Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study
Abstract
:1. Introduction
Problem Statement
2. Materials and Methods
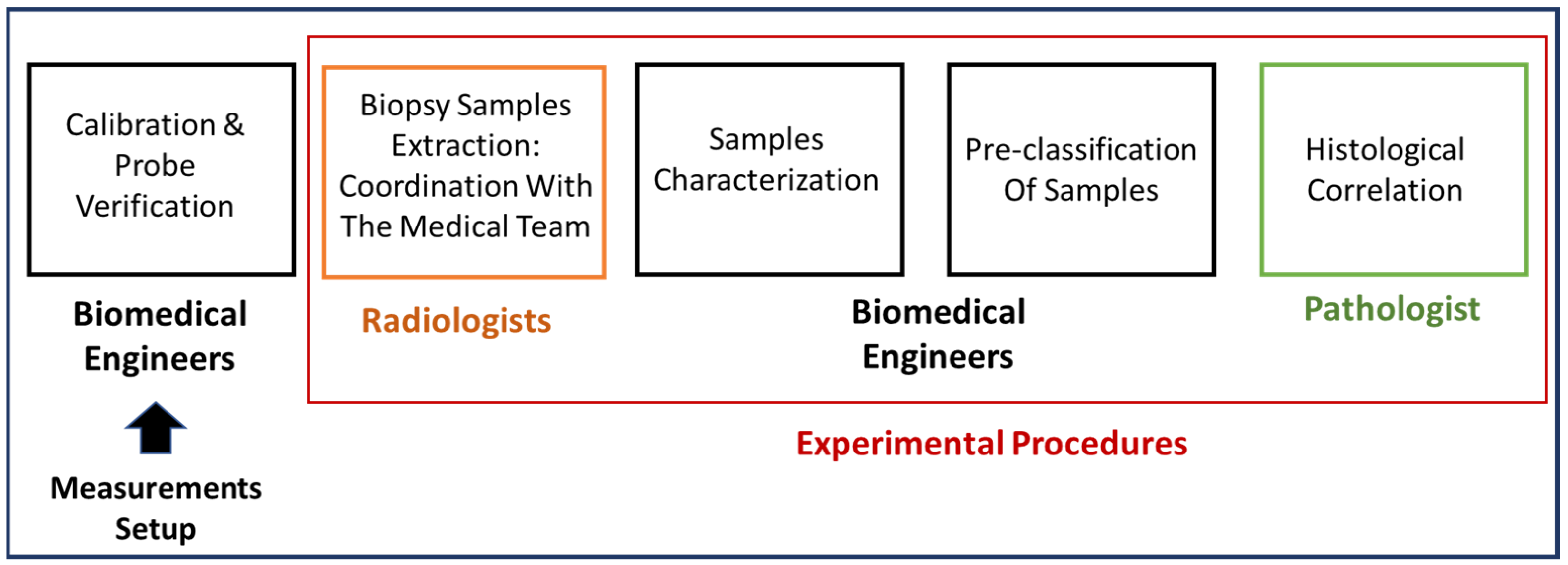
2.1. The Clinical Investigation
2.2. Dielectric Properties Acquisition Set-Up
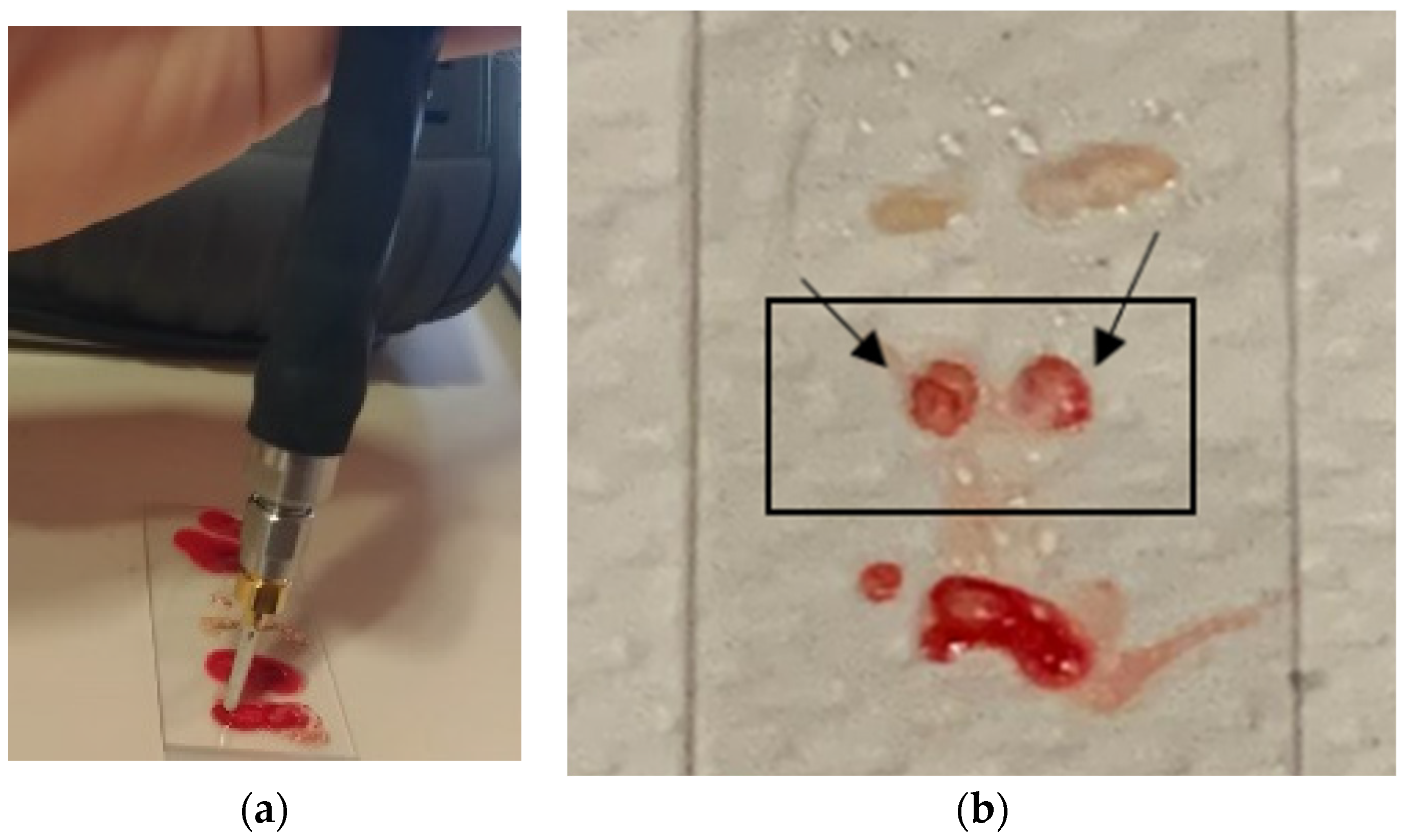
2.3. Experimental Procedure
3. Results and Discussion
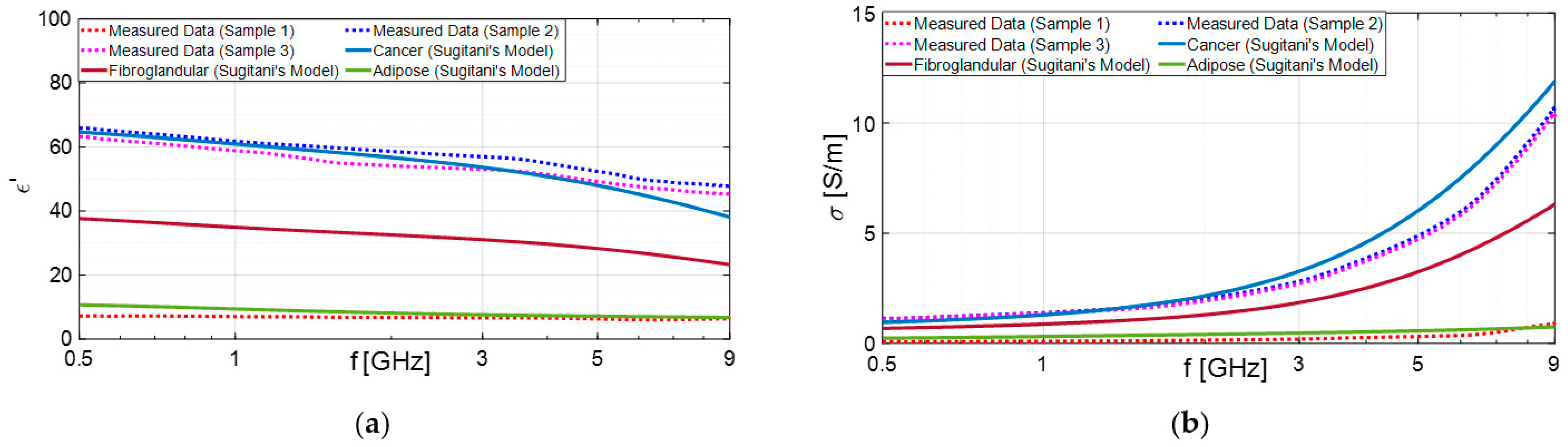
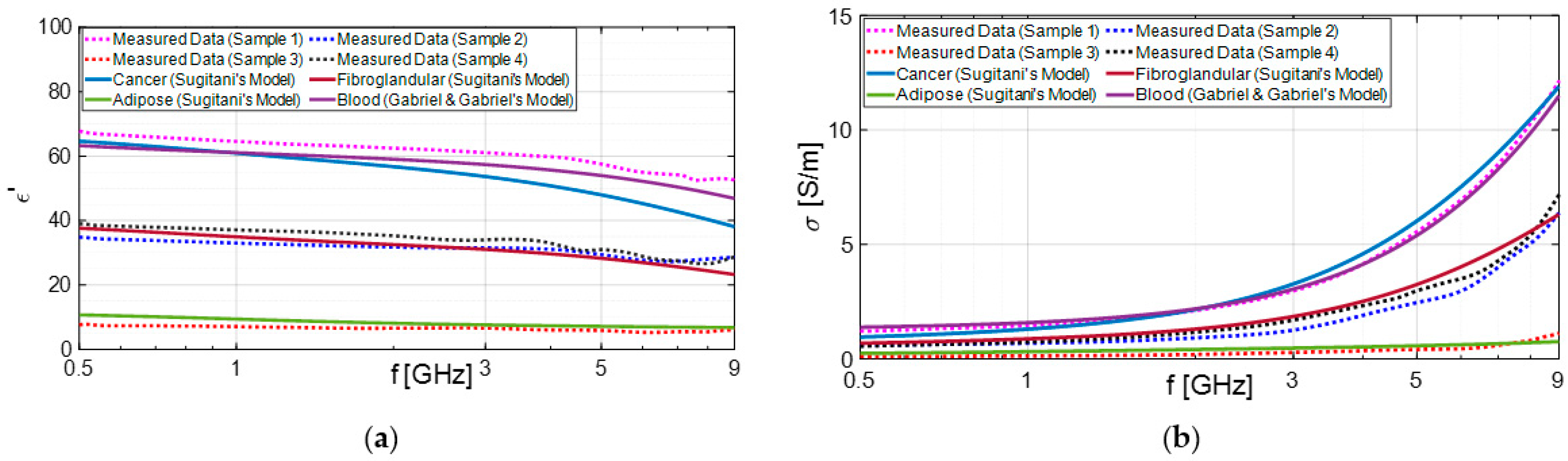
3.1. Experimental Results
3.2. Statistical Analysis
3.3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; Hannigan, A.; O’Brien, K.M. Mortality Reductions Due to Mammography Screening: Contemporary Population-Based Data. PLoS ONE 2017, 12, e0188947. [Google Scholar] [CrossRef]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E. The Impact of Mammographic Screening on Breast Cancer Mortality in Europe: A Review of Observational Studies. J. Med. Screen 2012, 19, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Kaucher, S.; Lorenz, E.; Bärnighausen, T.; Winkler, V. Development of Breast Cancer Mortality Considering the Implementation of Mammography Screening Programs—A Comparison of Western European Countries. BMC Public Health 2019, 19, 823. [Google Scholar] [CrossRef] [PubMed]
- Castellano, C.R.; Aguilar Angulo, P.M.; Hernández, L.C.; González-Carrato, P.S.-C.; González, R.G.; Alvarez, J.; Chacón, J.I.; Ruiz, J.; Fuentes Guillén, M.Á.; Gutiérrez Ávila, G. Breast Cancer Mortality after Eight Years of an Improved Screening Program Using Digital Breast Tomosynthesis. J. Med. Screen 2021, 28, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical Characteristics of Different Histologic Types of Breast Cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef]
