Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up
Abstract
:1. Introduction
1.1. Diabetes, Obesity, and Cardiovascular Disease—Global Context and Predicted Trends
1.2. Diastolic Dysfunction in Type 2 Diabetes Mellitus—Benefits of Antihyperglycemic Medication
1.3. Study Aims
2. Results
2.1. General Demographic and Clinical Characteristics
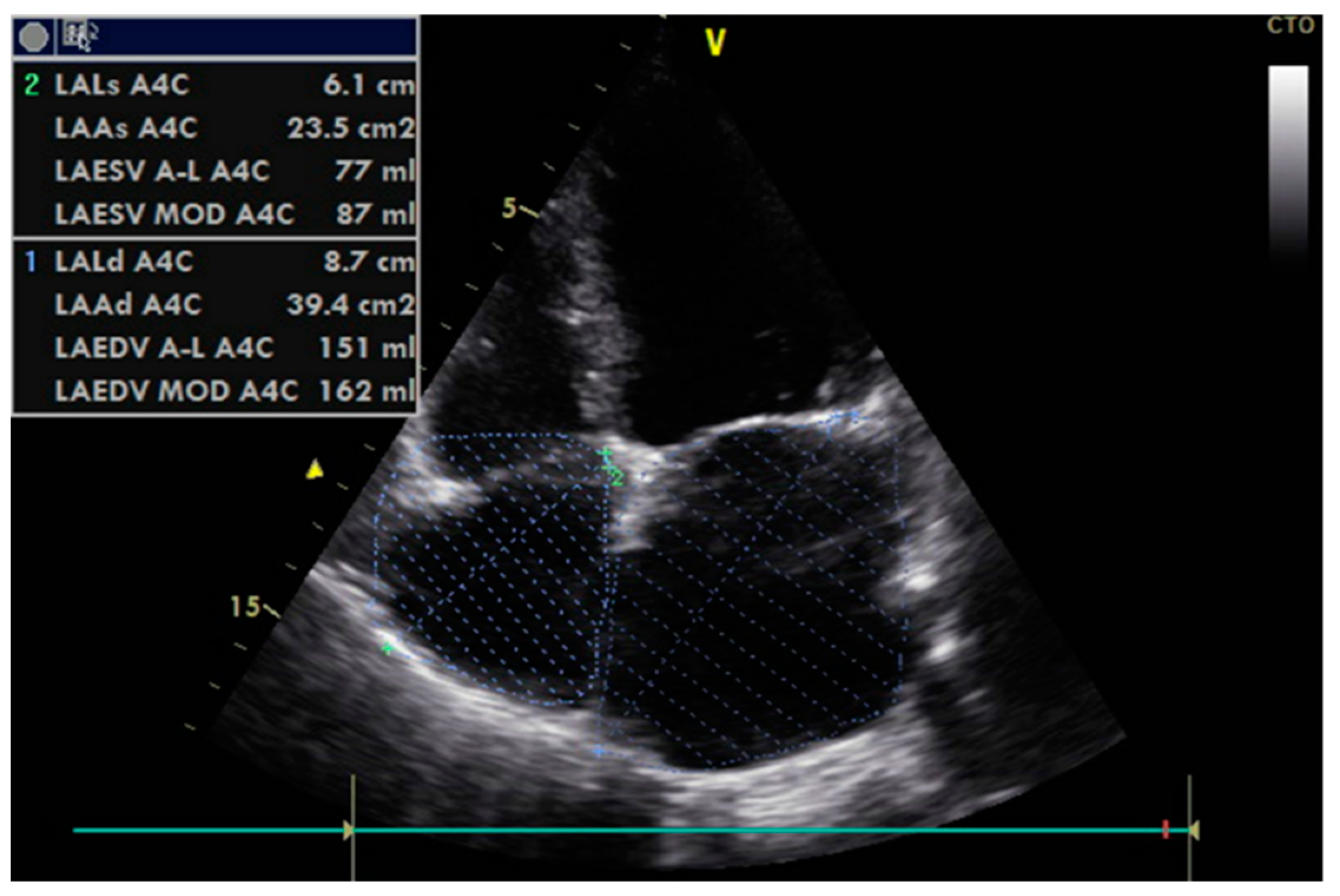
2.2. Echocardiographic Data and Assessment of Diastolic (Dys)Function
2.3. Subgroup Analysis Comparing Outcomes Based on Each Incretin-Based Drug Administered
2.4. Subgroup Analysis Based on the Patients’ Diastolic Function Assessments
3. Discussion
3.1. Diabetes-Related Results, Metabolic and Inflammatory Status
3.2. Diastolic (Dys)Function and Cardiovascular-Related Results
3.3. Study Limitations
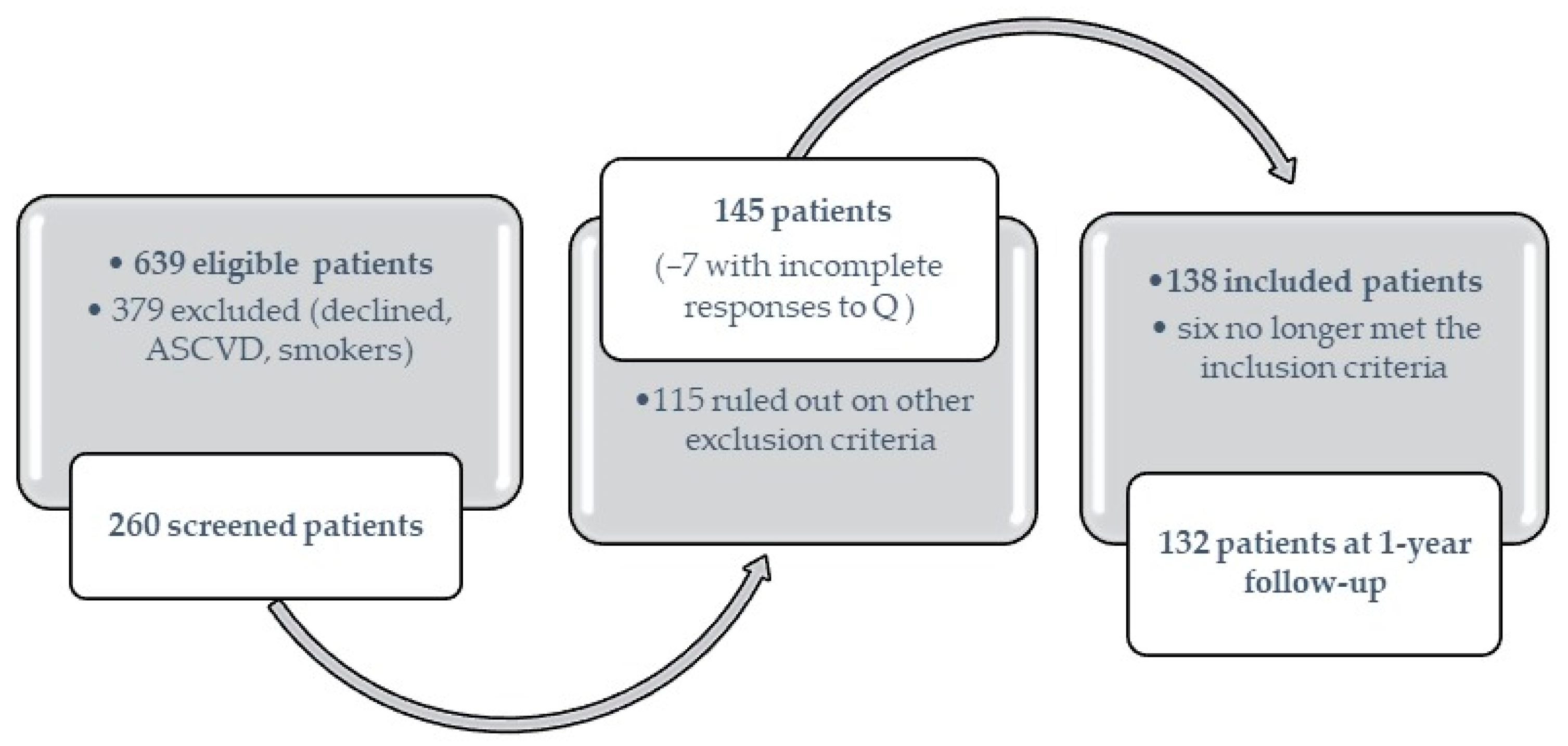
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 23 March 2023).
- The Lancet Diabetes: A defining disease of the 21st century. Lancet 2023, 401, 2087.
- Walker, A.F.; Graham, S.; Maple-Brown, L.; Egede, L.E.; Campbell, J.A.; Walker, R.J.; Wade, A.N.; Mbanya, J.C.; Long, J.A.; Yajnik, C.; et al. Interventions to address global inequity in diabetes: International progress. Lancet 2023, 402, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brindsen, H.; Gray, M. World Obesity Atlas; World Obesity Federation: London, UK, 2023; Available online: https://policycommons.net/artifacts/3454894/untitled/4255209/ (accessed on 23 May 2023).
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [PubMed]
- Cannataro, R.; Cione, E.; Cerullo, G.; Rondanelli, M.; Micheletti, P.; Crisafulli, O.; Micheli, M.L.; D’Antona, G. Type 1 diabetes management in a competitive athlete: A five-year case report. Physiol. Rep. 2023, 11, e15740. [Google Scholar]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic diet acts on body remodeling and MicroRNAs expression profile. MicroRNA 2019, 8, 116–126. [Google Scholar]
- Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar]
- Grigorescu, E.D.; Lăcătușu, C.M.; Floria, M.; Mihai, B.M.; Crețu, I.; Șorodoc, L. Left Ventricular Diastolic Dysfunction in Type 2 Diabetes-Progress and Perspectives. Diagnostics 2019, 9, 121. [Google Scholar]
- Chaudhary, A.K.; Aneja, G.K.; Shukla, S.; Razi, S.M. Study on Diastolic Dysfunction in Newly Diagnosed Type 2 Diabetes Mellitus and its Correlation with Glycosylated Haemoglobin (HbA1C). J. Clin. Diagn. Res. 2015, 9, OC20-2. [Google Scholar]
- Lindman, B.R.; Dávila-Román, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; de las Fuentes, L.; Joseph, S.M.; Vader, J.; Hernandez, A.F.; et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: A RELAX trial ancillary study. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [PubMed]
- Nogueira, K.C.; Furtado, M.; Fukui, R.T.; Correia, M.R.; Dos Santos, R.F.; Andrade, J.L.; Rossi da Silva, M.E. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor—A pilot study. Diabetol. Metab. Syndr. 2014, 6, 103. [Google Scholar] [PubMed]
- Dikshit, N.M.; Wadia, P.Z.; Shukla, D.K. Diastolic Dysfunction in Diabetes Mellitus. Natl. J. Med. Res. 2013, 3, 249–252. [Google Scholar]
- Wan, S.H.; Vogel, M.W.; Chen, H.H. Pre-clinical diastolic dysfunction. J. Am. Coll. Cardiol. 2014, 63, 407–416. [Google Scholar] [PubMed]
