Quantitative Analysis for Lung Disease on Thin-Section CT
Abstract
1. Introduction
2. Overview of Densitometric Analyses
3. Technical Considerations for Densitometric Analyses
3.1. Radiation Dose
3.2. Reconstruction Methods
3.3. Spatial Resolution
4. CT Analysis in Chronic Obstructive Pulmonary Disease
4.1. Densitometry Analysis
4.2. Airway Assessment
4.3. Air Trapping on Expiratory CT
4.4. Functional Small Airway Disease
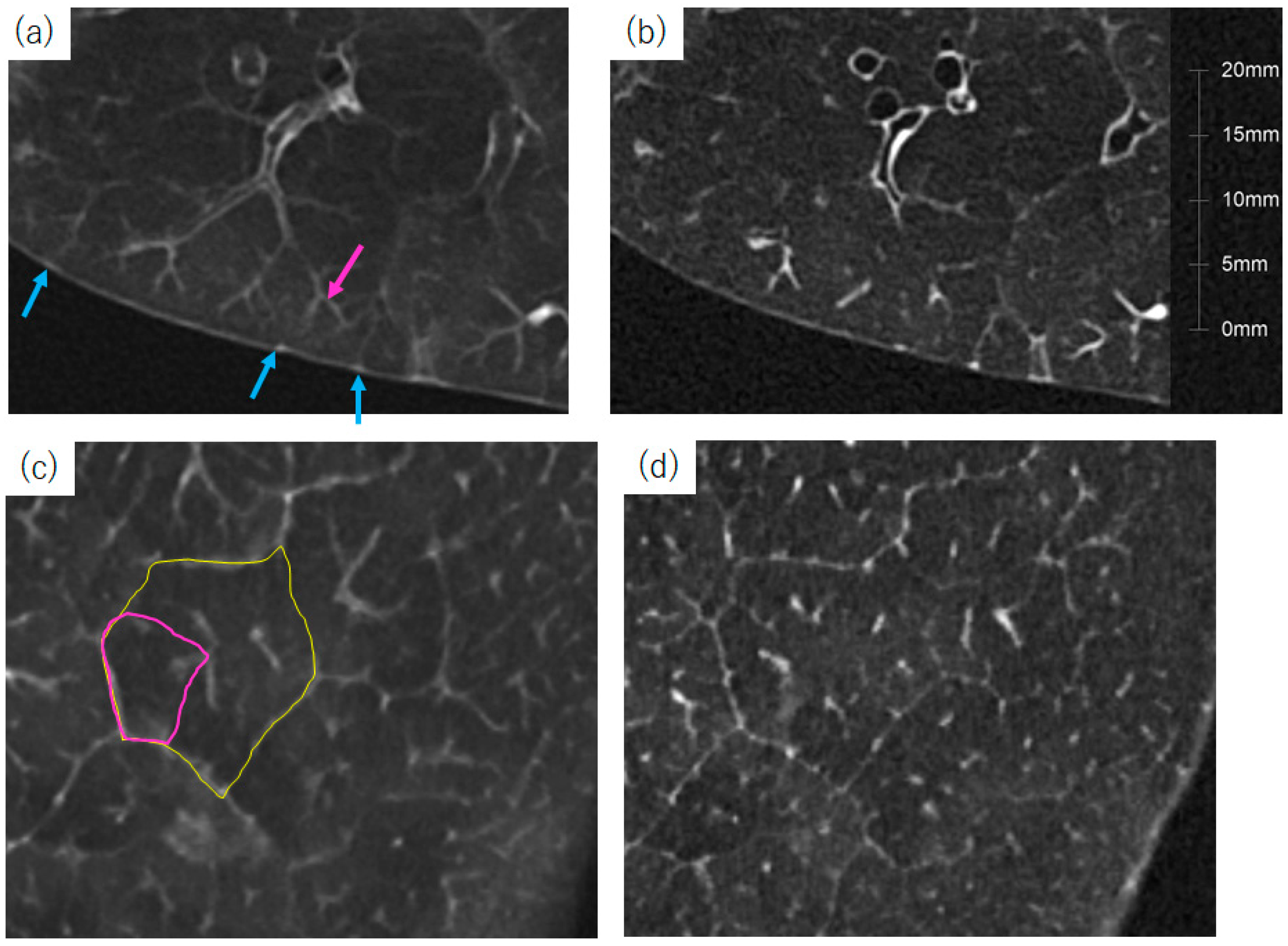
4.5. Vessel Volume Analysis
5. CT Analysis of ILD
5.1. Automatic Extraction of Various ILD Lesions in Thin-Section CT
5.2. CT Lung Volume in Interstitial Lung Disease
5.3. Evaluation of Disease Progression in Patients with ILD
5.4. Quantification of High-Attenuation Areas on Thin-Section CT
5.5. Identification of UIP Patterns
6. Thin-Section CT Analysis for COVID-19 Pneumonia
6.1. CT Findings of COVID-19 Pneumonia
6.2. Estimation of Respiratory Function Using CT in COVID-19 Pneumonia
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, W.R. Thin-section CT of the secondary pulmonary lobule: Anatomy and the image—The 2004 Fleischner lecture. Radiology 2006, 239, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Reid, L. The secondary lobule in the adult human lung, with special reference to its appearance in bronchograms. Thorax 1958, 13, 110–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reid, L.; Simon, G. The peripheral pattern in the normal bronchogram and its relation to peripheral pulmonary anatomy. Thorax 1958, 13, 103–109. [Google Scholar] [CrossRef][Green Version]
- Morita, Y.; Yamashiro, T.; Tsuchiya, N.; Tsubakimoto, M.; Murayama, S. Automatic bronchial segmentation on ultra-HRCT scans: Advantage of the 1024-matrix size with 0.25-mm slice thickness reconstruction. Jpn. J. Radiol. 2020, 38, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Sato, M.; Yamaya, T.; Sato, Y.; Uchida, Y.; Kitamura, H.; Hagiwara, E.; Komatsu, S.; Utsunomiya, D.; Ogura, T. Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn. J. Radiol. 2020, 38, 394–398. [Google Scholar] [CrossRef]
- Aoki, R.; Iwasawa, T.; Hagihara, E.; Komatsu, S.; Utsunomiya, D.; Ogura, T. Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity. Jpn. J. Radiol. 2021, 39, 451–458. [Google Scholar] [CrossRef]
- Arjomandi, M.; Zeng, S.; Chen, J.; Bhatt, S.P.; Abtin, F.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Buhr, R.G.; Criner, G.J.; et al. Changes in lung volumes with spirometric disease progression in COPD. Chronic Obstr. Pulm. Dis. 2023, 10, 270–285. [Google Scholar] [CrossRef]
- Müller, N.L.; Staples, C.A.; Miller, R.R.; Abboud, R.T. ‘Density mask’. An objective method to quantitate emphysema using computed tomography. Chest 1988, 94, 782–787. [Google Scholar] [CrossRef]
- Gevenois, P.A.; de Maertelaer, V.; De Vuyst, P.; Zanen, J.; Yernault, J.C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 1995, 152, 653–657. [Google Scholar] [CrossRef]
- Koo, M.C.; Tan, W.C.; Hogg, J.C.; Bourbeau, J.; Hague, C.J.; Leipsic, J.A.; Kirby, M. Quantitative computed tomography and visual emphysema scores: Association with lung function decline. ERJ Open Res. 2023, 9, 00523–2022. [Google Scholar] [CrossRef]
- Paoletti, M.; Cestelli, L.; Bigazzi, F.; Camiciottoli, G.; Pistolesi, M. Chronic obstructive pulmonary disease: Pulmonary function and CT lung attenuation do not show linear correlation. Radiology 2015, 276, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.S.; Baraghoshi, D.; Lynch, D.A.; Ash, S.Y.; Crapo, J.D.; Humphries, S.M.; COPDGene Investigators. Emphysema progression at CT by deep learning predicts functional impairment and mortality: Results from the COPDGene study. Radiology 2022, 304, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.J.; Barr, R.G.; Austin, J.H.M.; Kawut, S.M.; Raghu, G.; Sell, J.L.; Hoffman, E.A.; Newell, J.D., Jr.; Watts, J.R., Jr.; Nath, P.H.; et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: The Multi-Ethnic Study of Atherosclerosis. Thorax 2016, 71, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Ufuk, F.; Demirci, M.; Altinisik, G. Quantitative computed tomography assessment for systemic sclerosis-related interstitial lung disease: Comparison of different methods. Eur. Radiol. 2020, 30, 4369–4380. [Google Scholar] [CrossRef]
- Virdee, S.; Tan, W.C.; Hogg, J.C.; Bourbeau, J.; Hague, C.J.; Leipsic, J.A.; Kirby, M. Spatial dependence of CT emphysema in chronic obstructive pulmonary disease quantified by using join-count statistics. Radiology 2021, 301, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Mishima, M.; Hirai, T.; Itoh, H.; Nakano, Y.; Sakai, H.; Muro, S.; Nishimura, K.; Oku, Y.; Chin, K.; Ohi, M.; et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 1999, 96, 8829–8834. [Google Scholar] [CrossRef]
