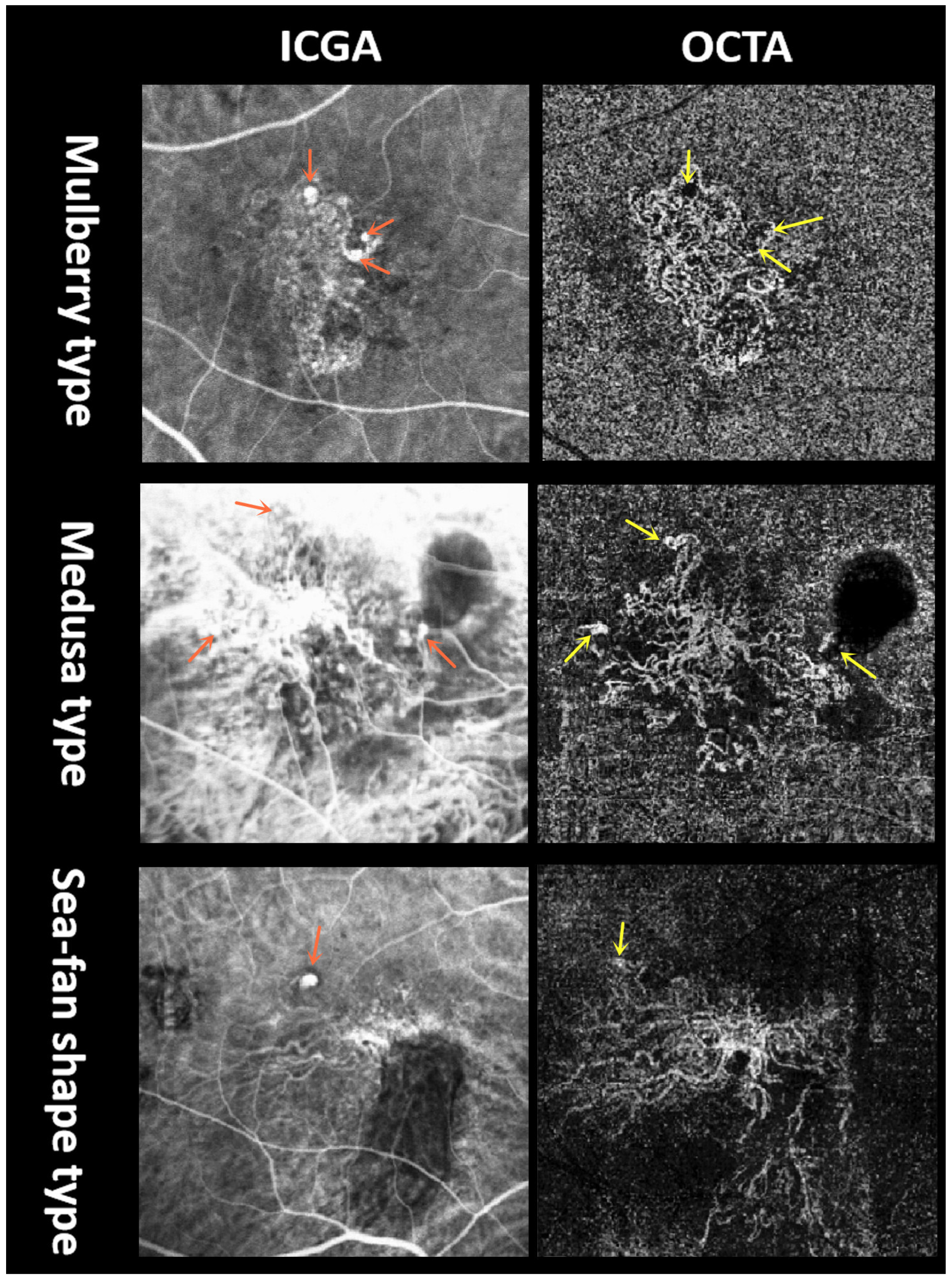
3.2.1. BVN Shape on OCTA
3.2.2. Polypoidal Lesions on OCTA
3.2.3. Changes in OCT-Related Parameters
3.3. Comparison of the Incidence of Imaging Parameters at Baseline
4. Discussion
4.1. Factors That Influence the Prognosis of BCVA
4.2. Variation and Correlation of Imaging Parameters
4.3. Comparison of the Incidence of Different Imaging Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCV | polypoidal choroidal vasculopathy |
| nAMD | neovascular age-related macular degeneration |
| BVN | branching vascular network |
| ICGA | indocyanine green angiography |
| OCT | optical coherence tomography |
| OCTA | optical coherence tomography angiography |
| VEGF | vascular endothelial growth factor |
| FA | fluorescein angiography |
| PRN | pro re nata |
| PED | pigment epithelium detachment |
| SRF | subretinal fluid |
| BCVA | best-corrected visual acuity |
| LGH | late geographic hyperfluorescence |
| SFCT | subfoveal choroidal thickness |
| DLS | double-layer sign |
| CFT | central foveal thickness |
| PLs | polypoidal lesions |
| CNV | choroidal neovascularization |
References
- Fenner, B.J.; Cheung, C.M.G.; Sim, S.S.; Lee, W.K.; Staurenghi, G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Kokame, G.; Yanagi, Y.; Teo, K.Y.C. Evolving treatment paradigms for PCV. Eye 2021, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.K.; Gal-Or, O.; Sadda, S.R.; Yannuzzi, L.A.; Freund, K.B. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): A lesson in the taxonomy of ‘expanded spectra’—A review. Clin. Exp. Ophthalmol. 2018, 46, 189–200. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Baek, J.; Dansingani, K.K.; Lee, J.H.; Freund, K.B. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina 2016, 36 (Suppl. S1), S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Ngo, W.K.; Chen, J.P.; Tan, N.W.; Lim, T.H. EVEREST study report 2: Imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2015, 99, 624–628. [Google Scholar] [CrossRef]
- Vyas, C.H.; Cheung, C.M.G.; Tan, C.; Chee, C.; Wong, K.; Jordan-Yu, J.M.N.; Wong, T.Y.; Tan, A.; Fenner, B.; Sim, S.; et al. Multicentre, randomised clinical trial comparing intravitreal aflibercept monotherapy versus aflibercept combined with reduced-fluence photodynamic therapy (RF-PDT) for the treatment of polypoidal choroidal vasculopathy. BMJ Open 2021, 11, e050252. [Google Scholar] [CrossRef]
- Koizumi, H.; Yamagishi, T.; Yamazaki, T.; Kinoshita, S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am. J. Ophthalmol. 2013, 155, 305–313. [Google Scholar] [CrossRef]