- From, A.M.; Scott, C.G.; Chen, H.H. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar]
- DeFronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.; Gavin, J.R., III; Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar]
- Ho, K.L.; Karwi, Q.G.; Connolly, D.; Pherwani, S.; Ketema, E.B.; Ussher, J.R.; Lopaschuk, G.D. Metabolic, structural and biochemical changes in diabetes and the development of heart failure. Diabetologia 2022, 65, 411–423. [Google Scholar]
- Gopal, K.; Chahade, J.J.; Kim, R.; Ussher, J.R. The Impact of Antidiabetic Therapies on Diastolic Dysfunction and Diabetic Cardiomyopathy. Front. Physiol. 2020, 11, 603247. [Google Scholar]
- Zhang, D.P.; Xu, L.; Wang, L.F.; Wang, H.J.; Jiang, F. Effects of antidiabetic drugs on left ventricular function/dysfunction: A systematic review and network meta-analysis. Cardiovasc. Diabetol. 2020, 19, 10. [Google Scholar]
- Ladeiras-Lopes, R.; Fontes-Carvalho, R.; Bettencourt, N.; Sampaio, F.; Gama, V.; Leite-Moreira, A.F. METformin in DIastolic dysfunction of MEtabolic syndrome (MET-DIME) trial: Rationale and study design: MET-DIME trial. Cardiovasc. Drugs. Ther. 2014, 28, 191–196. [Google Scholar] [PubMed]
- Andersson, C.; Søgaard, P.; Hoffmann, S.; Hansen, P.R.; Vaag, A.; Major-Pedersen, A.; Hansen, T.F.; Bech, J.; Køber, L.; Torp-Pedersen, C.; et al. Metformin is associated with improved left ventricular diastolic function measured by tissue doppler imaging in patients with diabetes. Eur. J. Endocrinol. 2010, 163, 593–599. [Google Scholar] [PubMed]
- Yamada, H.; Tanaka, A.; Kusunose, K.; Amano, R.; Matsuhisa, M.; Daida, H.; Ito, M.; Tsutsui, H.; Nanasato, M.; Kamiya, H.; et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: A subgroup analysis of the PROLOGUE study. Cardiovasc. Diabetol. 2017, 16, 63. [Google Scholar] [PubMed]
- Oe, H.; Nakamura, K.; Kihara, H.; Shimada, K.; Fukuda, S.; Takagi, T.; Miyoshi, T.; Hirata, K.; Yoshikawa, J.; Ito, H. Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type. 2 diabetes: Results of the. 3D trial. Cardiovasc. Diabetol. 2015, 14, 83. [Google Scholar]
- Cioffi, G.; Giorda, C.B.; Lucci, D.; Nada, E.; Ognibeni, F.; Mancusi, C.; Latini, R.; Maggioni, A.P. Effects of linagliptin on left ventricular DYsfunction in patients with type. 2 DiAbetes and concentric left ventricular geometry: Results of the DYDA 2 trial. Eur. J. Prev. Cardiol. 2020, 28, 8–17. [Google Scholar]
- Hiruma, S.; Shigiyama, F.; Kumashiro, N. Empagliflozin versus sitagliptin for ameliorating intrahepatic lipid content and tissue-specific insulin sensitivity in patients with early-stage type 2 diabetes with non-alcoholic fatty liver disease: A prospective randomized study. Diabetes Obes. Metab. 2023, 25, 1576–1588. [Google Scholar]
- Tadic, M.; Sala, C.; Saeed, S.; Grassi, G.; Mancia, G.; Rottbauer, W.; Cuspidi, C. New antidiabetic therapy and HFPEF: Light at the end of tunnel? Heart Fail. Rev. 2021, 27, 1137–1146. [Google Scholar]
- Cohen, N.D.; Gutman, S.J.; Briganti, E.M.; Taylor, A.J. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: A cardiac magnetic resonance study. Intern. Med. J. 2019, 49, 1006–1010. [Google Scholar] [CrossRef]
- Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 73. [Google Scholar]
