Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature
Abstract
1. Introduction
2. Materials and Methods
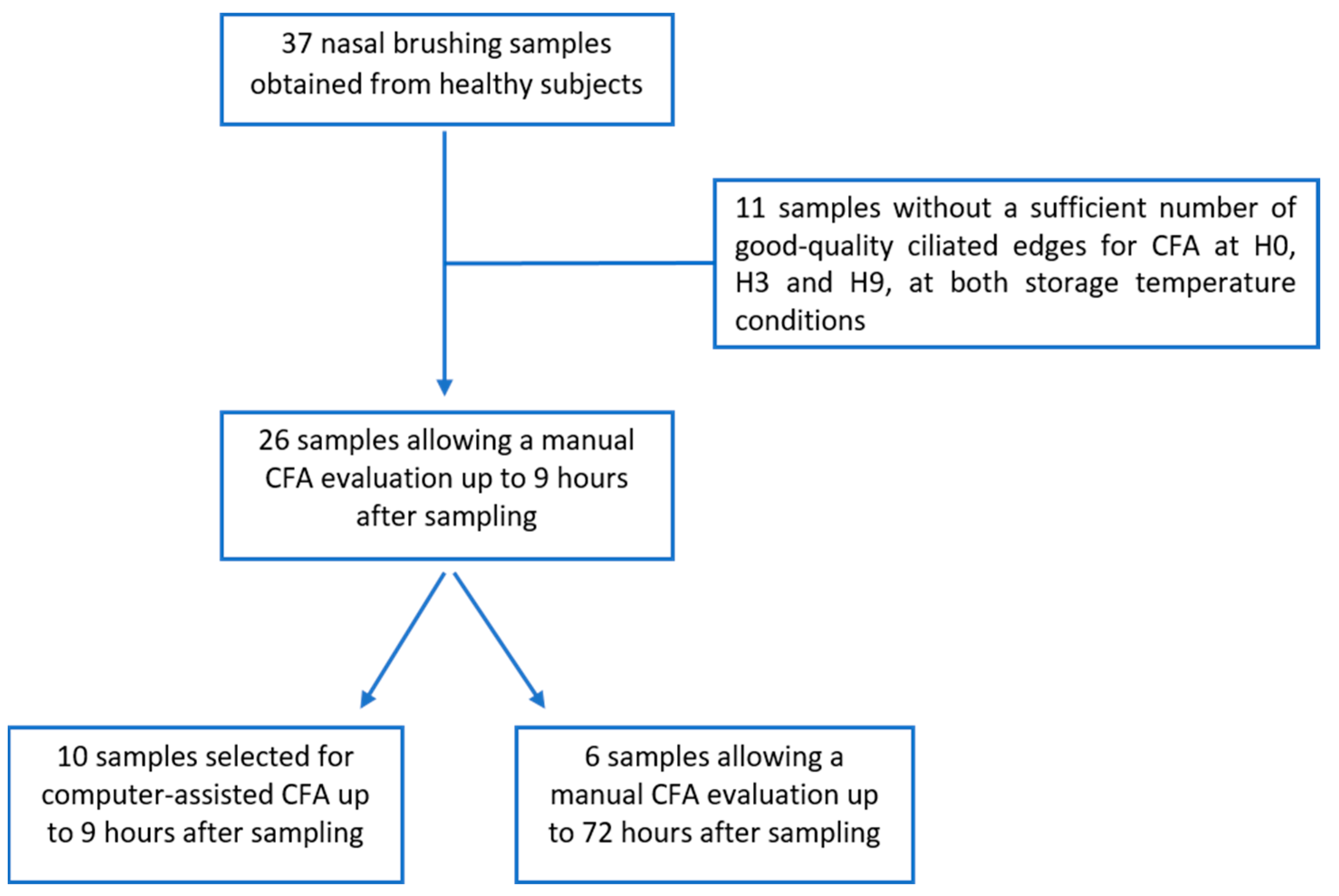
2.1. Study Design
2.2. Manual Ciliary Functional Evaluation
2.3. Computer-Assisted Ciliary Functional Evaluation
2.4. Statistical Analysis
3. Results
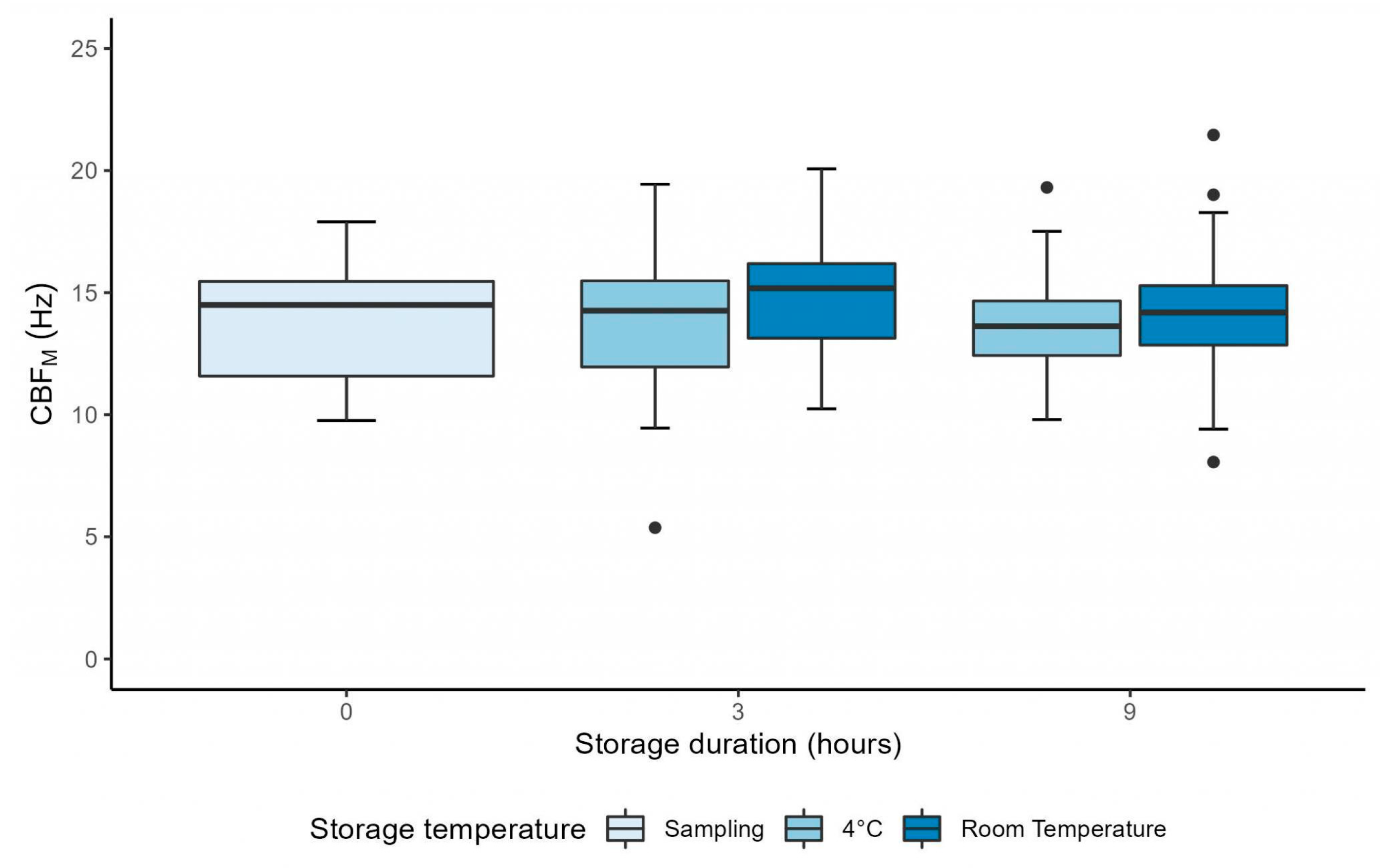
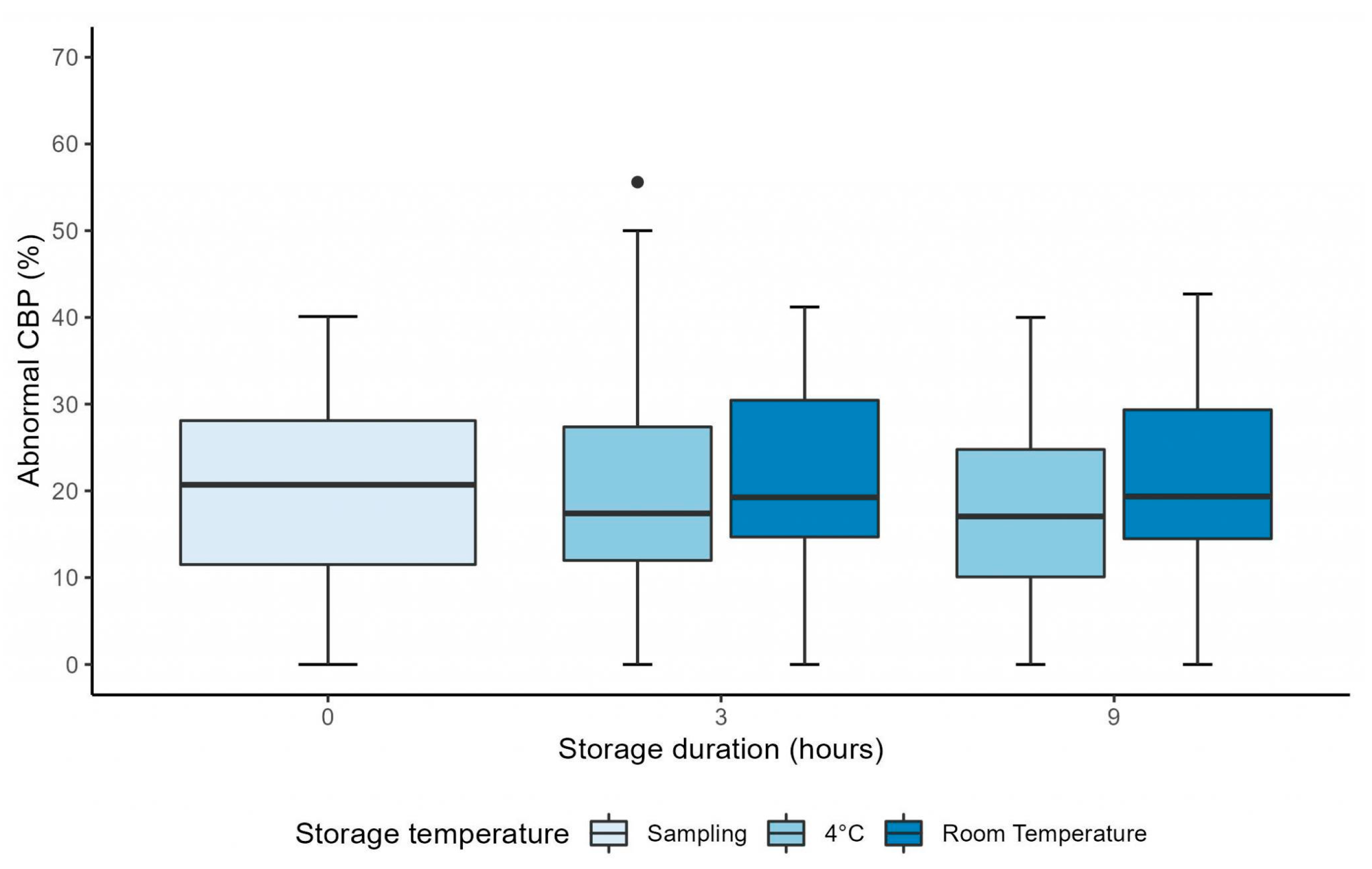
3.1. Manual CFA over 9 Hours
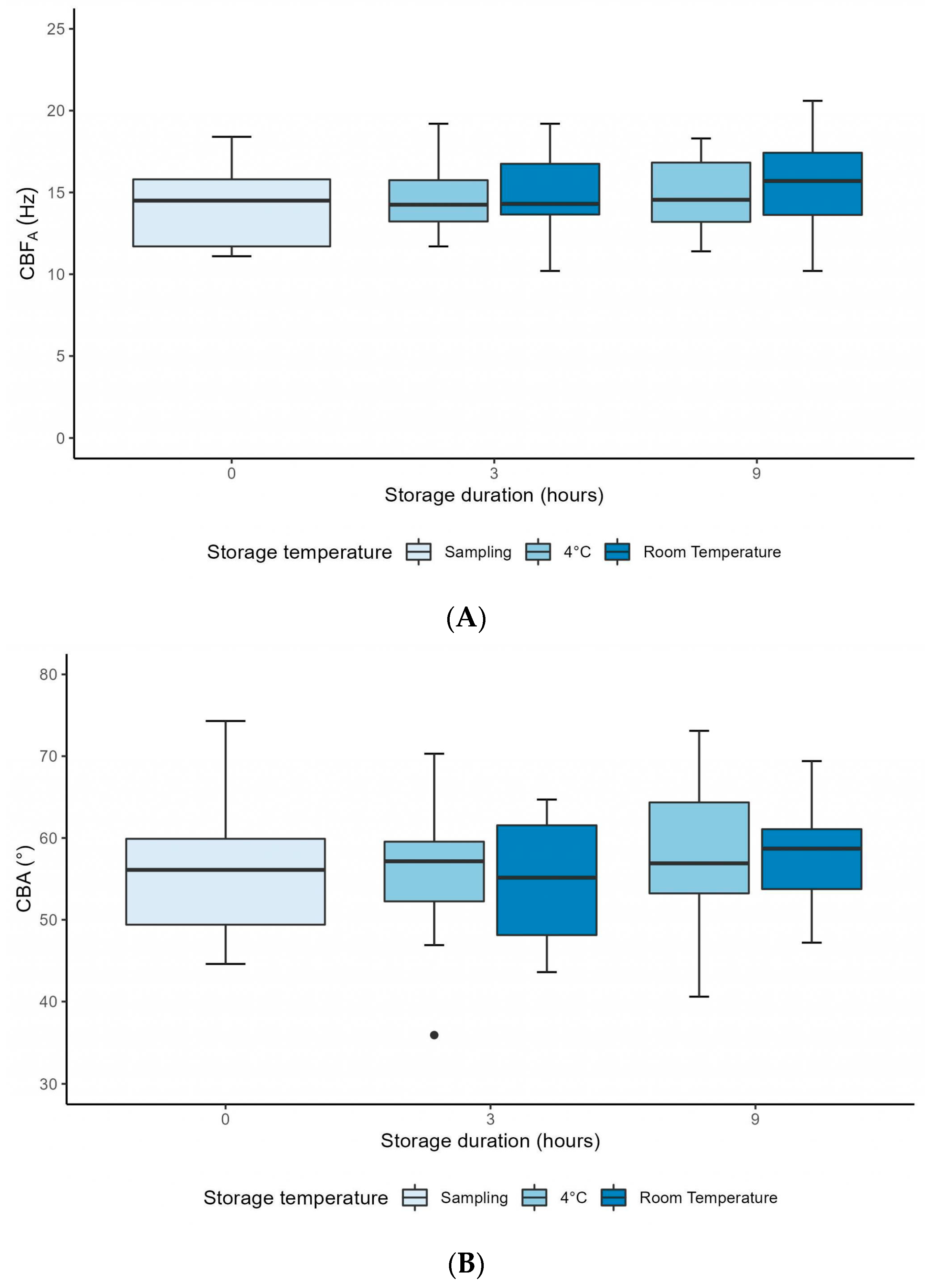
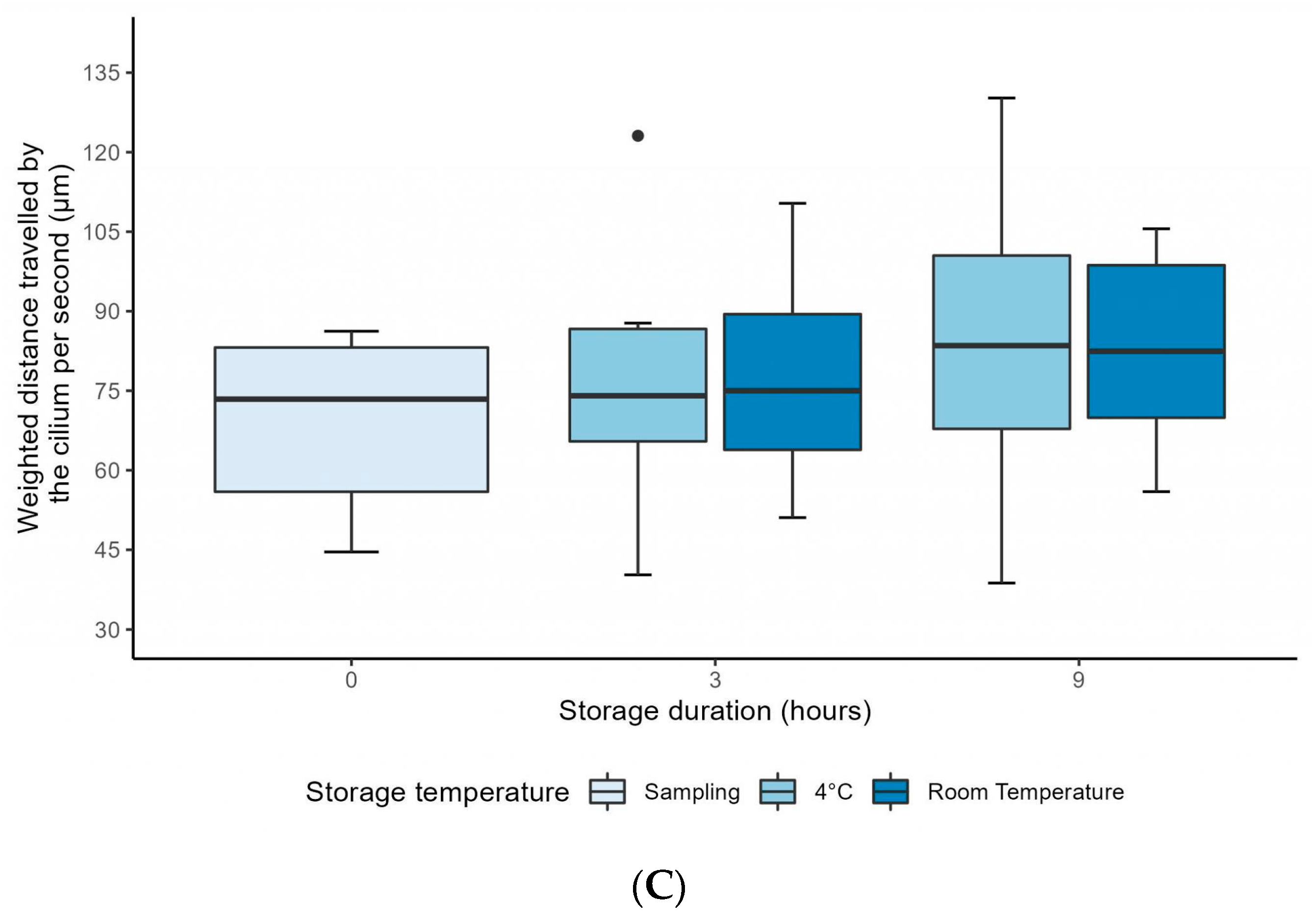
3.2. Computer-Assisted CFA over 9 Hours
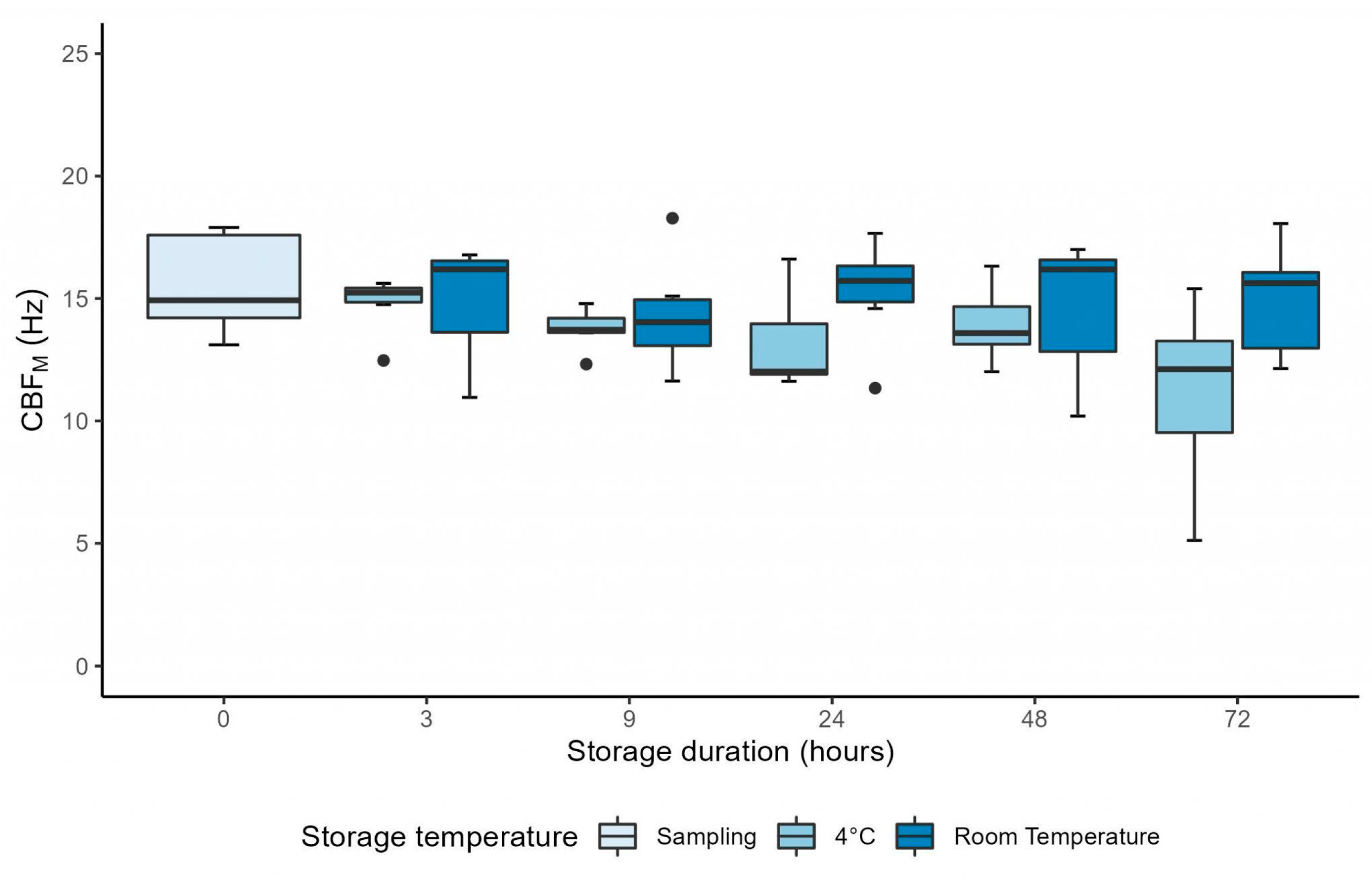
3.3. Manual CFA over 72 Hours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chilvers, M.A.; O’Callaghan, C. Local Mucociliary Defence Mechanisms. Paediatr. Respir. Rev. 2000, 1, 27–34. [Google Scholar] [CrossRef]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile Cilia and Airway Disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef]