- Shimizu, K.; Tanabe, N.; Tho, N.V.; Suzuki, M.; Makita, H.; Sato, S.; Muro, S.; Mishima, M.; Hirai, T.; Ogawa, E.; et al. Per cent low attenuation volume and fractal dimension of low attenuation clusters on CT predict different long-term outcomes in COPD. Thorax 2020, 75, 116–122. [Google Scholar] [CrossRef]
- Best, A.C.; Lynch, A.M.; Bozic, C.M.; Miller, D.; Grunwald, G.K.; Lynch, D.A. Quantitative CT indexes in idiopathic pulmonary fibrosis: Relationship with physiologic impairment. Radiology 2003, 228, 407–414. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; de Hoop, B.; Zanen, P.; Gietema, H.; Kruitwagen, C.L.; van Ginneken, B.; Isgum, I.; Mol, C.; van Klaveren, R.J.; Dijkstra, A.E.; et al. CT-quantified emphysema in male heavy smokers: Association with lung function decline. Thorax 2011, 66, 782–787. [Google Scholar] [CrossRef]
- Ash, S.Y.; San José Estépar, R.; Fain, S.B.; Tal-Singer, R.; Stockley, R.A.; Nordenmark, L.H.; Rennard, S.; Han, M.K.; Merrill, D.; Humphries, S.M.; et al. Relationship between emphysema progression at CT and mortality in ever-smokers: Results from the COPDGene and Eclipse cohorts. Radiology 2021, 299, 222–231. [Google Scholar] [CrossRef]
- Schroeder, J.D.; McKenzie, A.S.; Zach, J.A.; Wilson, C.G.; Curran-Everett, D.; Stinson, D.S.; Newell, J.D., Jr.; Lynch, D.A. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am. J. Roentgenol. 2013, 201, W460–W470. [Google Scholar] [CrossRef] [PubMed]
- Mets, O.M.; Murphy, K.; Zanen, P.; Gietema, H.A.; Lammers, J.W.; van Ginneken, B.; Prokop, M.; de Jong, P.A. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur. Radiol. 2012, 22, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.; Yin, Y.; Tschirren, J.; Tan, W.C.; Leipsic, J.; Hague, C.J.; Bourbeau, J.; Sin, D.D.; Hogg, J.C.; Coxson, H.O.; et al. A novel method of estimating small airway disease using inspiratory-to-expiratory computed tomography. Respiration 2017, 94, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Galbán, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galbán, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef]
- Nakano, Y.; Muro, S.; Sakai, H.; Hirai, T.; Chin, K.; Tsukino, M.; Nishimura, K.; Itoh, H.; Paré, P.D.; Hogg, J.C.; et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am. J. Respir. Crit. Care Med. 2000, 162, 1102–1108. [Google Scholar] [CrossRef]
- Nakano, Y.; Wong, J.C.; de Jong, P.A.; Buzatu, L.; Nagao, T.; Coxson, H.O.; Elliott, W.M.; Hogg, J.C.; Paré, P.D. The prediction of small airway dimensions using computed tomography. Am. J. Respir. Crit. Care Med. 2005, 171, 142–146. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Bodduluri, S.; Nakhmani, A.; Kim, Y.I.; Reinhardt, J.M.; Hoffman, E.A.; Motahari, A.; Wilson, C.G.; Humphries, S.M.; Regan, E.A.; et al. Sex differences in airways at chest CT: Results from the COPDGene cohort. Radiology 2022, 305, 699–708. [Google Scholar] [CrossRef]
- Kahnert, K.; Jörres, R.A.; Kauczor, H.U.; Alter, P.; Trudzinski, F.C.; Herth, F.; Jobst, B.; Weinheimer, O.; Nauck, S.; Mertsch, P.; et al. Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality. Ther. Adv. Respir. Dis. 2023, 17, 17534666221148663. [Google Scholar] [CrossRef]
- Kirby, M.; Tanabe, N.; Tan, W.C.; Zhou, G.; Obeidat, M.; Hague, C.J.; Leipsic, J.; Bourbeau, J.; Sin, D.D.; Hogg, J.C.; et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am. J. Respir. Crit. Care Med. 2018, 197, 56–65. [Google Scholar] [CrossRef]
- Matsuoka, S.; Washko, G.R.; Yamashiro, T.; Estepar, R.S.; Diaz, A.; Silverman, E.K.; Hoffman, E.; Fessler, H.E.; Criner, G.J.; Marchetti, N.; et al. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am. J. Respir. Crit. Care Med. 2010, 181, 218–225. [Google Scholar] [CrossRef]
- Estépar, R.S.; Kinney, G.L.; Black-Shinn, J.L.; Bowler, R.P.; Kindlmann, G.L.; Ross, J.C.; Kikinis, R.; Han, M.K.; Come, C.E.; Diaz, A.A.; et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am. J. Respir. Crit. Care Med. 2013, 188, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Pistenmaa, C.L.; Nardelli, P.; Ash, S.Y.; Come, C.E.; Diaz, A.A.; Rahaghi, F.N.; Barr, R.G.; Young, K.A.; Kinney, G.L.; Simmons, J.P.; et al. Pulmonary arterial pruning and longitudinal change in percent emphysema and lung function: The genetic epidemiology of COPD study. Chest 2021, 160, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; van Moorsel, C.H.M.; van Es, H.W.; van Beek, F.T.; Struik, M.H.L.; Kokosi, M.; Egashira, R.; Brun, A.L.; et al. Predicting outcomes in idiopathic pulmonary fibrosis using automated computed tomographic analysis. Am. J. Respir. Crit. Care Med. 2018, 198, 767–776. [Google Scholar] [CrossRef]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Kokosi, M.; Nair, A.; Karwoski, R.; Walsh, S.L.; Wells, A.U.; Hansell, D.M. Mortality prediction in idiopathic pulmonary fibrosis: Evaluation of computer-based CT analysis with conventional severity measures. Eur. Respir. J. 2017, 49, 1601011. [Google Scholar] [CrossRef] [PubMed]