- Verma, S.; Garg, A.; Yan, A.T.; Gupta, A.K.; Al-Omran, M.; Sabongui, A.; Teoh, H.; Mazer, C.D.; Connelly, K.A. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals with Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial? Diabetes Care 2016, 39, e212–e213. [Google Scholar]
- Otagaki, M.; Matsumura, K.; Kin, H.; Fujii, K.; Shibutani, H.; Matsumoto, H.; Takahashi, H.; Park, H.; Yamamoto, Y.; Sugiura, T.; et al. Effect of Tofogliflozin on Systolic and Diastolic Cardiac Function in Type 2 Diabetic Patients. Cardiovasc. Drugs Ther. 2019, 33, 435–442. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with tye 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55. [Google Scholar] [CrossRef]
- Saponaro, F.; Sonaglioni, A.; Rossi, A.; Montefusco, L.; Lombardo, M.; Adda, G.; Arosio, M. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment. Diabetes Res. Clin. Pract. 2016, 118, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Green, J.B.; Hernandez, A.F.; D’Agostino, R.B.; Granger, C.B.; Janmohamed, S.; Jones, N.P.; Leiter, L.A.; Noronha, D.; Russell, R.; Sigmon, K.; et al. Harmony Outcomes: A randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus-Rationale, design, and baseline characteristics. Am. Heart J. 2018, 203, 30–38. [Google Scholar] [CrossRef]
- Fan, W.; Tong, C.; Wong, N.D. LEADER Trial Eligibility and Preventable Cardiovascular Events in US Adults with Diabetes: The National Health and Nutrition Examination Surveys 2007–2016. Cardiovasc. Drugs Ther. 2020, 34, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Kotlyarov, S. Diversity of Lipid Function in Atherogenesis: A Focus on Endothelial Mechanobiology. Int. J. Mol. Sci. 2021, 22, 11545. [Google Scholar] [CrossRef]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H.; et al. Inhibition of Monocyte Adhesion to Endothelial Cells and Attenuation of Atherosclerotic Lesion by a Glucagon-like Peptide-1 Receptor Agonist, Exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Heuvelman, V.D.; Van Raalte, D.H.; Smits, M.M. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: From mechanistic studies in humans to clinical outcomes. Cardiovasc. Res. 2020, 116, 916–930. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular Actions and Clinical Outcomes with Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Grigorescu, E.-D.; Sorodoc, V.; Floria, M.; Anisie, E.; Popa, A.D.; Onofriescu, A.; Ceasovschih, A.; Sorodoc, L. The inflammatory marker HSCRP as a predictor of increased insulin resistance in type 2 diabetics without atherosclerotic manifestations. Rev. Chim. 2019, 70, 1791–1794. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. pharmacologic approaches to glycemic treatment: Standards of care in diabetes—2023. Diabetes Care 2022, 46 (Suppl. S1), S140–S157. [Google Scholar] [CrossRef]
- Bhavsar, S.; Mudaliar, S.; Cherrington, A. Evolution of exenatide as a diabetes therapeutic. Curr. Diabetes Rev. 2013, 9, 161–193. [Google Scholar]
- Klein, S.R.; Hobai, I.A. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: A case report. Can. J. Anesth. 2023, 70, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Guja, C.; Frías, J.P.; Somogyi, A.; Jabbour, S.; Wang, H.; Hardy, E.; Rosenstock, J. Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: The duration-7 Randomized Study. Diabetes Obes. Metab. 2018, 20, 1602–1614. [Google Scholar] [CrossRef]