- Zaha, D.C. Significance of Immunohistochemistry in Breast Cancer. World J. Clin. Oncol. 2014, 5, 382. [Google Scholar] [CrossRef]
- Bevers, T.B.; Anderson, B.O.; Bonaccio, E.; Buys, S.; Daly, M.B.; Dempsey, P.J.; Farrar, W.B.; Fleming, I.; Garber, J.E.; Harris, R.E.; et al. Breast Cancer Screening and Diagnosis. J. Natl. Compr. Cancer Netw. 2009, 7, 1060–1096. [Google Scholar] [CrossRef]
- O’Flynn, E.A.M.; Wilson, A.R.M.; Michell, M.J. Image-Guided Breast Biopsy: State-of-the-Art. Clin. Radiol. 2010, 65, 259–270. [Google Scholar] [CrossRef]
- Schueller, G.; Schueller-Weidekamm, C.; Helbich, T.H. Accuracy of Ultrasound-Guided, Large-Core Needle Breast Biopsy. Eur. Radiol. 2008, 18, 1761–1773. [Google Scholar] [CrossRef]
- Liberman, L. Percutaneous Image-Guided Core Breast Biopsy. Radiol. Clin. North Am. 2002, 40, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Helbich, T.H.; Matzek, W.; Fuchsjäger, M.H. Stereotactic and Ultrasound-Guided Breast Biopsy. Eur. Radiol. 2004, 14, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari Dowlatabad, H.; Mamdouh, A.; Yousefpour, N.; Mahdavi, R.; Zandi, A.; Hoseinpour, P.; Moosavi-Kiasari, S.M.S.; Abbasvandi, F.; Kordehlachin, Y.; Parniani, M.; et al. High-Frequency (30 MHz–6 GHz) Breast Tissue Characterization Stabilized by Suction Force for Intraoperative Tumor Margin Assessment. Diagnostics 2023, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Jilani, M.T.; Zaka, M.; Rehman, A.M.; Khan, M.T.; Khan, S.; Muzamil, A. A Brief Review of Measuring Techniques for Characterization of Dielectric Materials. Int. J. Inf. Technol. Electr. Eng. 2012, 1, 1–5. [Google Scholar]
- Costa, F.; Borgese, M.; Degiorgi, M.; Monorchio, A. Electromagnetic Characterisation of Materials by Using Transmission/Reflection (T/R) Devices. Electronics 2017, 6, 95. [Google Scholar] [CrossRef]
