The Role of Ultrasound in Epicutaneo-Caval Catheter Insertion in Neonates: Systematic Review, Meta-Analysis and Future Perspectives
Abstract
:1. Introduction
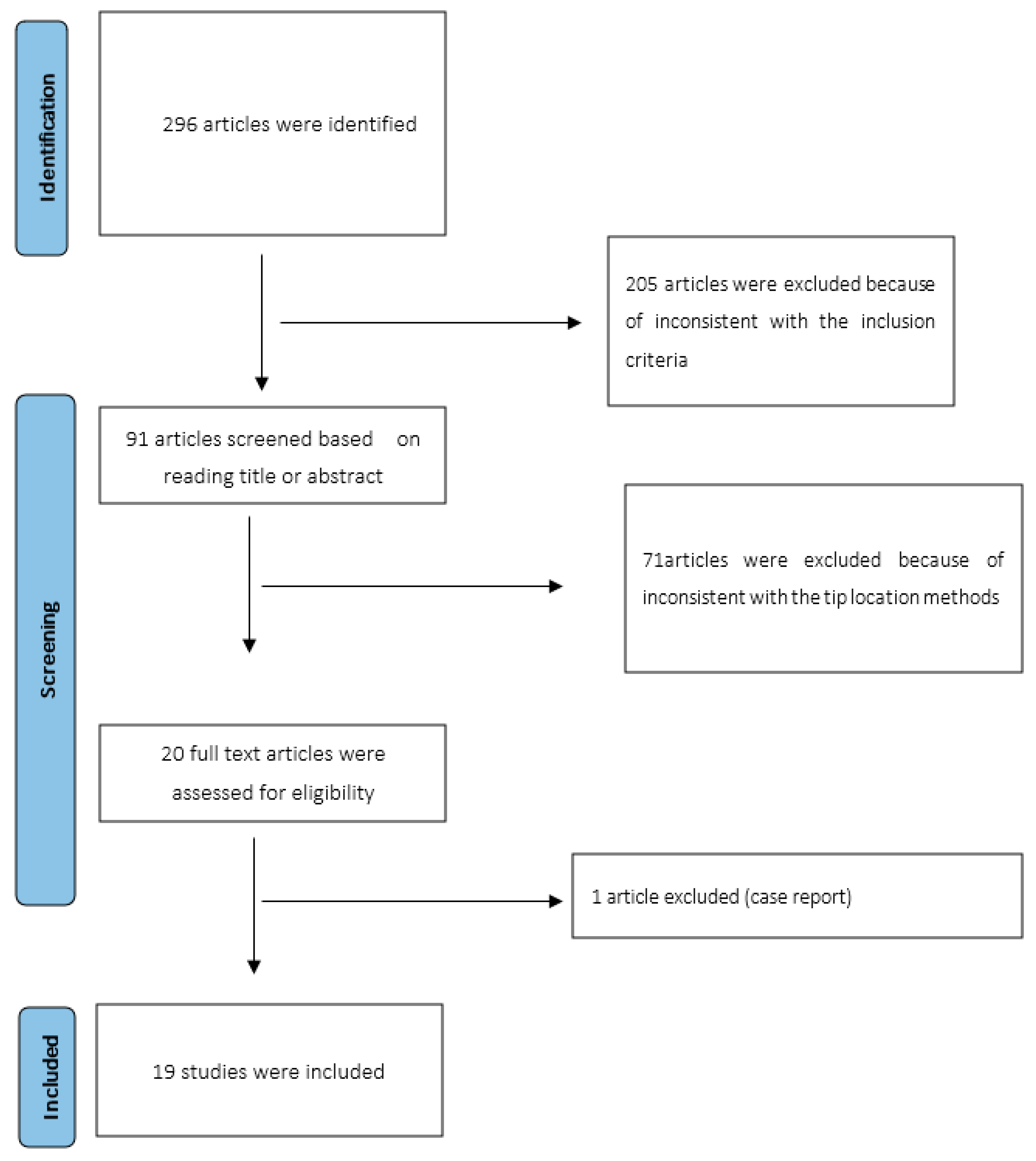
2. Materials and Methods
2.1. Inclusion Criteria and Exclusion Criteria
- Prospective, retrospective observational studies and clinical trials regarding the study of ECC tip position in neonates and infants;
- Studies comparing two procedures about tip position analysis, such as US or echocardiography and standard X-ray;
2.2. Data Analysis
2.3. Quality Assessment
| Item | Jain [1] | Motz [11] | Katheria [21] | Saul [22] | Zaghloul [23] | Johnson [24] | Oleti [25] | Ohki [26] | Tauzin [27] | Brissaud [28] | Ren [29] | Kuschel [30] | Telang [31] | Madar [32] | Diemer [33] | Shabeer [34] | Huang [35] | Motz [36] | Grasso [37] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2. Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 3. Prospective collection of data | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 |
| 4. Endpoints appropriate to the aim of the study | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| 5. Unbiased assessment of the study endpoint | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6. Follow-up period appropriate to the aim of the study | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7. Loss to follow-up less than 5% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8. Prospective calculation of the study size | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 2 |
| 9. An adequate control group | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 2 |
| 10. Contemporary groups | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| 11. Baseline equivalence of groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 12. Adequate statistical analyses | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 1 |
| Total score | 16 | 18 | 19 | 16 | 18 | 9 | 19 | 11 | 19 | 19 | 13 | 14 | 17 | 10 | 12 | 19 | 19 | 15 | 18 |
3. Results
3.1. Outcomes
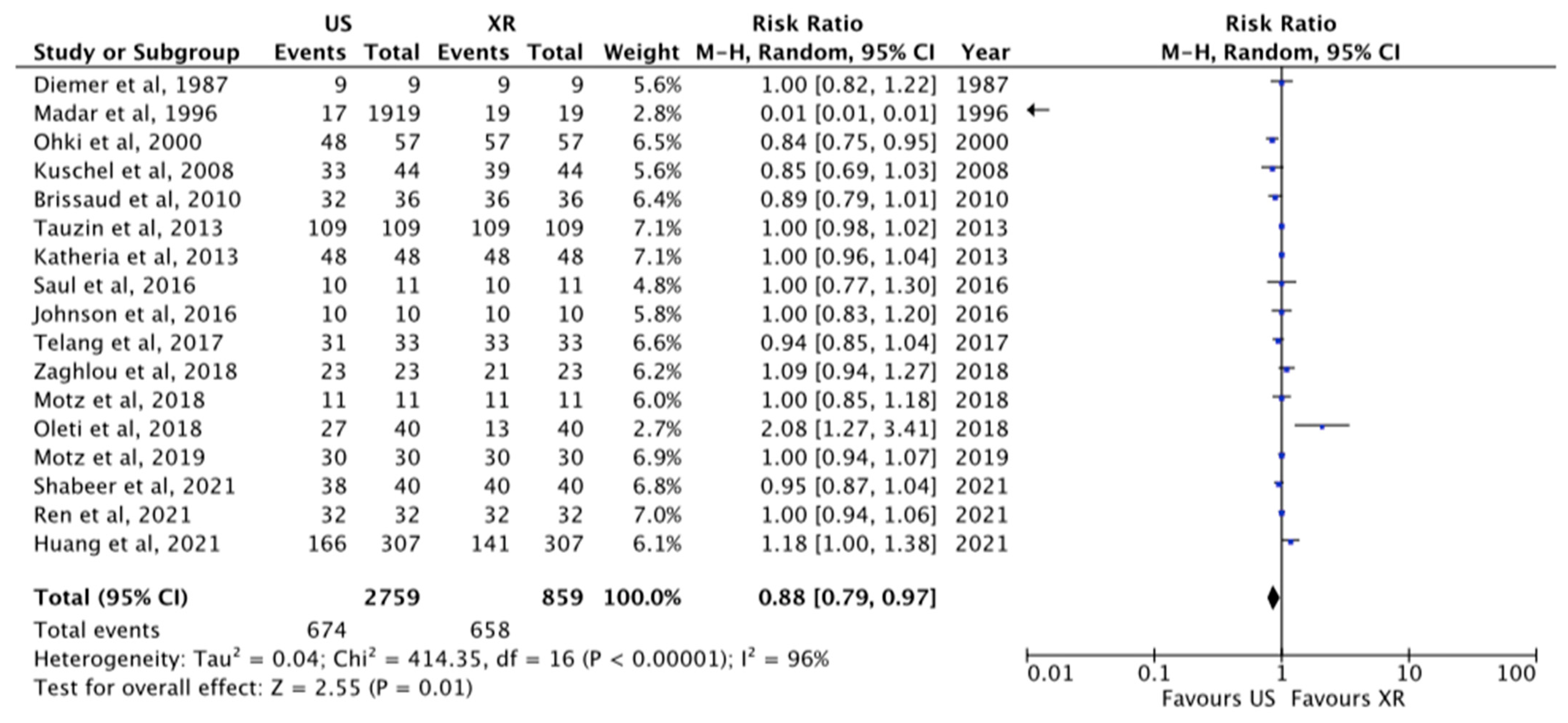
3.1.1. Tip Visualization
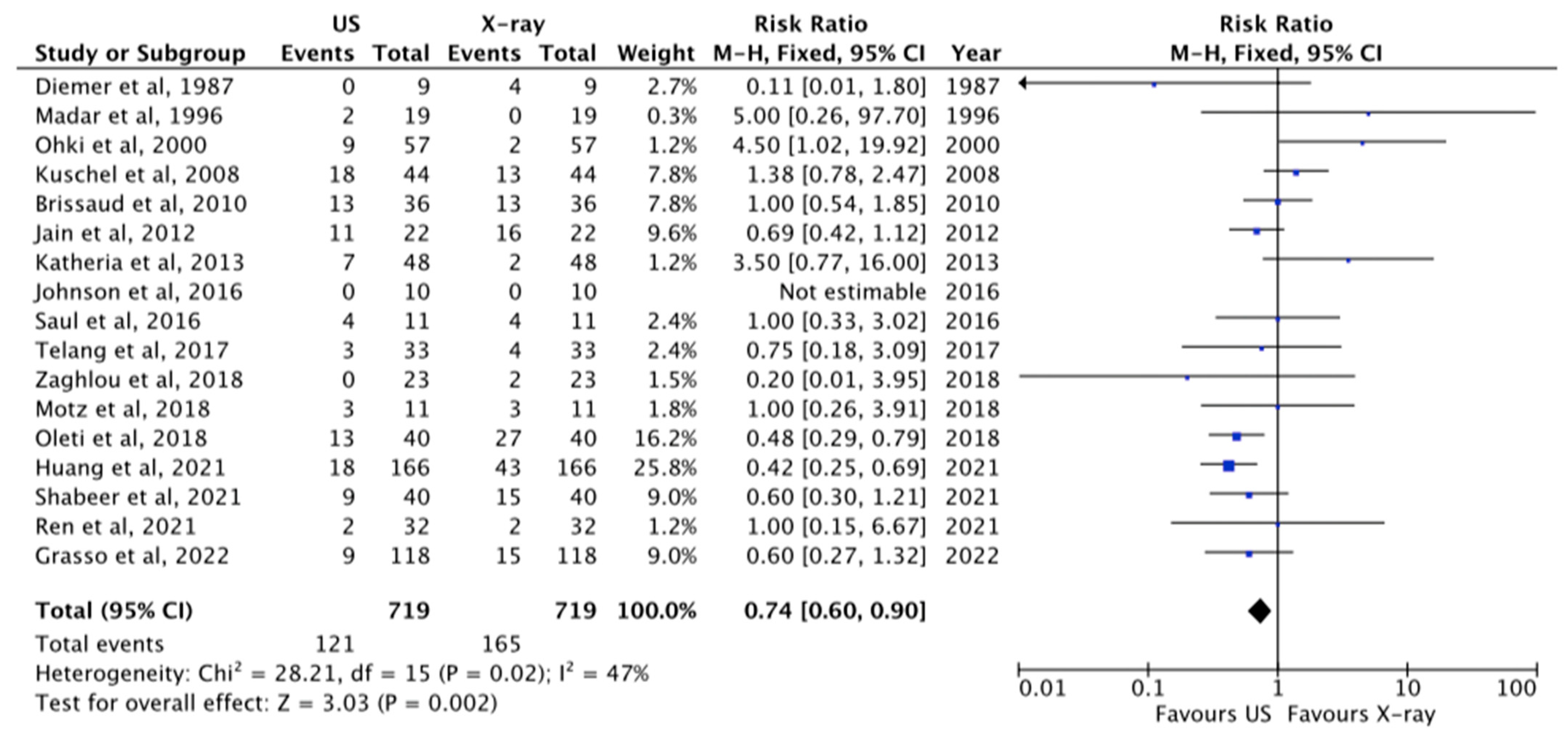
3.1.2. Correct Tip Position
3.1.3. Total Malposition