- Snider, G.L.; Kleinerman, J.; Thurlbeck, W.M.; Bengali, Z. The definition of emphysema. Am. Rev. Respir. Dis. 1985, 132, 182–185. [Google Scholar]
- Bankier, A.A.; De Maertelaer, V.; Keyzer, C.; Gevenois, P.A. Pulmonary emphysema: Subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999, 211, 851–858. [Google Scholar] [CrossRef]
- Madani, A.; Zanen, J.; de Maertelaer, V.; Gevenois, P.A. Pulmonary emphysema: Objective quantification at multi-detector row CT—Comparison with macroscopic and microscopic morphometry. Radiology 2006, 238, 1036–1043. [Google Scholar] [CrossRef]
- Klooster, K.; ten Hacken, N.H.; Hartman, J.E.; Kerstjens, H.A.; van Rikxoort, E.M.; Slebos, D.J. Endobronchial valves for emphysema without interlobar collateral ventilation. N. Engl. J. Med. 2015, 373, 2325–2335. [Google Scholar] [CrossRef]
- Cho, M.H.; Castaldi, P.J.; Hersh, C.P.; Hobbs, B.D.; Barr, R.G.; Tal-Singer, R.; Bakke, P.; Gulsvik, A.; San José Estépar, R.; Van Beek, E.J.; et al. A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am. J. Respir. Crit. Care Med. 2015, 192, 559–569. [Google Scholar] [CrossRef]
- Boueiz, A.; Lutz, S.M.; Cho, M.H.; Hersh, C.P.; Bowler, R.P.; Washko, G.R.; Halper-Stromberg, E.; Bakke, P.; Gulsvik, A.; Laird, N.M.; et al. Genome-wide association study of the genetic determinants of emphysema distribution. Am. J. Respir. Crit. Care Med. 2017, 195, 757–771. [Google Scholar] [CrossRef]
- Bodduluri, S.; Reinhardt, J.M.; Hoffman, E.A.; Newell, J.D., Jr.; Bhatt, S.P. Recent advances in computed tomography imaging in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2018, 15, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.M.; Mackintosh, J.A.; Jo, H.E.; Walsh, S.L.F.; Silva, M.; Calandriello, L.; Chapman, S.; Ellis, S.; Glaspole, I.; Goh, N.; et al. Quantitative computed tomography predicts outcomes in idiopathic pulmonary fibrosis. Respirology 2022, 27, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Sakane, H.; Ishida, M.; Shi, L.; Fukumoto, W.; Sakai, C.; Miyata, Y.; Ishida, T.; Akita, T.; Okada, M.; Awai, K.; et al. Biological effects of low-dose chest CT on chromosomal DNA. Radiology 2020, 295, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Fujisawa, Y.; Fujii, K.; Sugihara, N.; Kishida, Y.; Seki, S.; Yoshikawa, T. Effects of acquisition method and reconstruction algorithm for CT number measurement on standard-dose CT and reduced-dose CT: A QIBA phantom study. Jpn. J. Radiol. 2019, 37, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Abadi, E.; Jadick, G.; Lynch, D.A.; Segars, W.P.; Samei, E. Emphysema quantifications with CT scan: Assessing the effects of acquisition protocols and imaging parameters using virtual imaging trials. Chest 2023, 163, 1084–1100. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical principles and clinical prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Liu, L.P.; Shapira, N.; Chen, A.A.; Shinohara, R.T.; Sahbaee, P.; Schnall, M.; Litt, H.I.; Noël, P.B. First-generation clinical dual-source photon-counting CT: Ultra-low-dose quantitative spectral imaging. Eur. Radiol. 2022, 32, 8579–8587. [Google Scholar] [CrossRef]
- Willemink, M.J.; Leiner, T.; de Jong, P.A.; de Heer, L.M.; Nievelstein, R.A.; Schilham, A.M.; Budde, R.P. Iterative reconstruction techniques for computed tomography part 2: Initial results in dose reduction and image quality. Eur. Radiol. 2013, 23, 1632–1642. [Google Scholar] [CrossRef]
- De Boer, E.; Nijholt, I.M.; Jansen, S.; Edens, M.A.; Walen, S.; van den Berg, J.W.K.; Boomsma, M.F. Optimization of pulmonary emphysema quantification on CT scans of COPD patients using hybrid iterative and post processing techniques: Correlation with pulmonary function tests. Insights Imaging 2019, 10, 102. [Google Scholar] [CrossRef]
- Koetzier, L.R.; Mastrodicasa, D.; Szczykutowicz, T.P.; van der Werf, N.R.; Wang, A.S.; Sandfort, V.; van der Molen, A.J.; Fleischmann, D.; Willemink, M.J. Deep learning image reconstruction for CT: Technical principles and clinical prospects. Radiology 2023, 306, e221257. [Google Scholar] [CrossRef]