- Marsland, T.P.; Evans, S. Dielectric Measurements with an Open-Ended Coaxial Probe. IEE Proc. H Microw. Antennas Propag. 1987, 134, 341. [Google Scholar] [CrossRef]
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef]
- Fontana, N.; Canicatti, E.; Monorchio, A. An Application of the Virtual Transmission Line Model of an Open-Ended Coaxial Probe for Dielectric Properties Characterization of Biological Tissues. In Proceedings of the 2019 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 341–342. [Google Scholar]
- Guardiola, M.; Buitrago, S.; Fernández-Esparrach, G.; O’Callaghan, J.M.; Romeu, J.; Cuatrecasas, M.; Córdova, H.; González Ballester, M.Á.; Camara, O. Dielectric Properties of Colon Polyps, Cancer, and Normal Mucosa: Ex Vivo Measurements from 0.5 to 20 GHz. Med. Phys. 2018, 45, 3768–3782. [Google Scholar] [CrossRef]
- Schepps, J.L.; Foster, K.R. The UHF and Microwave Dielectric Properties of Normal and Tumour Tissues: Variation in Dielectric Properties with Tissue Water Content. Phys. Med. Biol. 1980, 25, 1149–1159. [Google Scholar] [CrossRef]
- O’Rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric Properties of Human Normal, Malignant and Cirrhotic Liver Tissue: In Vivo and Ex Vivo Measurements from 0.5 to 20 GHz Using a Precision Open-Ended Coaxial Probe. Phys. Med. Biol. 2007, 52, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The Measured Electrical Properties of Normal and Malignant Human Tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; McCartney, L.; Beasley, C.; Lazebnik, M.; Okoniewski, M.; Hagness, S.C.; Booske, J.H. Precision Open-Ended Coaxial Probes for in Vivo and Ex Vivo Dielectric Spectroscopy of Biological Tissues at Microwave Frequencies. IEEE Trans. Microw. Theory Tech. 2005, 53, 1713–1722. [Google Scholar] [CrossRef]