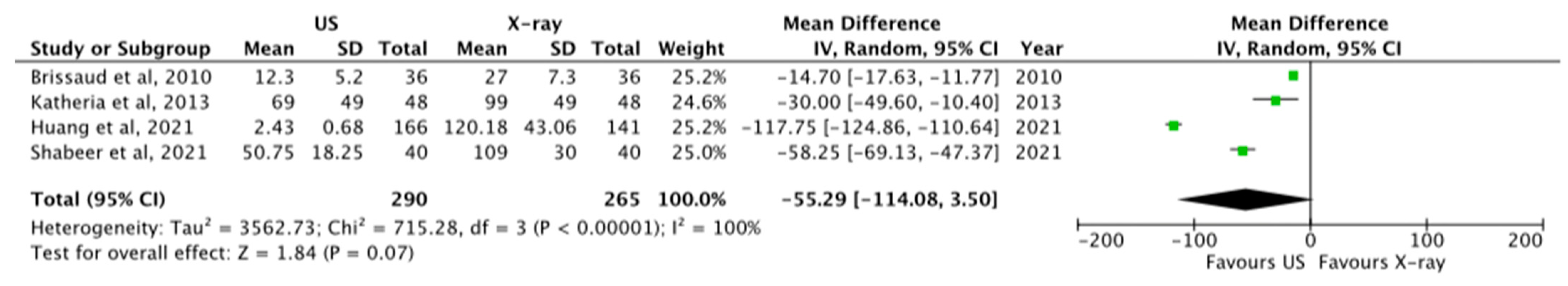
3.1.4. Timing of Insertion and Securing ECC
3.1.5. Saline Bolus
4. Discussion
- Sub-costal longitudinal view, (bicaval view) for the study of IVC, RA and SVC.
- Four-chambers apical view, for the study of the four cardiac chambers.
- Parasternal, long-axis view, for the study of SVC in long axis, RA and azygos vein.
- Subcostal longitudinal view, for the study of IVC and the RA.
- The type of papers included, with only three trials available.
- Most studies are old and might not reflect the actual ability in this new US era.
- The studies were performed in an era without a standard protocol for tip location.
- The training of health care providers was not considered.