- Nagayama, Y.; Emoto, T.; Kato, Y.; Kidoh, M.; Oda, S.; Sakabe, D.; Funama, Y.; Nakaura, T.; Hayashi, H.; Takada, S.; et al. Improving image quality with super-resolution deep-learning-based reconstruction in coronary CT angiography. Eur. Radiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gierada, D.S.; Pilgram, T.K.; Whiting, B.R.; Hong, C.; Bierhals, A.J.; Kim, J.H.; Bae, K.T. Comparison of standard- and low-radiation-dose CT for quantification of emphysema. AJR Am. J. Roentgenol. 2007, 188, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Radiological Society of North America. Quantitative Imaging Biomarkers Alliance. Available online: https://www.rsna.org/research/quantitative-imaging-biomarkers-alliance (accessed on 1 July 2023).
- Lee, S.M.; Lee, J.G.; Lee, G.; Choe, J.; Do, K.H.; Kim, N.; Seo, J.B. CT image conversion among different reconstruction kernels without a sinogram by using a convolutional neural network. Korean J. Radiol. 2019, 20, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Hata, A.; Yanagawa, M.; Honda, O.; Kikuchi, N.; Miyata, T.; Tsukagoshi, S.; Uranishi, A.; Tomiyama, N. Effect of matrix size on the image quality of ultrahigh-resolution CT of the lung: Comparison of 512 × 512, 1024 × 1024, and 2048 × 2048. Acad. Radiol. 2018, 25, 869–876. [Google Scholar] [CrossRef]
- Yanagawa, M.; Tsubamoto, M.; Satoh, Y.; Hata, A.; Miyata, T.; Yoshida, Y.; Kikuchi, N.; Kurakami, H.; Tomiyama, N. Lung adenocarcinoma at CT with 0.25-mm Section Thickness and a 2048 Matrix: High-Spatial-Resolution Imaging for Predicting Invasiveness. Radiology 2020, 297, 462–471. [Google Scholar] [CrossRef]
- Gaillandre, Y.; Duhamel, A.; Flohr, T.; Faivre, J.B.; Khung, S.; Hutt, A.; Felloni, P.; Remy, J.; Remy-Jardin, M. Ultra-high resolution CT imaging of interstitial lung disease: Impact of photon-counting CT in 112 patients. Eur. Radiol. 2023, 33, 5528–5539. [Google Scholar] [CrossRef]
- Tanabe, N.; Sato, S.; Oguma, T.; Shima, H.; Sato, A.; Muro, S.; Hirai, T. Associations of airway tree to lung volume ratio on computed tomography with lung function and symptoms in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 77. [Google Scholar] [CrossRef]
- Gould, G.A.; MacNee, W.; McLean, A.; Warren, P.M.; Redpath, A.; Best, J.J.; Lamb, D.; Flenley, D.C. CT measurements of lung density in life can quantitate distal airspace enlargement—An essential defining feature of human emphysema. Am. Rev. Respir. Dis. 1988, 137, 380–392. [Google Scholar] [CrossRef]
- Wang, M.; Aaron, C.P.; Madrigano, J.; Hoffman, E.A.; Angelini, E.; Yang, J.; Laine, A.; Vetterli, T.M.; Kinney, P.L.; Sampson, P.D.; et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA 2019, 322, 546–556. [Google Scholar] [CrossRef]
- Ito, I.; Nagai, S.; Handa, T.; Muro, S.; Hirai, T.; Tsukino, M.; Mishima, M. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am. J. Respir. Crit. Care Med. 2005, 172, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Polosukhin, V.V.; Gutor, S.S.; Du, R.H.; Richmond, B.W.; Massion, P.P.; Wu, P.; Cates, J.M.; Sandler, K.L.; Rennard, S.I.; Blackwell, T.S. Small airway determinants of airflow limitation in chronic obstructive pulmonary disease. Thorax 2021, 76, 1079–1088. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nasuhara, Y.; Onodera, Y.; Makita, H.; Nagai, K.; Fuke, S.; Ito, Y.; Betsuyaku, T.; Nishimura, M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 1309–1315. [Google Scholar] [CrossRef]
- Smith, B.M.; Hoffman, E.A.; Basner, R.C.; Kawut, S.M.; Kalhan, R.; Barr, R.G. Not all measures of hyperinflation are created equal: Lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. Chest 2014, 145, 1305–1315. [Google Scholar] [CrossRef]
- Lynch, D.A. Progress in imaging COPD, 2004–2014. Chronic Obstr. Pulm. Dis. 2014, 1, 73–82. [Google Scholar] [CrossRef][Green Version]
- Mets, O.M.; Buckens, C.F.; Zanen, P.; Isgum, I.; van Ginneken, B.; Prokop, M.; Gietema, H.A.; Lammers, J.W.; Vliegenthart, R.; Oudkerk, M.; et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA 2011, 306, 1775–1781. [Google Scholar] [CrossRef]
- Young, A.L.; Bragman, F.J.S.; Rangelov, B.; Han, M.K.; Galbán, C.J.; Lynch, D.A.; Hawkes, D.J.; Alexander, D.C.; Hurst, J.R.; COPDGene Investigators. Disease progression modeling in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020, 201, 294–302. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available online: https://goldcopd.org/2023-gold-report-2 (accessed on 1 July 2023).