- Augsburger, M.; Sarra, G.M.; Imesch, P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: A comparative study. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1889–1895. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Luo, M.Y.; Meng, L.H.; Zhang, W.F.; Li, B.; Wang, E.Q.; Liu, S.Z.; Yu, W.H.; Chen, Y.X. The Incidence, Characteristics, Management, Prognosis, and Classification of Breakthrough Vitreous Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy. Retina 2021, 41, 1675–1685. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yu, W.; Chen, Y. Activation of quiescent polypoidal choroidal vasculopathy after membrane peeling vitrectomy for epiretinal membrane: A case report. BMC Ophthalmol. 2021, 21, 321. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kobayashi, K.; Aoki, S.; Miyamoto, H.; Imaizumi, H. Comparative analysis of polypoidal choroidal vasculopathy with and without hemorrhage treated by anti-VEGF monotherapy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Schworm, B.; Luft, N.; Keidel, L.F.; Herold, T.R.; Wolf, A.; Priglinger, S.G.; Siedlecki, J. Ranibizumab non-response in pachychoroid neovasculopathy: Effects of switching to aflibercept. Sci. Rep. 2020, 10, 8439. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lai, T.Y.Y.; Teo, K.; Ruamviboonsuk, P.; Chen, S.J.; Kim, J.E.; Gomi, F.; Koh, A.H.; Kokame, G.; Jordan-Yu, J.M.; et al. Polypoidal Choroidal Vasculopathy: Consensus Nomenclature and Non-Indocyanine Green Angiograph Diagnostic Criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology 2021, 128, 443–452. [Google Scholar] [CrossRef]
- Bo, Q.; Yan, Q.; Shen, M.; Song, M.; Sun, M.; Yu, Y.; Rosenfeld, P.J.; Wang, F.; Sun, X. Appearance of Polypoidal Lesions in Patients With Polypoidal Choroidal Vasculopathy Using Swept-Source Optical Coherence Tomographic Angiography. JAMA Ophthalmol. 2019, 137, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Coscas, G.J.; Lupidi, M.; Coscas, F.; Cagini, C.; Souied, E.H. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: A New Diagnostic Challenge. Retina 2015, 35, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Karacorlu, M.; Sayman Muslubas, I.; Arf, S.; Hocaoglu, M.; Ersoz, M.G. Membrane patterns in eyes with choroidal neovascularization on optical coherence tomography angiography. Eye 2019, 33, 1280–1289. [Google Scholar] [CrossRef]
- Huang, Z.; Ding, Q.; Yan, M.; Lian, H.; Chen, Z.; Chen, X.; Song, Y. Short-term efficacy of conbercept and ranibizumab for polypoidal choroidal vasculopathy. Retina 2019, 39, 889–895. [Google Scholar] [CrossRef]
- Koh, A.; Lai, T.Y.Y.; Wei, W.B.; Mori, R.; Wakiyama, H.; Park, K.H.; Ngah, F.; Macfadden, W.; Dunger-Baldauf, C.; Parikh, S. Real-world effectiveness and safety of ranibizumab treatment in patients with and without polypoidal choroidal vasculopathy: Twelve-month results from the LUMINOUS Study. Retina 2020, 40, 1529–1539. [Google Scholar] [CrossRef]