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, A.; McNamara, P.; Ng, E.; El-Khuffash, A. The Use of Targeted Neonatal Echocardiography to Confirm Placement of Peripherally Inserted Central Catheters in Neonates. Am. J. Perinatol. 2012, 29, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Panda, S.; Jana, M.; Singh, A. Complications of peripherally inserted central venous catheters in neonates: Lesson learned over 2 years in a tertiary care centre in India. Afr. J. Paediatr. Surg. 2014, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.-Q.; Sun, J.; Zhu, L.-H.; Liao, Z.-Y.; Shen, P.; Zhao, L.-L.; Latour, J.M. Effectiveness of intracavitary electrocardiogram-guided peripherally inserted central catheter tip placement in premature infants: A multicentre pre-post intervention study. Eur. J. Pediatr. 2020, 179, 439–446. [Google Scholar] [CrossRef]
- Barone, G.; Pittiruti, M. Epicutaneo-caval catheters in neonates: New insights and new suggestions from the recent literature. J. Vasc. Access 2020, 21, 805–809. [Google Scholar] [CrossRef]
- Gorski, L.A.M.; Hadaway, L.M.; Hagle, M.E.P.; Broadhurst, D.M.; Clare, S.M.; Kleidon, T.M.P.; Meyer, B.M.P.; Nickel, B.A.-C.; Rowley, S.M.; Sharpe, E.D.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. 2021, 44, S1–S224. [Google Scholar] [CrossRef]
- Sharpe, E.L. Neonatal Peripherally Inserted Central Catheter Practices and Their Association with Demographics, Training, and Radiographic Monitoring. Adv. Neonatal Care 2014, 14, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bing, X.; Song, L.; Na, C.; Minghong, D.; Annuo, L. Intracavitary electrocardiogram guidance for placement of peripherally inserted central catheters in premature infants. Medicine 2019, 98, e18368. [Google Scholar] [CrossRef]
- Barone, G.; Pittiruti, M.; Biasucci, D.G.; Elisei, D.; Iacobone, E.; La Greca, A.; Marinosci, G.Z.; D’andrea, V. Neo-ECHOTIP: A structured protocol for ultrasound-based tip navigation and tip location during placement of central venous access devices in neonates. J. Vasc. Access 2022, 23, 5. [Google Scholar] [CrossRef]
- Srinivasan, H.; Tjin-A-Tam, A.; Galang, R.; Hecht, A.; Srinivasan, G. Migration Patterns of Peripherally Inserted Central Venous Catheters at 24 Hours Postinsertion in Neonates. Am. J. Perinatol. 2013, 30, 871–874. [Google Scholar] [CrossRef]
- Connolly, B.; Amaral, J.; Walsh, S.; Temple, M.; Chait, P.; Stephens, D. Influence of arm movement on central tip location of peripherally inserted central catheters (PICCs). Pediatr. Radiol. 2006, 36, 845–850. [Google Scholar] [CrossRef]
- Motz, P.; Arnim, A.V.S.A.V.; Likes, M.; Chabra, S.; Traudt, C.; Iyer, R.S.; Dighe, M. Limited Ultrasound Protocol for Upper Extremity Peripherally Inserted Central Catheter Monitoring: A Pilot Study in the Neonatal Intensive Care Unit. J. Ultrasound Med. 2019, 38, 1341–1347. [Google Scholar] [CrossRef]
- Nowlen, T.T.; Rosenthal, G.L.; Johnson, G.L.; Tom, D.J.; Vargo, T.A. Pericardial Effusion and Tamponade in Infants with Central Catheters. Pediatrics 2002, 110, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farahbakhsh, N.; Tabatabaii, S.A. Role of ultrasound for central catheter tip localization in neonates: A review of the current evidence. J. Matern.-Fetal Neonatal Med. 2019, 32, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, A.; Chick, J.F.B.; Srinivasa, R.N.; Hage, A.N.; Grove, J.J.; Gemmete, J.J.; Johnson, T.D. Chest Radiograph Measurement Technique Facilitates Accurate Bedside Peripherally Inserted Central Catheter Placement in Children. Cardiovasc. Interv. Radiol. 