- Onemli, E.; Joof, S.; Aydinalp, C.; Pastacı Özsobacı, N.; Ateş Alkan, F.; Kepil, N.; Rekik, I.; Akduman, I.; Yilmaz, T. Classification of Rat Mammary Carcinoma with Large Scale in Vivo Microwave Measurements. Sci. Rep. 2022, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Okoniewski, M.; Booske, J.H.; Hagness, S.C. Highly Accurate Debye Models for Normal and Malignant Breast Tissue Dielectric Properties at Microwave Frequencies. IEEE Microw. Wirel. Compon. Lett. 2007, 17, 822–824. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A Large-Scale Study of the Ultrawideband Microwave Dielectric Properties of Normal, Benign and Malignant Breast Tissues Obtained from Cancer Surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A Large-Scale Study of the Ultrawideband Microwave Dielectric Properties of Normal Breast Tissue Obtained from Reduction Surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef]
- Martellosio, A.; Bellomi, M.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L. Dielectric Properties Characterization From 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Campbell, A.M.; Land, D. V Dielectric Properties of Female Human Breast Tissue Measured in Vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193–210. [Google Scholar] [CrossRef]
- Hussein, M.; Awwad, F.; Jithin, D.; El Hasasna, H.; Athamneh, K.; Iratni, R. Breast Cancer Cells Exhibits Specific Dielectric Signature In Vitro Using the Open-Ended Coaxial Probe Technique from 200 MHz to 13.6 GHz. Sci. Rep. 2019, 9, 4681. [Google Scholar] [CrossRef]