- Apan, B.-H.; Bala, C.; Cristina, A.; Buzoianu, A.D. The Effects of Exenatide on Serum CRP Levels in Patients with Type 2 Diabetes: A Systematic Review of Randomized Controlled Trials. Rom. J. Diabetes Nutr. Metab. Dis. 2020, 27, 66–72. [Google Scholar]
- Satoh-Asahara, N.; Sasaki, Y.; Wada, H.; Tochiya, M.; Iguchi, A.; Nakagawachi, R.; Odori, S.; Kono, S.; Hasegawa, K.; Shimatsu, A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 2013, 62, 347–351. [Google Scholar] [CrossRef]
- Yadava, S.K.; Dolma, N.; Lamichhane, G.; Poudel, N.; Barakoti, M.; Karki, D.B. Prevalence of Diastolic Dysfunction in Type 2 Diabetes Mellitus. Kathmandu Univ. Med. J. (KUMJ) 2017, 15, 212–216. [Google Scholar]
- Randhawa, F.A.; Hussnain, M.T.; Nazir, S.; Masud, F. Frequency of diastolic dysfunction in asymptomatic, normotensive type 2 diabetic patients. J. Ayub Med. Coll. Abbottabad 2014, 26, 35–37. [Google Scholar]
- Patil, V.C.; Patil, H.V.; Avhad, A.B.; Kulkarni, A.R. A Comparative Study of Diastolic Dysfunction by Echocardiography among Diabetic and Non-Diabetic Subjects. J. Krishna Inst. Med. Sci. Univ. 2020, 9, 50–66. [Google Scholar]
- Chee, K.H.; Tan, K.L.; Luqman, I.; Saiful, S.S.; Chew, Y.Y.; Chinna, K.; Tan, A.T. Prevalence and predictors of left ventricular diastolic dysfunction in Malaysian patients with type 2 diabetes mellitus without prior known cardiovascular disease. Front. Cardiovasc. Med. 2021, 8, 676862. [Google Scholar] [CrossRef]
- Fontes-Carvalho, R.; Ladeiras-Lopes, R.; Bettencourt, P.; Leite-Moreira, A.; Azevedo, A. Diastolic dysfunction in the diabetic continuum: Association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 4. [Google Scholar] [CrossRef]
- Levelt, E.; Mahmod, M.; Piechnik, S.K.; Ariga, R.; Francis, J.M.; Rodgers, C.T.; Clarke, W.T.; Sabharwal, N.; Schneider, J.E.; Karamitsos, T.D.; et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 2016, 65, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Guria, R.T.; Prasad, M.K.; Mishra, B.; Marandi, S.; Kumar, A.; Dungdung, A. Association of glycosylated haemoglobin (hba1c) level with left ventricular diastolic dysfunction in patients with type 2 diabetes. Cureus 2022, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Liu, X.; Chen, R.; Ou, H.; Lai, J.; Zhang, Y.; Yan, D. An in-depth analysis of glycosylated haemoglobin level, body mass index and left ventricular diastolic dysfunction in patients with type 2 diabetes. BMC Endocr. Disord. 2019, 19, 88. [Google Scholar] [CrossRef]
- Dzhun, Y.; Mankovsky, G.; Rudenko, N.; Marushko, Y.; Saenko, Y.; Mankovsky, B. Glycemic variability is associated with diastolic dysfunction in patients with type 2 diabetes. J. Diabetes Its Complicat. 2023, 18, 166. [Google Scholar] [CrossRef]
- Bergerot, C.; Davidsen, E.S.; Amaz, C.; Thibault, H.; Altman, M.; Bellaton, A.; Moulin, P.; Derumeaux, G.; Ernande, L. Diastolic function deterioration in type 2 diabetes mellitus: Predictive factors over a 3-year follow-up. Eur. Heart J.-Cardiovasc. Imaging 2017, 19, 67–73. [Google Scholar] [CrossRef]
- Leung, M.; Wong, V.W.; Hudson, M.; Leung, D.Y. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ. Cardiovasc. Imaging 2016, 9, e003643. [Google Scholar] [CrossRef] [PubMed]