- Gupta, P.; Ting, D.S.W.; Thakku, S.G.; Wong, T.Y.; Cheng, C.Y.; Wong, E.; Mathur, R.; Wong, D.; Yeo, I.; Gemmy Cheung, C.M. Detailed characterization of choroidal morphologic and vascular features in age-related macular degeneration and polypoidal choroidal vasculopathy. Retina 2017, 37, 2269–2280. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Deak, G.G.; Kundi, M.; Simader, C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 2015, 122, 822–832. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tsujikawa, A.; Mizukami, S.; Miyoshi, N.; Yoshimura, N. Cystoid macular edema in polypoidal choroidal vasculopathy viewed by a scanning laser ophthalmoscope: CME in PCV viewed by SLO. Int. Ophthalmol. 2009, 29, 503–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, C.M.G.; Tan, C.S.; Patalauskaite, R.; Margaron, P.; Lai, T.Y.Y. Ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: Predictors of Visual and Anatomical Response in the EVEREST II Study. Retina 2021, 41, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Duan, J.; Zhang, M. Pachychoroid Spectrum Disease: Underlying Pathology, Classification, and Phenotypes. Curr. Eye Res. 2021, 46, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Martin, D.F.; Toth, C.A.; Daniel, E.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Huang, J. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2013, 120, 1860–1870. [Google Scholar] [CrossRef]
- Li, X.; Qu, J.; Su, G.; Yu, S.; Zhang, Y.; Sadda, S.V.; Group, S.S. The comparison of two different strategies of intravitreal conbercept for polypoidal choroidal vasculopathy in Chinese patients results from a 48-week randomized phase 4 study: STAR study. Acta Ophthalmol. 2023, 101, e327–e337. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.C.; Kwon, K.Y.; Lee, J.H.; Koh, H.J.; Byeon, S.H.; Kim, S.S.; Kim, M.; Lee, C.S. Subfoveal choroidal thickness as a predictor of treatment response to anti-vascular endothelial growth factor therapy for polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1497–1503. [Google Scholar] [CrossRef]
- Fan, D.; Hua, R. Different imaging characteristics between unilateral and bilateral polypoidal choroidal vasculopathy. Photodiagnosis Photodyn. Ther. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Minami, S.; Kurihara, T.; Kamoshita, M.; Sonobe, H.; Watanabe, K.; Uchida, A.; Shinoda, H.; Tsubota, K.; et al. Dynamic changes in choroidal conditions during anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Sci. Rep. 2019, 9, 11389. [Google Scholar] [CrossRef]
- Liu, K.; Lai, T.Y.; Chiang, S.W.; Chan, V.C.; Young, A.L.; Tam, P.O.; Pang, C.P.; Chen, L.J. Gender specific association of a complement component 3 polymorphism with polypoidal choroidal vasculopathy. Sci. Rep. 2014, 4, 7018. [Google Scholar] [CrossRef]