- Choi, J.W.; Cho, J.; Lee, Y.; Yim, J.; Kang, B.; Keun Oh, K.; Hee Jung, W.; Jung Kim, H.; Cheon, C.; Lee, H.-D.; et al. Microwave Detection of Metastasized Breast Cancer Cells in the Lymph Node; Potential Application for Sentinel Lymphadenectomy. Breast Cancer Res. Treat. 2004, 86, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Kılıç, M.A.; Erdoğan, M.; Çayören, M.; Tunaoğlu, D.; Kurtoğlu, İ.; Yaslan, Y.; Çayören, H.; Arıkan, A.E.; Teksöz, S.; et al. Machine Learning Aided Diagnosis of Hepatic Malignancies through in Vivo Dielectric Measurements with Microwaves. Phys. Med. Biol. 2016, 61, 5089–5102. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Mobashsher, A.T.; Mohammed, B.; Foong, D.; Abbosh, A. Benign and Malignant Skin Lesions: Dielectric Characterization, Modelling and Analysis in Frequency Band 1 to 14 GHz. IEEE Trans. Biomed. Eng. 2023, 70, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T. Multiclass Classification of Hepatic Anomalies with Dielectric Properties: From Phantom Materials to Rat Hepatic Tissues. Sensors 2020, 20, 530. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, N.; Sánchez-Bayuela, D.Á.; Sani, L.; Vispa, A.; Bigotti, A.; Badia, M.; Papini, L.; Raspa, G.; Rana, S.P.; Romero Castellano, C.; et al. MammoWave Breast Imaging Device: An International and Multicentric Clinical Investigation. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023; pp. 1–5. [Google Scholar]
- Álvarez Sánchez-Bayuela, D.; Ghavami, N.; Tiberi, G.; Sani, L.; Vispa, A.; Bigotti, A.; Raspa, G.; Badia, M.; Papini, L.; Ghavami, M.; et al. A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Evaluate MammoWave’s Ability in Breast Lesions Detection. PLoS ONE 2023, 18, e0288312. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice (ISO Standard No. ISO 14155:2020). 2020. Available online: https://www.iso.org/standard/71690.html (accessed on 10 September 2021).