- Skali, H.; Shah, A.; Gupta, D.K.; Cheng, S.; Claggett, B.; Liu, J.; Bello, N.; Aguilar, D.; Vardeny, O.; Matsushita, K.; et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease. Circ. Heart Fail. 2015, 8, 448–454. [Google Scholar] [CrossRef]
- Kim, G.S.; Park, J.H.; Won, J.C. The role of glucagon-like peptide 1 receptor agonists and sodium-glucose cotransporter 2 inhibitors in reducing cardiovascular events in patients with type 2 diabetes. Endocrinol. Metab. 2019, 34, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, R.L.; Moreau, K.L.; Ozemek, C.; Herlache, L.; McMillin, S.; Gilligan, S.; Huebschmann, A.G.; Bauer, T.A.; Dorosz, J.; Reusch, J.E.B.; et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 449–455. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Ponikowski, P.; Bolli, G.B.; Lukashevich, V.; Kozlovski, P.; Kothny, W.; Lewsey, J.D.; Krum, H. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure. JACC Heart Fail. 2018, 6, 8–17. [Google Scholar] [CrossRef]
- de Almeida Salles, T.; Zogbi, C.; de Lima, T.M.; de Godoi Carneiro, C.; Garcez, A.T.; Barbeiro, H.V.; Antonio, E.L.; dos Santos, L.; da Costa Pereira, A.; Tucci, P.J.; et al. The contributions of dipeptidyl peptidase IV to inflammation in heart failure. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H1760–H1772. [Google Scholar] [CrossRef]
- Hiruma, S.; Shigiyama, F.; Hisatake, S.; Mizumura, S.; Shiraga, N.; Hori, M.; Ikeda, T.; Hirose, T.; Kumashiro, N. A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: The asset study. Cardiovasc. Diabetol. 2021, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.; Bistas, K.G.; Jacobs, T.F. Exenatide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518981/ (accessed on 17 August 2023).
- Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-dpp-4-inhibitors-type-2-diabetes-may-cause-severe-joint-pain (accessed on 17 August 2023).
- Radbakhsh, S.; Atkin, S.L.; Simental-Mendia, L.E.; Sahebkar, A. The role of incretins and incretin-based drugs in autoimmune diseases. Int. Immunopharmacol. 2021, 98, 107845. [Google Scholar] [CrossRef]
- Fralick, M.; Jenkins, A.J.; Khunti, K.; Mbanya, J.C.; Mohan, V.; Schmidt, M.I. Global accessibility of therapeutics for diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 199–204. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, E.-D.; Lăcătușu, C.-M.; Crețu, I.; Floria, M.; Onofriescu, A.; Ceasovschih, A.; Mihai, B.-M.; Șorodoc, L. Self-Reported Satisfaction to Treatment, Quality of Life and General Health of Type 2 Diabetes Patients with Inadequate Glycemic Control from North-Eastern Romania. Int. J. Environ. Res. Public Health 2021, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, E.D.; Lăcătușu, C.M.; Floria, M.; Cazac, G.D.; Onofriescu, A.; Ceasovschih, A.; Crețu, I.; Mihai, B.M.; Șorodoc, L. Association of Inflammatory and Metabolic Biomarkers with Mitral Annular Calcification in Type 2 Diabetes Patients. J. Pers. Med. 2022, 12, 1484. [Google Scholar] [CrossRef] [PubMed]

| Studied Variables | Incretin Treatment Group | Control Group | p-Value | |
|---|---|---|---|---|
| Diabetes duration * (years) | 5.5 (7) | 4.5 (9) | 0.470 | |
| Age (years) | T0 | 57.12 ± 9.28 | 59.33 ± 7.27 | 0.167 |
| T1 | 58.11 ± 9.29 | 60.33 ± 7.77 | 0.179 | |
| Δ | 0.98 ± 0.18, p < 0.001 ** | 1 ± 0.00, p < 0.001 ** | 0.691 | |
| BMI (kg/m2) | T0 | 33.19 ± 5.91 | 31.65 ± 4.51 | 0.090 |