- Chang, C.J.; Huang, Y.M.; Hsieh, M.H.; Li, A.F.; Chen, S.J. Flow signal change in polyps after anti-vascular endothelial growth factor therapy. PLoS ONE 2020, 15, e0241230. [Google Scholar] [CrossRef]
- Spaide, R.F.; Ledesma-Gil, G.; Gemmy Cheung, C.M. Intervortex venous anastomosis in pachychoroid-related disorders. Retina 2021, 41, 997–1004. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kang, S.W.; Chung, S.E.; Kong, M.G.; Kim, J.H. Development of polypoidal choroidal vasculopathy in unaffected fellow eyes. Br. J. Ophthalmol. 2012, 96, 1217–1221. [Google Scholar] [CrossRef]
- Siedlecki, J.; Schworm, B.; Priglinger, S.G. The Pachychoroid Disease Spectrum-and the Need for a Uniform Classification System. Ophthalmol. Retina 2019, 3, 1013–1015. [Google Scholar] [CrossRef]
- Takayama, K.; Ito, Y.; Kaneko, H.; Kataoka, K.; Sugita, T.; Maruko, R.; Hattori, K.; Ra, E.; Haga, F.; Terasaki, H. Comparison of indocyanine green angiography and optical coherence tomographic angiography in polypoidal choroidal vasculopathy. Eye 2017, 31, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Yanagi, Y.; Mohla, A.; Lee, S.Y.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y. Characterization and differentiation of polypoidal choroidal vasculopathy using swept source optical coherence tomography angiography. Retina 2017, 37, 1464–1474. [Google Scholar] [CrossRef]
- Hua, R.; Wang, H. Dark Signals in the Choroidal Vasculature on Optical Coherence Tomography Angiography: An Artefact or Not? J. Ophthalmol. 2017, 2017, 5498125. [Google Scholar] [CrossRef] [PubMed]
- Chong Teo, K.Y.; Sadda, S.R.; Gemmy Cheung, C.M.; Chakravarthy, U.; Staurenghi, G.; Invernizzi, A.; Ogura, Y.; Ruamviboonsuk, P.; Chen, S.J.; Gupta, V.; et al. Non-ICGA treatment criteria for Suboptimal Anti-VEGF Response for Polypoidal Choroidal Vasculopathy: APOIS PCV Workgroup Report 2. Ophthalmol. Retina 2021, 5, 945–953. [Google Scholar] [CrossRef] [PubMed]
| Variable | Baseline | Last Follow-Up | p Value |
|---|---|---|---|
| LogMAR BCVA | 0.70 (interquartile range: 0.40–1.00) | 0.60 (interquartile range: 0.40–1.00) | z = 3.093, p = 0.002 * |
| Macular edema (OCT) | 12/51 | 12/51 | z = 0.000, p = 1.000 |
| BVN (OCTA) | Ill-defined type, 15 eyes | Ill-defined type, 14 eyes | |
| Mulberry type, 23 eyes | Mulberry type, 20 eyes | z = 0.855, p = 0.393 | |
| Medusa or sea-fan shape type, 12 eyes | Medusa or sea-fan shape type, 14 eyes | ||
| Dead tree type, 1 eye | Dead tree type, 3 eyes |
| Variable | Ill-Defined Group (n = 15) | Well-Defined Group (n = 35) | p |
|---|---|---|---|
| Age, years | 66.00 [interquartile range: 62.00 to 73.00] | 64.00 [interquartile range: 61.00 to 70.00] | 0.379 |
| Gender, male/female | Male = 6/9 | Male = 21/14 | 0.193 |
| The number of injections (n) | 5 [interquartile range: 4 to 7] | 5 [interquartile range: 4 to9] | 0.522 |