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary Ciliary Dyskinesia: Recent Advances in Diagnostics, Genetics, and Characterization of Clinical Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Onnebrink, J.G.; Omran, H. Diagnosis and Management of Primary Ciliary Dyskinesia. Cilia 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Ciliary Beat Pattern Is Associated with Specific Ultrastructural Defects in Primary Ciliary Dyskinesia. J. Allergy Clin. Immunol. 2003, 112, 518–524. [Google Scholar] [CrossRef]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society Guidelines for the Diagnosis of Primary Ciliary Dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef]
- Shoemark, A.; Dell, S.; Shapiro, A.; Lucas, J.S. ERS and ATS Diagnostic Guidelines for Primary Ciliary Dyskinesia: Similarities and Differences in Approach to Diagnosis. Eur. Respir. J. 2019, 54, 1901066. [Google Scholar] [CrossRef]
- Chilvers, M.A.; O’Callaghan, C. Analysis of Ciliary Beat Pattern and Beat Frequency Using Digital High Speed Imaging: Comparison with the Photomultiplier and Photodiode Methods. Thorax 2000, 55, 314–317. [Google Scholar] [CrossRef]
- Raidt, J.; Wallmeier, J.; Hjeij, R.; Onnebrink, J.G.; Pennekamp, P.; Loges, N.T.; Olbrich, H.; Häffner, K.; Dougherty, G.W.; Omran, H.; et al. Ciliary Beat Pattern and Frequency in Genetic Variants of Primary Ciliary Dyskinesia. Eur. Respir. J. 2014, 44, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Dehlink, E.; Hogg, C.; Carr, S.B.; Bush, A. Clinical Phenotype and Current Diagnostic Criteria for Primary Ciliary Dyskinesia. Expert Rev. Respir. Med. 2016, 10, 1163–1175. [Google Scholar] [CrossRef]
- Jackson, C.L.; Behan, L.; Collins, S.A.; Goggin, P.M.; Adam, E.C.; Coles, J.L.; Evans, H.J.; Harris, A.; Lackie, P.; Packham, S.; et al. Accuracy of Diagnostic Testing in Primary Ciliary Dyskinesia. Eur. Respir. J. 2016, 47, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, B.; Shoemark, A.; Jackson, C.L.; Hirst, R.; Thompson, J.; Hayes, J.; Frost, E.; Copeland, F.; Hogg, C.; O’Callaghan, C.; et al. Accuracy of High-Speed Video Analysis to Diagnose Primary Ciliary Dyskinesia. Chest 2019, 155, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Stannard, W.A.; Chilvers, M.A.; Rutman, A.R.; Williams, C.D.; O’Callaghan, C. Diagnostic