| T1 | 34.01 ± 15.75 | 31.15 ± 4.70 | 0.246 | |
| Δ | 0.89 ± 15.55, p = 0.591 | −0.5 ± 1.98, p = 0.105 | 0.560 | |
| Waist circumference (cm) | T0 | 110.30 ± 10.81 | 108.64 ± 10.22 | 0.167 |
| T1 | 108.10 ± 9.82 | 107.45 ± 9.84 | 0.725 | |
| Δ | −2.19 ± 5.37, p < 0.001 ** | −1.19 ± 5.25, p = 0.150 | 0.318 | |
| HbA1c * (%) | T0 | 7.8 (1.08) | 7.55 (1.40) | 0.039 ** |
| T1 | 7.2 (1.10) | 7.2 (1.10) | 0.936 | |
| Δ | −0.66 (1.30), p < 0.001 ** | −0.45 (1.23), p = 0.001 ** | 0.262 | |
| Fasting glycemia * (mg/dL) | T0 | 165 (46) | 162 (50) | 0.285 |
| T1 | 148 (42) | 142.50 (41) | 0.599 | |
| Δ | −14 (53.75), p < 0.001 ** | −9 (42.25), p = 0.07 | 0.310 | |
| Insulin * (µIU/mL) | T0 | 13.85 (10.57) | 9.45 (7.44) | 0.021 ** |
| T1 | 11.10 (9.82) | 9.2 (7.05) | 0.296 | |
| Δ | −1.46 (10.57), p = 0.017 ** | −0.59 (5.65), p = 0.337 | 0.511 | |
| C-peptide (ng/mL) | T0 | 3.35 ± 1.45 | 3.07 ± 1.32 | 0.236 |
| T1 | 3.42 ± 1.67 | 2.44 ± 1.21 | 0.001 ** | |
| Δ | 0.059 ± 1.97, p = 0.777 | −0.64 ± 1.77, p = 0.022 ** | 0.050 | |
| HOMA-IR | T0 | 6.26 ± 4.18 | 4.60 ± 2.95 | 0.014 ** |
| T1 | 4.40 ± 3.26 | 3.48 ±2.26 | 0.089 | |
| Δ | −1.86 ± 4.04, p < 0.001 ** | −1.12 ± 3.44, p = 0.031 ** | 0.292 | |
| HOMA C-peptide | T0 | 4.24 ± 2.03 | 3.60 ± 2.22 | 0.091 |
| T1 | 3.54 ± 2.01 | 2.42 ± 1.47 | 0.001 ** | |
| Δ | −0.70 ± 2.58, p = 0.011 ** | −1.17 ± 2.83, p = 0.007 ** | 0.332 | |
| Index C-peptide * | T0 | 0.23 (0.16) | 0.28 (0.30) | 0.003 ** |
| T1 | 0.24 (0.18) | 0.32 (0.32) | 0.004 ** | |
| Δ | 0.01 (0.22), p = 0.306 | 0.004 (0.34), p = 0.507 | 1.000 | |
| Total cholesterol (mg/dL) | T0 | 192.22 ± 45.06 | 197.95 ± 44.91 | 0.721 |
| T1 | 191.02 ± 44.22 | 184.60 ± 41.77 | 0.429 | |
| Δ | −1.19 ± 45.43, p = 0.805 | −13.34 ± 43.81, p = 0.052 | 0.148 | |
| LDL cholesterol (mg/dL) | T0 | 102.72 ± 38.58 | 102.63 ± 38.73 | 0.623 |
| T1 | 95.13 ± 35.63 | 91.23 ± 35.99 | 0.576 | |
| Δ | −7.58 ± 38.41, p = 0.087 | −11.40 ± 42.86, p = 0.100 | 0.626 | |
| HDL cholesterol (mg/dL) | T0 | 55.51 ± 15.01 | 60.23 ± 16.24 | 0.130 |
| T1 | 57.17 ± 14.77 | 55.63 ± 13.82 | 0.568 | |
| Δ | 1.65 ± 13.57, p = 0.258 | −4.60 ± 14.27, p = 0.041 ** | 0.016 ** | |
| Triglycerides (mg/dL) | T0 | 205.61 ± 95.34 | 183.21 ± 69.06 | 0.167 |
| T1 | 191.63 ± 84.81 | 184.60 ± 78.98 | 0.650 | |
| Δ | −13.98 ± 84.41, p = 0.124 | 1.39 ± 71.41, p = 0.899 | 0.306 | |
| eGFR (mL/min/1.73 m2) | T0 | 83.84 ± 18.14 | 78.39 ± 16.71 | 0.106 |
| T1 | 77.10 ± 18.24 | 77.56 ± 16.58 | 0.892 | |
| Δ | −6.73 ± 11.60, p = 0.001 ** | −0.82 ± 13.00, p = 0.685 | 0.011 ** | |
| UACR (mg/g) | T0 | 27.10 ± 47.01 | 23.52 ± 16.58 | 0.559 |
| T1 | 16.02 ± 20.95 | 12.20 ± 47.84 | 0.993 | |
| Δ | −11.07 ± 31.88, p = 0.009 ** | −11.31 ± 43.18, p = 0.148 | 0.978 | |
| Uric acid (mg/dL) | T0 | 5.28 ± 1.34 | 5.70 ± 1.48 | 0.387 |
| T1 | 5.28 ± 1.34 | 5.66 ± 1.07 | 0.106 | |
| Δ | −0.10 ± 1.07, p = 0.355 | −0.03 ± 1.46, p = 0.876 | 0.752 | |
| hsCRP * (mg/L) | T0 | 5.33 (8.24) | 5.7 (12.09) | 0.983 |
| T1 | 3.57 (4.98) | 5.96 (8.93) | 0.196 | |
| Δ | −1.33 (4.91), p = 0.001 ** | 0 (10.77) | 0.238 | |
| IL-6 (pg/mL) | T0 | 2.98 ± 1.90 | 3.50 ± 2.89 | 0.089 |
| T1 | 3.55 ± 2.43 | 4.23 ± 2.39 | 0.102 | |
| Δ | 0.56 ± 2.81, p = 0.061 | 0.72 ± 3.89, p = 0.237 | 0.816 | |
| TNF-α (pg/mL) | T0 | 8.12 ± 5.63 | 7.41 ± 3.30 | 0.578 |
| T1 | 9.56 ± 6.92 | 8.57 ± 3.15 | 0.585 | |