- Han, M.K.; Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.M.G.; Roche, N.; Papi, A.; Stockley, R.A.; Wedzicha, J.; Vogelmeier, C.F. From GOLD 0 to pre-COPD. Am. J. Respir. Crit. Care Med. 2021, 203, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Peinado, V.I.; Barberá, J.A.; Abate, P.; Ramírez, J.; Roca, J.; Santos, S.; Rodriguez-Roisin, R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Vogel-Claussen, J.; Parikh, M.A.; Austin, J.H.; Bluemke, D.A.; Carr, J.; Choi, J.; Goldstein, T.A.; Gomes, A.S.; Hoffman, E.A.; et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am. J. Respir. Crit. Care Med. 2015, 192, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, R.; Hoffman, E.A.; Sonka, M.; Hunninghake, G.W.; McLennan, G. Interstitial lung disease: A quantitative study using the adaptive multiple feature method. Am. J. Respir. Crit. Care Med. 1999, 159, 519–525. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Lynch, D.A.; van Beek, E.J.; Kazerooni, E.A.; Guo, J.; Xia, M.; Murray, S.; Anstrom, K.J.; Yow, E.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis: Adaptive multiple features method fibrosis association with outcomes. Am. J. Respir. Crit. Care Med. 2017, 195, 921–929. [Google Scholar] [CrossRef]
- Iwasawa, T.; Asakura, A.; Sakai, F.; Kanauchi, T.; Gotoh, T.; Ogura, T.; Yazawa, T.; Nishimura, J.; Inoue, T. Assessment of prognosis of patients with idiopathic pulmonary fibrosis by computer-aided analysis of CT images. J. Thorac. Imaging 2009, 24, 216–222. [Google Scholar] [CrossRef]
- Iwasawa, T.; Ogura, T.; Sakai, F.; Kanauchi, T.; Komagata, T.; Baba, T.; Gotoh, T.; Morita, S.; Yazawa, T.; Inoue, T. CT analysis of the effect of pirfenidone in patients with idiopathic pulmonary fibrosis. Eur. J. Radiol. 2014, 83, 32–38. [Google Scholar] [CrossRef]
- Iwasawa, T.; Okudela, K.; Takemura, T.; Fukuda, T.; Matsushita, S.; Baba, T.; Ogura, T.; Tajiri, M.; Yoshizawa, A. Computer-aided Quantification of Pulmonary Fibrosis in Patients with Lung Cancer: Relationship to Disease-free Survival. Radiology 2019, 292, 489–498. [Google Scholar] [CrossRef]
- Maldonado, F.; Moua, T.; Rajagopalan, S.; Karwoski, R.A.; Raghunath, S.; Decker, P.A.; Hartman, T.E.; Bartholmai, B.J.; Robb, R.A.; Ryu, J.H. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 204–212. [Google Scholar] [CrossRef]
- Handa, T.; Tanizawa, K.; Oguma, T.; Uozumi, R.; Watanabe, K.; Tanabe, N.; Niwamoto, T.; Shima, H.; Mori, R.; Nobashi, T.W.; et al. Novel artificial intelligence-based technology for chest computed tomography analysis of idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2022, 19, 399–406. [Google Scholar] [CrossRef]
- Ohno, Y.; Aoyagi, K.; Takenaka, D.; Yoshikawa, T.; Ikezaki, A.; Fujisawa, Y.; Murayama, K.; Hattori, H.; Toyama, H. Machine learning for lung CT texture analysis: Improvement of inter-observer agreement for radiological finding classification in patients with pulmonary diseases. Eur. J. Radiol. 2021, 134, 109410. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Aoyagi, K.; Takenaka, D.; Yoshikawa, T.; Fujisawa, Y.; Sugihara, N.; Hamabuchi, N.; Hanamatsu, S.; Obama, Y.; Ueda, T.; et al. Machine learning for lung texture analysis on thin-section CT: Capability for assessments of disease severity and therapeutic effect for connective tissue disease patients in comparison with expert panel evaluations. Acta Radiol. 2022, 63, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Iwasawa, T.; Saka, T.; Yamashiro, T.; Utsunomiya, D.; Misumi, T.; Baba, T.; Ogura, T. Effects of automatic deep-learning-based lung analysis on quantification of interstitial lung disease: Correlation with pulmonary function test results and prognosis. Diagnostics 2022, 12, 3038. [Google Scholar] [CrossRef]
- Chae, K.J.; Lim, S.; Seo, J.B.; Hwang, H.J.; Choi, H.; Lynch, D.; Jin, G.Y. Interstitial lung abnormalities at CT in the Korean national lung cancer screening program: Prevalence and deep learning-based texture analysis. Radiology 2023, 307, e222828. [Google Scholar] [CrossRef]
- Iwasawa, T.; Takemura, T.; Okudera, K.; Gotoh, T.; Iwao, Y.; Kitamura, H.; Baba, T.; Ogura, T.; Oba, M.S. The importance of subpleural fibrosis in the prognosis of patients with idiopathic interstitial pneumonias. Eur. J. Radiol. 2017, 90, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Robbie, H.; Wells, A.U.; Jacob, J.; Walsh, S.L.F.; Nair, A.; Srikanthan, A.; Tazoniero, P.; Devaraj, A. Visual and automated CT measurements of lung volume loss in idiopathic pulmonary fibrosis. AJR Am. J. Roentgenol. 2019, 213, 318–324. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Nasser, M.; Colevray, M.; Nempont, O.; Lartaud, P.J.; Vlachomitrou, A.; Broussaud, T.; Ahmad, K.; Traclet, J.; Cottin, V.; et al. Automatic quantitative computed tomography measurement of longitudinal lung volume loss in interstitial lung diseases. Eur. Radiol. 2022, 32, 4292–4303. [Google Scholar] [CrossRef]
- Negroni, D.; Zagaria, D.; Paladini, A.; Falaschi, Z.; Arcoraci, A.; Barini, M.; Carriero, A. COVID-19 CT scan lung segmentation: How we do It. J. Digit. Imaging 2022, 35, 424–431. [Google Scholar] [CrossRef]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Kokosi, M.; Nair, A.; Karwoski, R.; Raghunath, S.M.; Walsh, S.L.; Wells, A.U.; Hansell, D.M. Automated quantitative computed tomography versus visual computed tomography scoring in idiopathic pulmonary fibrosis: Validation against pulmonary function. J. Thorac. Imaging 2016, 31, 304–311. [Google Scholar] [CrossRef]