2018, 41, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Chen, H.; Tang, M.; Qu, Y.; Tang, B. Accuracy and Safety Study of Intracavitary Electrocardiographic Guidance for Peripherally Inserted Central Catheter Placement in Neonates. J. Perinat. Neonatal Nurs. 2019, 33, 89–95. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Liang, J.; Xu, M.; Yu, J. Effectiveness of Intracavitary Electrocardiogram Guidance in Peripherally Inserted Central Catheter Tip Placement in Neonates. J. Perinat. Neonatal Nurs. 2017, 31, 326–331. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Katheria, A.C.; Fleming, S.E.; Kim, J.H. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J. Perinatol. 2013, 33, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Ajayi, S.; Schutzman, D.L.; Horrow, M.M. Sonography for Complete Evaluation of Neonatal Intensive Care Unit Central Support Devices. J. Ultrasound Med. 2016, 35, 1465–1473. [Google Scholar] [CrossRef]
- Zaghloul, N.; Watkins, L.; Choi-Rosen, J.; Perveen, S.; Kurepa, D. The superiority of point of care ultrasound in localizing central venous line tip position over time. Eur. J. Pediatr. 2019, 178, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.N.; Thomas, T.; Grove, J.; Jarboe, M.D. Insertion of peripherally inserted central catheters in neonates less than 1.5 kg using ultrasound guidance. Pediatr. Surg. Int. 2016, 32, 1053–1057. [Google Scholar] [CrossRef]
- Oleti, T.; Sankar, M.J.; Thukral, A.; Sreenivas, V.; Gupta, A.K.; Agarwal, R.; Deorari, A.K.; Paul, V.K. Does ultrasound guidance for peripherally inserted central catheter (PICC) insertion reduce the incidence of tip malposition?—A randomized trial. J. Perinatol. 2019, 39, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ohki, Y. Ultrasonographic detection of very thin percutaneous central venous catheter in neonates. Acta Paediatr. 2000, 89, 1381–1384. [Google Scholar] [CrossRef]
- Tauzin, L.; Sigur, N.; Joubert, C.; Parra, J.; Hassid, S.; Moulies, M.-E. Echocardiography allows more accurate placement of peripherally inserted central catheters in low birthweight infants. Acta Paediatr. 2013, 102, 703–706. [Google Scholar] [CrossRef]
- Brissaud, O.; Harper, L.; Lamireau, D.; Jouvencel, P.; Fayon, M. Sonography-guided positioning of intravenous long lines in neonates. Eur. J. Radiol. 2010, 74, e18–e21. [Google Scholar] [CrossRef]
- Ren, X.-L.; Li, H.-L.; Liu, J.; Chen, Y.-J.; Wang, M.; Qiu, R.-X. Ultrasound to Localize the Peripherally Inserted Central Catheter Tip Position in Newborn Infants. Am. J. Perinatol. 2021, 38, 122–125. [Google Scholar] [CrossRef]
- Kuschel, C.A.; Bach, K.P.; Webster, N.J.; Page, B.; Groves, A.M.; Battin, M.R. The reliability of 2D and colour Doppler ultrasound in localising longline position. J. Paediatr. Child Health 2008, 44, 483–487. [Google Scholar] [CrossRef]
- Telang, N.; Sharma, D.; Pratap, O.T.; Kandraju, H.; Murki, S. Use of real-time ultrasound for locating tip position in neonates undergoing peripherally inserted central catheter insertion: A pilot study. Indian J. Med. Res. 2017, 145, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Madar, R.J.; Deshpande, S.A. Reappraisal of ultrasound imaging of neonatal intravascular catheters. Arch. Dis. Child Fetal Neonatal. Ed. 1996, 75, F62–F64. [Google Scholar] [CrossRef] [PubMed]
- Diemer, A. Central venous silastic catheters in newborns: Localization by sonography and radiology. Pediatr. Radiol. 1984, 17, 15–17. [Google Scholar] [CrossRef]