Testing of Patients Suspected of Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2010, 181, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Papon, J.F.; Bassinet, L.; Cariou-Patron, G.; Zerah-Lancner, F.; Vojtek, A.M.; Blanchon, S.; Crestani, B.; Amselem, S.; Coste, A.; Housset, B.; et al. Quantitative Analysis of Ciliary Beating in Primary Ciliary Dyskinesia: A Pilot Study. Orphanet J. Rare Dis. 2012, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Kempeneers, C.; Seaton, C.; Garcia Espinosa, B.; Chilvers, M.A. Ciliary Functional Analysis: Beating a Path towards Standardization. Pediatr. Pulmonol. 2019, 54, 1627–1638. [Google Scholar] [CrossRef]
- Bricmont, N.; Alexandru, M.; Louis, B.; Papon, J.-F.; Kempeneers, C. Ciliary Videomicroscopy: A Long Beat from the European Respiratory Society Guidelines to the Recognition as a Confirmatory Test for Primary Ciliary Dyskinesia. Diagnostics 2021, 11, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kempeneers, C.; Seaton, C.; Chilvers, M.A. Variation of Ciliary Beat Pattern in Three Different Beating Planes in Healthy Subjects. Chest 2017, 151, 993–1001. [Google Scholar] [CrossRef]
- Sommer, J.U.; Gross, S.; Hörmann, K.; Stuck, B.A. Time-Dependent Changes in Nasal Ciliary Beat Frequency. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 1383–1387. [Google Scholar] [CrossRef]
- Reula, A.; Pitarch-Fabregat, J.; Milara, J.; Cortijo, J.; Mata-Roig, M.; Milian, L.; Armengot, M. High-Speed Video Microscopy for Primary Ciliary Dyskinesia Diagnosis: A Study of Ciliary Motility Variations with Time and Temperature. Diagnostics 2021, 11, 1301. [Google Scholar] [CrossRef]
- Jackson, C.L.; Goggin, P.M.; Lucas, J.S. Ciliary Beat Pattern Analysis Below 37°C May Increase Risk of Primary Ciliary Dyskinesia Misdiagnosis. Chest 2012, 142, 543–544. [Google Scholar] [CrossRef][Green Version]
- Bricmont, N.; Benchimol, L.; Poirrier, A.-L.; Grignet, C.; Seaton, C.; Chilvers, M.A.; Seghaye, M.