- O’Donnell, C.R.; Bankier, A.A.; Stiebellehner, L.; Reilly, J.J.; Brown, R.; Loring, S.H. Comparison of plethysmographic and helium dilution lung volumes: Which is best for COPD? Chest 2010, 137, 1108–1115. [Google Scholar] [CrossRef]
- Kauczor, H.U.; Heussel, C.P.; Fischer, B.; Klamm, R.; Mildenberger, P.; Thelen, M. Assessment of lung volumes using helical CT at inspiration and expiration: Comparison with pulmonary function tests. AJR Am. J. Roentgenol. 1998, 171, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Zach, J.A.; Newell, J.D., Jr.; Schroeder, J.; Murphy, J.R.; Curran-Everett, D.; Hoffman, E.A.; Westgate, P.M.; Han, M.K.; Silverman, E.K.; Crapo, J.D.; et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Investig. Radiol. 2012, 47, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Jin, G.Y.; Li, Y.Z.; Lee, J.E.; Shin, H.S. CT Quantification of lungs and airways in normal Korean subjects. Korean J. Radiol. 2017, 18, 739–748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Oldham, J.M.; Lee, C.T.; Wu, Z.; Bowman, W.S.; Pugashetti, J.V.; Dao, N.; Tonkin, J.; Seede, H.; Echt, G.; Adegunsoye, A.; et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur. Respir. J. 2022, 59, 2101396. [Google Scholar] [CrossRef]
- Galvin, J.R.; Frazier, A.A.; Franks, T.J. Collaborative radiologic and histopathologic assessment of fibrotic lung disease. Radiology 2010, 255, 692–706. [Google Scholar] [CrossRef]
- Chassagnon, G.; Vakalopoulou, M.; Régent, A.; Sahasrabudhe, M.; Marini, R.; Hoang-Thi, T.N.; Dinh-Xuan, A.T.; Dunogué, B.; Mouthon, L.; Paragios, N.; et al. Elastic registration-driven deep learning for longitudinal assessment of systemic sclerosis interstitial lung disease at CT. Radiology 2021, 298, 189–198. [Google Scholar] [CrossRef]
- Ohkubo, H.; Kanemitsu, Y.; Uemura, T.; Takakuwa, O.; Takemura, M.; Maeno, K.; Ito, Y.; Oguri, T.; Kazawa, N.; Mikami, R.; et al. Normal lung quantification in usual interstitial pneumonia pattern: The impact of threshold-based volumetric CT analysis for the staging of idiopathic pulmonary fibrosis. PLoS ONE 2016, 11, e0152505. [Google Scholar] [CrossRef]
- Best, A.C.; Meng, J.; Lynch, A.M.; Bozic, C.M.; Miller, D.; Grunwald, G.K.; Lynch, D.A. Idiopathic pulmonary fibrosis: Physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 2008, 246, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Hatabu, H.; Hunninghake, G.M.; Richeldi, L.; Brown, K.K.; Wells, A.U.; Remy-Jardin, M.; Verschakelen, J.; Nicholson, A.G.; Beasley, M.B.; Christiani, D.C.; et al. Interstitial lung abnormalities detected incidentally on CT: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2020, 8, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A. Interstitial lung abnormality incidentally detected on CT: An important prognostic indicator. Chest 2021, 159, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Putman, R.K.; Hatabu, H.; Araki, T.; Gudmundsson, G.; Gao, W.; Nishino, M.; Okajima, Y.; Dupuis, J.; Latourelle, J.C.; Cho, M.H.; et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA 2016, 315, 672–681. [Google Scholar] [CrossRef]
- Washko, G.R.; Hunninghake, G.M.; Fernandez, I.E.; Nishino, M.; Okajima, Y.; Yamashiro, T.; Ross, J.C.; Estépar, R.S.; Lynch, D.A.; Brehm, J.M.; et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N. Engl. J. Med. 2011, 364, 897–906. [Google Scholar] [CrossRef]
- Easthausen, I.; Podolanczuk, A.; Hoffman, E.; Kawut, S.; Oelsner, E.; Kim, J.S.; Raghu, G.; Stukovsky, K.H.; Redline, S.; McClelland, R.L.; et al. Reference values for high attenuation areas on chest CT in a healthy, never-smoker, multi-ethnic sample: The MESA study. Respirology 2020, 25, 855–862. [Google Scholar] [CrossRef]
- Kim, J.S.; Manichaikul, A.W.; Hoffman, E.A.; Balte, P.; Anderson, M.R.; Bernstein, E.J.; Madahar, P.; Oelsner, E.C.; Kawut, S.M.; Wysoczanski, A.; et al. MUC5B, telomere length and longitudinal quantitative interstitial lung changes: The MESA Lung Study. Thorax 2023, 78, 566–573. [Google Scholar] [CrossRef]
- Cottin, V.; Selman, M.; Inoue, Y.; Wong, A.W.; Corte, T.J.; Flaherty, K.R.; Han, M.K.; Jacob, J.; Johannson, K.A.; Kitaichi, M.; et al. Syndrome of combined pulmonary fibrosis and emphysema: An official ATS/ERS/JRS/ALAT research statement. Am. J. Respir. Crit. Care Med. 2022, 206, e7–e41. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawabata, Y.; Kanauchi, T.; Hoshi, E.; Kurashima, K.; Koyama, S.; Colby, T.V. Multiple, thin-walled cysts are one of the HRCT features of airspace enlargement with fibrosis. Eur. J. Radiol. 2015, 84, 986–992. [Google Scholar] [CrossRef]
- Matsuoka, S.; Yamashiro, T.; Matsushita, S.; Kotoku, A.; Fujikawa, A.; Yagihashi, K.; Nakajima, Y. Quantitative CT evaluation in patients with combined pulmonary fibrosis and emphysema: Correlation with pulmonary function. Acad. Radiol. 2015, 22, 626–631. [Google Scholar] [CrossRef]