- Shabeer, M.P.; Abiramalatha, T.; Gibikote, S.; Rebekah, G.; Thomas, N. Bedside sonography performed by neonatology residents to confirm central vascular catheter position in neonates—A Prospective Diagnostic Evaluation study. J. Neonatal Perinat. Med. 2021, 14, 101–107. [Google Scholar] [CrossRef]
- Huang, H.-C.; Su, L.-T.; Liu, Y.-C.; Chang, H.-Y.; Ou-Yang, M.-C.; Chung, M.-Y.; Chen, F.-S.; Chen, C.-C.; Chen, I.-L. The role of ultrasonography for detecting tip location of percutaneous central venous catheters in neonates—A single-center, prospective cohort study. Pediatr. Neonatol. 2021, 62, 265–270. [Google Scholar] [CrossRef]
- Motz, P.; Von Saint Andre Von Arnim, A.; Iyer, R.S.; Chabra, S.; Likes, M.; Dighe, M. Point-of-care ultrasound for peripherally inserted central catheter monitoring: A pilot study. J. Perinat. Med. 2019, 47, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Grasso, F.; Capasso, A.; Pacella, D.; Borgia, F.; Salomè, S.; Capasso, L.; Raimondi, F. Ultrasound Guided Catheter Tip Location in Neonates: A Prospective Cohort Study. J. Pediatr. 2022, 244, 86–91.e2. [Google Scholar] [CrossRef]
- Mertens, L.; Seri, I.; Marek, J.; Arlettaz, R.; Barker, P.; McNamara, P.; Moon-Grady, A.J.; Coon, P.D.; Noori, S.; Simpson, J.; et al. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: Practice Guidelines and Recommendations for Training: Writing group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). Eur. J. Echocardiogr. 2011, 12, 715–736. [Google Scholar] [CrossRef]
- Jumani, K.; Advani, S.; Reich, N.G.; Gosey, L.; Milstone, A.M. Risk Factors for Peripherally Inserted Central Venous Catheter Complications in Children. JAMA Pediatr. 2013, 167, 429. [Google Scholar] [CrossRef]
- Pet, G.C.; Eickhoff, J.C.; McNevin, K.E.; Do, J.; McAdams, R.M. Risk factors for peripherally inserted central catheter complications in neonates. J. Perinatol. 2020, 40, 581–588. [Google Scholar] [CrossRef]
- Cartwright, D.W. Central venous lines in neonates: A study of 2186 catheters. Arch. Dis. Child Fetal. Neonatal. Ed. 2004, 89, F504–F508. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Wang, H.; Hu, X. Complications of upper extremity versus lower extremity placed peripherally inserted central catheters in neonatal intensive care units: A meta-analysis. Intensive Crit. Care Nurs. 2020, 56, 102753. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Pittiruti, M.; D’Andrea, V. Ultrasound-guided catheter tip location in neonatal central venous access. Focus on well-defined protocols and proper ultrasound training. J. Pediatr. 2022, 247, 181. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, V.; Prontera, G.; Cota, F.; Russo, R.; Barone, G.; Vento, G. Real-Time Ultrasound Tip Location Reduces Malposition and Radiation Exposure during Epicutaneo-Caval Catheter Placement in Neonates. Am. J. Perinatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Njere, I.; Islam, S.; Parish, D.; Kuna, J.; Keshtgar, A.S. Outcome of peripherally inserted central venous catheters in surgical and medical neonates. J. Pediatr. Surg. 2011, 46, 946–950. [Google Scholar] [CrossRef]
- Elmekkawi, A.; Maulidi, H.; Mak, W.; Aziz, A.; Lee, K.-S. Outcomes of upper extremity versus lower extremity placed peripherally inserted central catheters in a medical-surgical neonatal intensive care unit1. J. Neonatal Perinat. Med. 2019, 12, 57–63. [Google Scholar] [CrossRef]