| Δ | 1.43 ± 3.61, p = 0.001 ** | 0.36 ± 3.93, p = 0.547 | 0.125 |
| Studied Variables | Incretin Treatment Group | Control Group | p-Value | |
|---|---|---|---|---|
| LAVi (mL/m2) | T0 | 43.40 ± 11.57 | 45.15 ± 12.42 | 0.595 |
| T1 | 43.32 ± 10.55 | 45.49 ± 12.55 | 0.496 | |
| Δ | −0.082 ± 8.80, p = 0.937 | 0.341 ± 8.53, p = 0.799 | 0.804 | |
| LA area (cm2) | T0 | 24.49 ± 4.34 | 24.14 ± 4.34 | 0.605 |
| T1 | 24.15 ± 4.19 | 24.23 ± 4.50 | 0.913 | |
| Δ | −0.34 ± 3.07, p = 0.288 | 0.09 ± 2.55, p = 0.812 | 0.417 | |
| E | T0 | 51.92 ± 12.19 | 50.95 ± 11.01 | 0.686 |
| T1 | 53.38 ± 11.15 | 52.91 ± 11.42 | 0.820 | |
| Δ | 1.46 ± 9.94, p = 0.170 | 1.95 ± 8.08, p = 0.121 | 0.778 | |
| A | T0 | 53.01 ± 17.88 | 50.96 24.76 | 0.680 |
| T1 | 55.01 ± 19.12 | 54.91 24.11 | 0.983 | |
| Δ | 2.00 ± 10.98, p = 0.072 | 3.96 ± 12.29, p = 0.118 | 0.401 | |
| E/A | T0 | 1.09 ± 0.44 | 1.13 0.53 | 0.249 |
| T1 | 1.07 ± 0.40 | 1.17 0.53 | 0.675 | |
| Δ | −0.02 ± 0.32, p = 0.496 | 0.03 ± 0.25, p = 0.395 | 0.276 | |
| EDT | T0 | 192.05 ± 42.31 | 198.17 ± 44.81 | 0.363 |
| T1 | 199.83 ± 44.45 | 206.19 ± 41.90 | 0.442 | |
| Δ | 7.77 ± 37.83, p = 0.060 | 8.02 ± 25.13, p = 0.045 ** | 0.965 | |
| IVRT (ms) | T0 | 102.99 ± 18.81 | 105.77 ± 18.53 | 0.642 |
| T1 | 106.97 ± 18.74 | 109.30 ± 21.17 | 0.141 | |
| Δ | 3.97 ± 18.76, p = 0.049 ** | 3.53 ± 15.45, p = 0.141 | 0.894 | |
| Lateral e’ | T0 | 9.20 ± 2.53 | 9.14 ± 2.50 | 0.922 |
| T1 | 9.35 ± 2.43 | 9.14 ± 2.70 | 0.756 | |
| Δ | 0.15 ± 0.92, p = 0.468 | 0 ± 1.58, p = 1 | 0.622 | |
| Septal e’ | T0 | 7.45 ± 1.72 | 7.51 ± 1.48 | 0.456 |
| T1 | 7.09 ± 1.66 | 7.33 ± 1.79 | 0.649 | |
| Δ | −0.35 ± 1.55, p = 0.032 ** | −0.18 ± 0.32, p = 0.376 | 0.522 | |
| Mean e’ | T0 | 8.27 ± 1.96 | 8.30 ± 1.70 | 0.964 |
| T1 | 8.29 ± 1.80 | 8.24 ± 1.95 | 0.928 | |
| Δ | 0.01 ± 1.57, p = 0.931 | −0.06 ± 1.23, p = 0.750 | 0.784 | |
| E/e’ * | T0 | 6.40 (2.41) | 6.17 (2.06) | 0.274 |
| T1 | 6.29 (2.14) | 6.47 (1.38) | 0.656 | |
| Δ | 0.20 (1.51), p = 0.308 | 0.37 (0.86), p = 0.019 ** | 0.442 | |
| Lateral s’ * | T0 | 6.55 (1.4) | 6.8 (1.8) | 0.493 |
| T1 | 6.95 (1.8) | 7 (1.8) | 0.697 | |
| Δ | 0.4 (0.95), p = 0.07 | 0.2 (0.4), p = 0.02 ** | 0.537 | |
| IVS | T0 | 11.45 ± 1.68 | 11.19 ± 1.57 | 0.477 |
| T1 | 11.55 ± 1.55 | 11.55 ± 1.46 | 0.996 | |
| Δ | 0.09 ± 1.58, p = 0.572 | 0.35 ± 0.97, p = 0.021 ** | 0.245 | |
| LVPW | T0 | 11.01 ± 1.52 | 10.93 ± 1.35 | 0.935 |
| T1 | 11.13 ± 1.53 | 11.36 ± 1.48 | 0.424 | |
| Δ | 0.12 ± 1.50, p = 0.441 | 0.43 ± 0.87, p = 0.002 ** | 0.143 | |
| LV ejection fraction (%) | T0 | 67.48 ± 9.56 | 66.46 ± 8.97 | 0.547 |
| T1 | 67.19 ± 7.06 | 65.58 ± 7.25 | 0.226 | |
| Δ | −0.65 ± 8.37, p = 0.462 | −0.76 ± 7.48, p = 0.505 | 0.943 | |
| LV fractional shortening (%) | T0 | 38.57 ± 8.08 | 37.43 ± 7.88 | 0.480 |
| T1 | 38.37 ± 6.79 | 37.10 ± 7.65 | 0.489 | |
| Δ | −0.20 ± 6.90, p = 0.789 | −0.32 ± 5.46, p = 0.709 | 0.922 | |
| LVEDD (mm) * | T0 | 47 (10) | 50 (9) | 0.030 ** |
| T1 | 49 (11) | 51 (8) | 0.119 | |
| Δ | 2 (3), p = 0.05 ** | 0 (1), p = 0.09 | 0.984 | |
| LVESD (mm) | T0 | 27.84 ± 7.53 | 29.44 ± 8.34 | 0.293 |
| T1 | 29.48 ± 8.12 | 29.24 ± 8.17 | 0.969 | |
| Δ | 1.64 ± 6.41, p = 0.022 ** | −0.19 ± 6.54, p = 0.850 | 0.140 |