- International Organization for Standardization. Medical Devices—Application of Risk Management to Medical Devices (ISO Standard No. ISO 14971:2019). 2019. Available online: https://www.iso.org/standard/72704.html (accessed on 10 September 2021).
- Canicatti, E.; Brizi, D.; Monorchio, A.; Fontana, N.; Tiberi, G. Dielectric Characterization of Biological Samples by Using an Open-Ended Coaxial Probe. In Proceedings of the 2020 IEEE International Symposium on Antennas and Propagation and North American Radio Science Meeting, Montreal, QC, Canada, 5–10 July 2020; pp. 639–640. [Google Scholar]
- Canicatti, E.; Fontana, N.; Barmada, S.; Monorchio, A. Dielectric Characterization Improvement of Biopsy Samples Via a Coated Open-Ended Coaxial Probe. In Proceedings of the 2022 Microwave Mediterranean Symposium (MMS), Pizzo Calabro, Italy, 9–13 May 2022; pp. 1–4. [Google Scholar]
- Canicatti, E.; Sanchez-Bayuela, D.A.; Castellano, C.R.; Gonzalez, R.G.; Hernandez, L.M.C.; Angulo, P.M.A.; Martin, J.R.; Monorchio, A.; Tiberi, G. Dielectric Characterization of Small Breast Biopsy Via Miniaturized Open-Ended Coaxial Probe. In Proceedings of the 2022 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (AP-S/URSI), Denver, CO, USA, 10–15 July 2022; pp. 1332–1333. [Google Scholar]
- Otto, G.P.; Chew, W.C. Improved Calibration of a Large Open-Ended Coaxial Probe for Dielectric Measurements. IEEE Trans. Instrum. Meas. 1991, 40, 742–746. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Sugitani, T.; Kubota, S.; Kuroki, S.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex Permittivities of Breast Tumor Tissues Obtained from Cancer Surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: III. Parametric Models for the Dielectric Spectrum of Tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- O’Halloran, M.; Byrne, D.; Conceição, R.C.; Jones, E.; Glavin, M. Anatomy and Dielectric Properties of the Breast and Breast Cancer. In An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Cham, Switzerland, 2016; pp. 5–16. [Google Scholar]
- Kuhn, M.J.; Awida, M.; Mahfouz, M.R.; Fathy, A.E. Open-Ended Coaxial Probe Measurements for Breast Cancer Detection. In Proceedings of the 2010 IEEE Radio and Wireless Symposium (RWS), New Orleans, LA, USA, 10–14 January 2010; pp. 512–515. [Google Scholar]
- Lalkhen, A.G.; McCluskey, A. Clinical Tests: Sensitivity and Specificity. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 221–223. [Google Scholar] [CrossRef]
- Florkowski, C.M. Sensitivity, Specificity, Receiver-Operating Characteristic (ROC) Curves and Likelihood Ratios: Communicating the Performance of Diagnostic Tests. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S83–S87. [Google Scholar]
- Mulita, F.; Verras, G.I.; Anagnostopoulos, C.N.; Kotis, K. A Smarter Health through the Internet of Surgical Things. Sensors 2022, 22, 4577. [Google Scholar] [CrossRef]




| Subject ID | Number of Excised Tissue Samples | Number of Points Analyzed | Mammographic Lesion’s BI-RADS Score | Probe Rule-of-Thumb Output | Histological Outcomes |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | Both samples positive (P) | No evidence of neoplasia (Benign) |
| 2 | 6 | 11 | 4B | 3 positive (P) and 3 negative (N) samples | Invasive ductal carcinoma, luminal (Malignant) in all analyzed samples |