- Suzuki, M.; Kawata, N.; Abe, M.; Yokota, H.; Anazawa, R.; Matsuura, Y.; Ikari, J.; Matsuoka, S.; Tsushima, K.; Tatsumi, K. Objective quantitative multidetector computed tomography assessments in patients with combined pulmonary fibrosis with emphysema: Relationship with pulmonary function and clinical events. PLoS ONE 2020, 15, e0239066. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nagatani, Y.; Takahashi, M.; Ogawa, E.; Tho, N.V.; Ryujin, Y.; Nagao, T.; Nakano, Y. Quantitative CT analysis of honeycombing area in idiopathic pulmonary fibrosis: Correlations with pulmonary function tests. Eur. J. Radiol. 2016, 85, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Teramukai, S.; Kondo, H.; Watanabe, A.; Ebina, M.; Kishi, K.; Fujii, Y.; Mitsudomi, T.; Yoshimura, M.; Maniwa, T.; et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 147, 1604–1611.e3. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Song, J.W.; Yoon, H.Y.; Cross, G.; Barnett, J.; Woo, W.L.; Adams, F.; Kokosi, M.; Devaraj, A.; Renzoni, E.; et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. eBiomedicine 2018, 28, 303–310. [Google Scholar] [CrossRef]
- Fukihara, J.; Kondoh, Y.; Brown, K.K.; Kimura, T.; Kataoka, K.; Matsuda, T.; Yamano, Y.; Suzuki, A.; Furukawa, T.; Sumikawa, H.; et al. Probable usual interstitial pneumonia pattern on chest CT: Is it sufficient for a diagnosis of idiopathic pulmonary fibrosis? Eur. Respir. J. 2020, 55, 1802465. [Google Scholar] [CrossRef]
- Raghu, G. Idiopathic pulmonary fibrosis: Shifting the concept to irreversible pulmonary fibrosis of many entities. Lancet Respir. Med. 2019, 7, 926–929. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; Wells, A.U. Usual interstitial pneumonia as a stand-alone diagnostic entity: The case for a paradigm shift? Lancet Respir. Med. 2023, 11, 188–196. [Google Scholar] [CrossRef]
- Takemura, T.; Baba, T.; Niwa, T.; Ogura, T. Histology for Transbronchial Lung Cryobiopsy Samples. In Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Disease, 1st ed.; Poletti, V., Ed.; Springer: Cham, Switzerland, 2019; pp. 67–73. [Google Scholar]
- Murata, K.; Itoh, H.; Todo, G.; Kanaoka, M.; Noma, S.; Itoh, T.; Furuta, M.; Asamoto, H.; Torizuka, K. Centrilobular lesions of the lung: Demonstration by high-resolution CT and pathologic correlation. Radiology 1986, 161, 641–645. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Lynch, D.A.; Galvin, J.R.; Gross, B.H.; Müller, N.; Schwartz, D.A.; King, T.E., Jr.; Lynch, J.P., 3rd; Hegele, R.; Waldron, J.; et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest 2003, 124, 1215–1223. [Google Scholar] [CrossRef]
- Lynch, D.A.; Travis, W.D.; Müller, N.L.; Galvin, J.R.; Hansell, D.M.; Grenier, P.A.; King, T.E., Jr. Idiopathic interstitial pneumonias: CT features. Radiology 2005, 236, 10–21. [Google Scholar] [CrossRef]
- Umakoshi, H.; Iwano, S.; Inoue, T.; Li, Y.; Naganawa, S. Quantitative evaluation of interstitial pneumonia using 3D-curved high-resolution CT imaging parallel to the chest wall: A pilot study. PLoS ONE 2017, 12, e0185532. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the radiologist needs to know. RadioGraphics 2020, 40, 1848–1865. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Fan, W.; Li, Z.; Shi, M.; Liang, Y. Imaging characteristics of initial chest computed tomography and clinical manifestations of patients with COVID-19 pneumonia. Jpn. J. Radiol. 2020, 38, 533–538. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT findings in coronavirus Disease-19 (COVID-19): Relationship to duration of infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [PubMed]
- Kawata, N.; Iwao, Y.; Matsuura, Y.; Suzuki, M.; Ema, R.; Sekiguchi, Y.; Sato, H.; Nishiyama, A.; Nagayoshi, M.; Takiguchi, Y.; et al. Prediction of oxygen supplementation by a deep-learning model integrating clinical parameters and chest CT images in COVID-19. Jpn. J. Radiol. 2023. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.C.; Diao, K.Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef]
- Annoni, A.D.; Conte, E.; Mancini, M.E.; Gigante, C.; Agalbato, C.; Formenti, A.; Muscogiuri, G.; Mushtaq, S.; Guglielmo, M.; Baggiano, A.; et al. Quantitative evaluation of COVID-19 pneumonia lung extension by specific software and correlation with patient clinical outcome. Diagnostics 2021, 11, 265. [Google Scholar] [CrossRef]
- Colombi, D.; Bodini, F.C.; Petrini, M.; Maffi, G.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 2020, 296, E86–E96. [Google Scholar] [CrossRef]
- Kang, J.; Kang, J.; Seo, W.J.; Park, S.H.; Kang, H.K.; Park, H.K.; Hyun, J.; Song, J.E.; Kwak, Y.G.; Kim, K.H.; et al. Prediction models for respiratory outcomes in patients with COVID-19: Integration of quantitative computed tomography parameters, demographics, and laboratory features. J. Thorac. Dis. 2023, 15, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Koh, J.; Jeon, Y.K.; Goo, J.M.; Yoon, S.H. An integrated radiologic-pathologic understanding of COVID-19 pneumonia. Radiology 2023, 306, e222600. [Google Scholar] [CrossRef]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-Mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, X.; Bian, Y.; Ji, X.; Lu, J. Relationship between clinical types and radiological subgroups defined by latent class analysis in 2019 novel coronavirus pneumonia caused by SARS-CoV-2. Eur. Radiol. 2020, 30, 6139–6150. [Google Scholar] [CrossRef]