- Erhard, D.M.; Nguyen, S.; Guy, K.J.; Casalaz, D.M.; König, K. Dwell times and risk of non-elective removal of 1-French peripherally inserted central catheters according to catheter tip position in very preterm infants. Eur. J. Pediatr. 2017, 176, 407–411. [Google Scholar] [CrossRef]
- Bashir, R.; Swarnam, K.; Vayalthrikkovil, S.; Yee, W.; Soraisham, A. Association between Peripherally Inserted Central Venous Catheter Insertion Site and Complication Rates in Preterm Infants. Am. J. Perinatol. 2016, 33, 945–950. [Google Scholar] [CrossRef]
- Brescia, F.; Pittiruti, M.; Spencer, T.R.; Dawson, R.B. The SIP protocol update: Eight strategies, incorporating Rapid Peripheral Vein Assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J. Vasc. Access 2022. ahead of print. [Google Scholar] [CrossRef]
- Spencer, T.R.; Pittiruti, M. Rapid Central Vein Assessment (RaCeVA): A systematic, standardized approach for ultrasound assessment before central venous catheterization. J. Vasc. Access 2019, 20, 239–249. [Google Scholar] [CrossRef]
- Lai, W.W.; Geva, T.; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M.; Pignatelli, R.H.; Rychik, J. Guidelines and Standards for Performance of a Pediatric Echocardiogram: A Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 1413–1430. [Google Scholar] [CrossRef] [PubMed]


| Quality Assessment | No. of Patients | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Cases | Controls | Relative (95% CI) | |
| Tip visualization at US and X-ray | US | X-ray | ||||||||
| 17 | OS | Moderate | Considerable | Not serious | Serious | None | 2759 | 859 | RR 0.88 [0.79–0.97] | ⊗⊗OO LOW |
| Correct TIP position at US and X-ray | US | X-ray | ||||||||
| 17 | OS | Moderate | Moderate | Not serious | Serious | None | 719 | 719 | RR 0.74 [0.60–0.90] | ⊗⊗⊗O MODERATE |
| Malposition visualized at US and X-ray | US | X-ray | ||||||||
| 15 | OS | Moderate | Substantial | Not serious | Serious | None | 302 | 266 | RR 0.13 [0.02–0.93] | ⊗⊗OO LOW |
| Timing of TIP location for US and X-ray | US | X-ray | ||||||||
| 4 | OS | Moderate | Considerable | Not serious | Serious | None | 290 | 265 | MD −55.29 [−114.08, 3.50] | ⊗⊗OO LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andrea, V.; Cascini, V.; Russo, R.; Perri, A.; Prontera, G.; Ancora, G.; Vento, G.; Lisi, G.; Barone, G. The Role of Ultrasound in Epicutaneo-Caval Catheter Insertion in Neonates: Systematic Review, Meta-Analysis and Future Perspectives. Diagnostics 2023, 13, 2850. https://doi.org/10.3390/diagnostics13172850
D’Andrea V, Cascini V, Russo R, Perri A, Prontera G, Ancora G, Vento G, Lisi G, Barone G. The Role of Ultrasound in Epicutaneo-Caval Catheter Insertion in Neonates: Systematic Review, Meta-Analysis and Future Perspectives. Diagnostics. 2023; 13(17):2850. https://doi.org/10.3390/diagnostics13172850
Chicago/Turabian StyleD’Andrea, Vito, Valentina Cascini, Rosellina Russo, Alessandro Perri, Giorgia Prontera, Gina Ancora, Giovanni Vento, Gabriele Lisi, and Giovanni Barone. 2023. "The Role of Ultrasound in Epicutaneo-Caval Catheter Insertion in Neonates: Systematic Review, Meta-Analysis and Future Perspectives" Diagnostics 13, no. 17: 2850. https://doi.org/10.3390/diagnostics13172850
APA StyleD’Andrea, V., Cascini, V., Russo, R., Perri, A., Prontera, G., Ancora, G., Vento, G., Lisi, G., & Barone, G. (2023). The Role of Ultrasound in Epicutaneo-Caval Catheter Insertion in Neonates: Systematic Review, Meta-Analysis and Future Perspectives. Diagnostics, 13(17), 2850. https://doi.org/10.3390/diagnostics13172850