| 3a (Left breast, inner quadrant) | 4 | 10 | 4B | 1 positive (P) and 3 negative (N) samples | Fibrosis (Benign) in all analyzed samples |
| 3b (Left breast, outer quadrant) | 4 | 6 | 4B | 2 positive (P) and 2 negative (N) samples | Fibrosis (Benign) in all analyzed samples |
| 4 | 5 | 11 | 5 | All samples positive (P) | Invasive ductal carcinoma (with in situ component) grade II, Her2- |
| 5 | 3 | 7 | 5 | All samples positive (P) | Invasive ductal carcinoma grade II, luminal |
| 6 | 3 | 10 | 5 | All samples positive (P) | Invasive ductal carcinoma (with in situ component) grade II, Her2+ |
| 7 | 2 | 16 | 3 | All samples negative (N) | Stromal collagenization compatible with stromal hyperplasia |
| 8 | 3 | 6 | 3 | All samples negative (N) | Benign fibroepithelial lesion in all analyzed samples |
| 9 | 5 | 13 | 5 | All samples positive (P) | Invasive ductal carcinoma (with in situ component) grade II |
| 10 | 3 | 8 | 5 | 2 positive (P) and 1 negative (N) samples | Invasive ductal carcinoma grade II in (A), adipose tissue in (B) |
| 11 | 5 | 12 | 5 | All samples positive (P) | Invasive ductal carcinoma grade II, intermediate luminal |
| 12a (Right Breast) | 3 | 8 | 5 | 1 positive (P) and 2 negative (N) samples | Microinvasive carcinoma grade I, luminal A |
| 12b (Left Breast) | 3 | 6 | 5 | 2 positive (P) and 1 negative (N) samples | Microinvasive carcinoma grade I, luminal A |
| 13 | 7 | 20 | 4B | 2 positive (P) and 5 negative (N) samples | Adenosis and mastopathy in all analyzed samples |
| 14 | 3 | 7 | 4B | 2 positive (P) and 1 negative (N) samples | Invasive ductal carcinoma grade II, luminal A in all analyzed samples |
| 15 | 3 | 8 | 5 | 3 positive (P) samples | Fibroadenoma in all analyzed samples |
| Status of Patient According to “Gold Standard (Histological Assessment)” | |||
|---|---|---|---|
| Presence of Malignant Lesions | Absence of Malignant Lesions | ||
| Presented Rule-of-Thumb Output | Positive | TP 31 | FN 7 |
| Negative | FP 10 | TN 16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canicattì, E.; Sánchez-Bayuela, D.Á.; Romero Castellano, C.; Aguilar Angulo, P.M.; Giovanetti González, R.; Cruz Hernández, L.M.; Ruiz Martín, J.; Tiberi, G.; Monorchio, A. Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study. Diagnostics 2023, 13, 3015. https://doi.org/10.3390/diagnostics13183015
Canicattì E, Sánchez-Bayuela DÁ, Romero Castellano C, Aguilar Angulo PM, Giovanetti González R, Cruz Hernández LM, Ruiz Martín J, Tiberi G, Monorchio A. Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study. Diagnostics. 2023; 13(18):3015. https://doi.org/10.3390/diagnostics13183015
Chicago/Turabian StyleCanicattì, Eliana, Daniel Álvarez Sánchez-Bayuela, Cristina Romero Castellano, Paul Martín Aguilar Angulo, Rubén Giovanetti González, Lina Marcela Cruz Hernández, Juan Ruiz Martín, Gianluigi Tiberi, and Agostino Monorchio. 2023. "Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study" Diagnostics 13, no. 18: 3015. https://doi.org/10.3390/diagnostics13183015
APA StyleCanicattì, E., Sánchez-Bayuela, D. Á., Romero Castellano, C., Aguilar Angulo, P. M., Giovanetti González, R., Cruz Hernández, L. M., Ruiz Martín, J., Tiberi, G., & Monorchio, A. (2023). Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study. Diagnostics, 13(18), 3015. https://doi.org/10.3390/diagnostics13183015