- Lins, M.; Vandevenne, J.; Thillai, M.; Lavon, B.R.; Lanclus, M.; Bonte, S.; Godon, R.; Kendall, I.; De Backer, J.; De Backer, W. Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad. Radiol. 2020, 27, 1449–1455. [Google Scholar] [CrossRef]
- Morris, M.F.; Pershad, Y.; Kang, P.; Ridenour, L.; Lavon, B.; Lanclus, M.; Godon, R.; De Backer, J.; Glassberg, M.K. Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease. Eur. Respir. J. 2021, 58, 2004133. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Polidori, T.; Rucci, C.; Guido, G.; Bracci, B.; de Dominicis, C.; Laghi, A. Chest CT features of COVID-19 in Rome, Italy. Radiology 2020, 296, E79–E85. [Google Scholar] [CrossRef]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.B.; Wang, D.C.; Mei, J.; et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Som, A.; Mendoza, D.P.; Flores, E.J.; Reid, N.; Carey, D.; Li, M.D.; Witkin, A.; Rodriguez-Lopez, J.M.; Shepard, J.O.; et al. Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020, 20, 1365–1366. [Google Scholar] [CrossRef]
- Damiani, E.; Carsetti, A.; Casarotta, E.; Scorcella, C.; Domizi, R.; Adrario, E.; Donati, A. Microvascular alterations in patients with SARS-CoV-2 severe pneumonia. Ann. Intensive Care 2020, 10, 60. [Google Scholar] [CrossRef]
- Herrmann, J.; Mori, V.; Bates, J.H.T.; Suki, B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat. Commun. 2020, 11, 4883. [Google Scholar] [CrossRef] [PubMed]














| Index | Method | Target Lesion | Reference |
|---|---|---|---|
| Lung volume | Lung volume measured in thin-section CT | COPD and ILD | [7] |
| %LAA | Volume ratio of LAAs below a certain threshold (usually −950 HU) in inspiratory CT | Emphysema | [8,9,10,11,12] |
| %HAA | Volume ratio of HAAs over a certain threshold (usually −700 HU) in inspiratory CT | ILD | [13,14] |
| LAC | Low-attenuation clusters analysis | Emphysema | [15] |
| Fractal dimension | Size distribution of clusters of emphysematous regions | Emphysema | [16,17] |
| MLA | Mean lung attenuation in inspiratory CT | COPD and ILD | [18] |
| Perc15 | HU at the 15th percentile of the histogram in inspiratory CT | Emphysema | [19,20] |
| Skewness and kurtosis | Histogram indices in inspiratory CT | ILD | [18] |
| Air trapping | LAAs below a certain threshold (usually −856 HU) in full-expiration CT | Small airway disease | [21,22] |
| PRM and DPM | Inspiratory and expiratory CT images are registered, and each voxel is classified as emphysema, gas trapping, or normal | Identification of fSAD and pre-COPD | [23,24] |
| WA% | Wall area percentage defined as Ao − Ai/Ao × 100 (Ai; luminal area, Ao; total area of the airways) | Airway narrowing | [25,26] |
| Pi10 | Pi10 calculation: The square root of the wall area is plotted against the internal perimeter for each measured airway. | Airway narrowing | [26,27,28] |
| TAC | Total airway count; the sum of all airway segments from the segmented airway tree | Central airway narrowing | [28,29] |
| %CSA | Total area of small pulmonary vessels (usually less than 5 mm in diameter) in 2D images | Pulmonary vessels | [30] |
| Vessel volume | Vessel volume in 3D images | Pulmonary vessels | [31,32] |
| Vessel-related structures | Vessel volume measured using CALIPER | Pulmonary vessels in ILD | [33,34] |
| System | Method | References |
|---|---|---|
| Adaptive multiple feature method (AMFM) | Texture-based method with 17 texture parameters | [76,77] |
| Gaussian Histogram Normalized Correlation (GHNC) | Use of local histograms in original and differential images | [78,79,80] |
| Computer-Aided Lung Informatics for Pathology Evaluation and Rating (CALIPER) | Biomedical Imaging Resource (Mayo Clinic, Rochester, MN, USA) | [33,34,81] |
| Novel artificial intelligence-based quantitative CT image analysis software (AIQCT) | Deep Learning-based Texture Analysis (Fujifilm corporation, Tokyo, Japan) | [82] |
| CT Lung Parenchyma Analysis | Three-dimensional machine learning for CT texture analysis (Canon Medical Systems, Tochigi, Japan) | [83,84] |
| QZIP-ILD | Deep Learning-based Texture Analysis (Ziosoft, Inc., Tokyo, Japan). | [85] |
| AVIEW Lung Texture interstitial lung abnormalities | Deep Learning-based Texture Analysis (Coreline, Seoul, Republic of Korea). | [86] |
| Data-driven texture analysis (DTA) | Convolutional neural network algorithms | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasawa, T.; Matsushita, S.; Hirayama, M.; Baba, T.; Ogura, T. Quantitative Analysis for Lung Disease on Thin-Section CT. Diagnostics 2023, 13, 2988. https://doi.org/10.3390/diagnostics13182988
Iwasawa T, Matsushita S, Hirayama M, Baba T, Ogura T. Quantitative Analysis for Lung Disease on Thin-Section CT. Diagnostics. 2023; 13(18):2988. https://doi.org/10.3390/diagnostics13182988
Chicago/Turabian StyleIwasawa, Tae, Shoichiro Matsushita, Mariko Hirayama, Tomohisa Baba, and Takashi Ogura. 2023. "Quantitative Analysis for Lung Disease on Thin-Section CT" Diagnostics 13, no. 18: 2988. https://doi.org/10.3390/diagnostics13182988
APA StyleIwasawa, T., Matsushita, S., Hirayama, M., Baba, T., & Ogura, T. (2023). Quantitative Analysis for Lung Disease on Thin-Section CT. Diagnostics, 13(18), 2988. https://doi.org/10.3390/diagnostics13182988