| Baseline BCVA, logMAR | 0.70 [interquartile range: 0.30 to 1.22] | 0.70 [interquartile range: 0.52 to 1.00] | 0.907 |
| Baseline SFCT, μm | 356.00 [interquartile range: 187.00 to 389.00] | 259.00 [interquartile range: 191.00 to 360.00] | 0.244 |
| Baseline CFT, μm | 446.00 [interquartile range: 376.00 to 722.00] | 424.00 [interquartile range: 307.00 to 598.00] | 0.295 |
| Baseline SRF, μm | 128.00 [interquartile range: 0.00 to 297.00] | 145.00 [interquartile range: 0.00 to 257.00] | 0.923 |
| Baseline PED height, μm | 41.00 [interquartile range: 0.00 to 114.00] | 63.00 [interquartile range: 0.00 to 252.00] | 0.418 |
| BCVA improvement, logMAR | −0.18 [interquartile range: −0.40 to 0.00] | 0.00 [interquartile range: −0.15 to 0.00] | 0.032 * |
| SFCT improvement, μm | 21.00 [interquartile range: −26.00 to 80.00] | 4.00 [interquartile range: −34.00 to 38.00] | 0.295 |
| CFT improvement, μm | 211.00 [interquartile range: 73.00 to 305.00] | 68.00 [interquartile range: −14.00 to 189.00] | 0.018 * |
| SRF improvement, μm | 84.00 [interquartile range: 0.00 to 259.00] | 0.00 [interquartile range: 0.00 to 110.00] | 0.087 |
| PED height improvement, μm | 0.00 [interquartile range: 0.00 to 108.00] | 0.00 [interquartile range: 0.00 to 32.00] | 0.500 |
| B | OR | 95%CI | p | |
|---|---|---|---|---|
| Age | 0.050 | 1.051 | 0.962–1.148 | 0.274 |
| Sex | 2.577 | 13.153 | 2.187–79.100 | 0.005 * |
| PLs on OCTA at baseline | 2.364 | 10.637 | 1.840–61.485 | 0.008 * |
| The number of injections | −0.412 | 0.663 | 0.454–0.967 | 0.033 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, F.; Xing, P.; Zhang, H.; Niu, T.; Wang, Q.; Hua, R. Factors Influencing Visual Acuity in Patients with Active Subfoveal Circumscribed Polypoidal Choroidal Vasculopathy and Changes in Imaging Parameters. Diagnostics 2023, 13, 3017. https://doi.org/10.3390/diagnostics13183017
Xia F, Xing P, Zhang H, Niu T, Wang Q, Hua R. Factors Influencing Visual Acuity in Patients with Active Subfoveal Circumscribed Polypoidal Choroidal Vasculopathy and Changes in Imaging Parameters. Diagnostics. 2023; 13(18):3017. https://doi.org/10.3390/diagnostics13183017
Chicago/Turabian StyleXia, Fan, Peiyu Xing, Hao Zhang, Tongtong Niu, Qi Wang, and Rui Hua. 2023. "Factors Influencing Visual Acuity in Patients with Active Subfoveal Circumscribed Polypoidal Choroidal Vasculopathy and Changes in Imaging Parameters" Diagnostics 13, no. 18: 3017. https://doi.org/10.3390/diagnostics13183017
APA StyleXia, F., Xing, P., Zhang, H., Niu, T., Wang, Q., & Hua, R. (2023). Factors Influencing Visual Acuity in Patients with Active Subfoveal Circumscribed Polypoidal Choroidal Vasculopathy and Changes in Imaging Parameters. Diagnostics, 13(18), 3017. https://doi.org/10.3390/diagnostics13183017