-C.; Louis, R.; Lefebvre, P.; Kempeneers, C. Nasal Brushing Sampling and Processing Using Digital High Speed Ciliary Videomicroscopy—Adaptation for the COVID-19 Pandemic. JoVE 2020, 10, e61949. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Rutman, A.; O’Callaghan, C. Disrupted Ciliated Epithelium Shows Slower Ciliary Beat Frequency and Increased Dyskinesia. Eur. Respir. J. 2009, 34, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Functional Analysis of Cilia and Ciliated Epithelial Ultrastructure in Healthy Children and Young Adults. Thorax 2003, 58, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Blanchon, S.; Legendre, M.; Bottier, M.; Tamalet, A.; Montantin, G.; Collot, N.; Faucon, C.; Dastot, F.; Copin, B.; Clement, A.; et al. Deep Phenotyping, Including Quantitative Ciliary Beating Parameters, and Extensive Genotyping in Primary Ciliary Dyskinesia. J. Med. Genet. 2019, 57, jmedgenet-2019-106424. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Hirst, R.A.; Bankart, M.J.; Jones, D.W.; Easton, A.J.; Andrew, P.W.; O’Callaghan, C. Cooling of Cilia Allows Functional Analysis of the Beat Pattern for Diagnostic Testing. Chest 2011, 140, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Nikolaizik, W.; Hahn, J.; Bauck, M.; Weber, S. Comparison of Ciliary Beat Frequencies at Different Temperatures in Young Adults. ERJ Open Res. 2020, 6, 00477–02020. [Google Scholar] [CrossRef] [PubMed]

| H0 | H3 | p-Value | ||
|---|---|---|---|---|
| CBFM (Hz) (n = 26) | 4 °C | 14.01 ± 2.36 | 13.65 ± 2.90 | 0.56 |
| RT | 14.76 ± 2.17 | 0.18 | ||
| Percentage of abnormal CBP (%) (n = 26) | 4 °C | 19.81 ± 9.52 | 21.40 ± 14.09 | 0.55 |
| RT | 20.95 ± 11.48 | 0.69 | ||
| CBFA (Hz) (n = 10) | 4 °C | 14.33 ± 2.45 | 14.56 ± 2.16 | 0.83 |
| RT | 15.03 ± 2.68 | 0.42 | ||
| CBA (°) (n = 10) | 4 °C | 56.08 ± 8.59 | 55.80 ± 9.59 | 0.94 |
| RT | 54.58 ± 7.86 | 0.69 | ||
| DTSW (µm) (n = 10) | 4 °C | 70.73 ± 14.23 | 76.63 ± 21.69 | 0.47 |
| RT | 77.04 ± 18.33 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bricmont, N.; Bonhiver, R.; Benchimol, L.; Louis, B.; Papon, J.-F.; Monseur, J.; Donneau, A.-F.; Moermans, C.; Schleich, F.; Calmès, D.; et al. Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature. Diagnostics 2023, 13, 2974. https://doi.org/10.3390/diagnostics13182974
Bricmont N, Bonhiver R, Benchimol L, Louis B, Papon J-F, Monseur J, Donneau A-F, Moermans C, Schleich F, Calmès D, et al. Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature. Diagnostics. 2023; 13(18):2974. https://doi.org/10.3390/diagnostics13182974
Chicago/Turabian StyleBricmont, Noemie, Romane Bonhiver, Lionel Benchimol, Bruno Louis, Jean-François Papon, Justine Monseur, Anne-Françoise Donneau, Catherine Moermans, Florence Schleich, Doriane Calmès, and et al. 2023. "Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature" Diagnostics 13, no. 18: 2974. https://doi.org/10.3390/diagnostics13182974
APA StyleBricmont, N., Bonhiver, R., Benchimol, L., Louis, B., Papon, J.-F., Monseur, J., Donneau, A.-F., Moermans, C., Schleich, F., Calmès, D., Poirrier, A.-L., Louis, R., Seghaye, M.-C., & Kempeneers, C. (2023). Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature. Diagnostics, 13(18), 2974. https://doi.org/10.3390/diagnostics13182974