| LV Diastolic Dysfunction | DD Parameters | Age | Disease Duration | BMI | WC | HbA1c | HOMA-IR | HOMA C Peptide | Index C-Peptide | UACR | TNF-α | IL-6 | hsCRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | −0.135 | −0.077 | 0.373 * | 0.209 | −0.194 | 0.408 ** | 0.352 * | −0.224 | 0.136 | 0.094 | 0.201 | 0.214 | |
| Present | E | −0.142 | −0.103 | 0.196 | 0.145 | −0.086 | 0.050 | −0.125 | 0.124 | 0.061 | 0.121 | 0.164 | 0.002 |
| Indeterminate | 0.218 | −0.033 | 0.464 | 0.471 | 0.101 | 0.110 | 0.159 | −0.309 | 0.107 | 0.300 | 0.413 | 0.249 | |
| Absent | −0.262 | −0.063 | 0.292 | 0.289 | −0.184 | 0.161 | 0.213 | −0.285 | 0.356 * | −0.112 | −0.039 | 0.093 | |
| Present | E/A | −0.367 ** | −0.361 ** | −0.007 | 0.013 | −0.168 | 0.087 | −0.070 | 0.174 | −0.027 | −0.070 | 0.233 * | −0.010 |
| Indeterminate | −0.330 | 0.377 | 0.225 | 0.011 | 0.101 | −0.492 | −0.498 | 0.536 * | 0.216 | −0.252 | 0.242 | 0.249 | |
| Absent | −0.249 | −0.109 | 0.237 | 0.355 * | 0.030 | 0.226 | 0.299 | −0.291 | 0.119 | −0.014 | 0.101 | 0.204 | |
| Present | e’ | −0.240 * | −0.340 ** | −0.180 | −0.108 | −0.203 | −0.126 | −0.132 | 0.133 | −0.005 | −0.049 | 0.084 | −0.012 |
| Indeterminate | −0.765 ** | −0.175 | 0.183 | −0.083 | −0.199 | −0.161 | −0.240 | 0.240 | −0.771 | −0.186 | 0.065 | 0.214 | |
| Absent | 0.033 | 0.042 | 0.030 | −0.075 | −0.038 | −0.007 | 0.014 | −0.012 | 0.159 | −0.020 | 0.195 | −0.015 | |
| Present | E/e’ | 0.139 | 0.255 * | 0.145 | 0.144 | 0.004 | −0.086 | −0.100 | 0.100 | 0.027 | 0.075 | 0.036 | −0.088 |
| Indeterminate | 0.796 ** | 0.258 | 0.187 | 0.326 | 0.251 | 0.125 | 0.284 | −0.284 | 0.886 * | 0.484 | 0.066 | −0.042 | |
| Absent | 0.189 | −0.245 | −0.029 | 0.078 | 0.000 | −0.254 | −0.160 | 0.149 | 0.096 | 0.129 | −0.050 | −0.237 | |
| Present | EDT | 0.322 ** | 0.236 * | −0.041 | 0.075 | 0.238 * | −0.009 | 0.105 | −0.155 | 0.095 | 0.082 | −0.041 | −0.013 |
| Indeterminate | 0.373 | 0.399 | −0.437 | −0.371 | 0.214 | −0.402 | −0.339 | 0.408 | 0.240 | −0.122 | −0.183 | −0.622 * | |
| Absent | −0.132 | 0.026 | 0.043 | 0.051 | 0.020 | 0.097 | 0.195 | −0.154 | 0.135 | 0.125 | 0.097 | −0.054 | |
| Present | IVRT | −0.036 | 0.107 | 0.071 | 0.112 | −0.084 | 0.142 | 0.050 | −0.045 | −0.044 | −0.099 | 0.123 | 0.090 |
| Indeterminate | 0.482 | −0.079 | −0.003 | 0.079 | 0.346 | 0.032 | 0.298 | −0.263 | 0.326 | −0.035 | 0.508 | 0.047 | |
| Absent | 0.181 | 0.217 | 0.036 | −0.019 | −0.169 | 0.158 | 0.095 | −0.115 | 0.213 | −0.145 | −0.021 | 0.070 | |
| Present | LAVi | 0.170 | −0.022 | 0.185 | 0.174 | −0.194 | −0.100 | −0.249 * | 0.224 * | −0.115 | −0.022 | −0.053 | −0.129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorescu, E.-D.; Lăcătușu, C.-M.; Floria, M.; Cazac, G.-D.; Onofriescu, A.; Sauciuc, L.-A.; Ceasovschih, A.; Crețu, I.; Mihai, B.-M.; Șorodoc, L. Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up. Diagnostics 2023, 13, 2817. https://doi.org/10.3390/diagnostics13172817
Grigorescu E-D, Lăcătușu C-M, Floria M, Cazac G-D, Onofriescu A, Sauciuc L-A, Ceasovschih A, Crețu I, Mihai B-M, Șorodoc L. Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up. Diagnostics. 2023; 13(17):2817. https://doi.org/10.3390/diagnostics13172817
Chicago/Turabian StyleGrigorescu, Elena-Daniela, Cristina-Mihaela Lăcătușu, Mariana Floria, Georgiana-Diana Cazac, Alina Onofriescu, Livia-Amira Sauciuc, Alexandr Ceasovschih, Ioana Crețu, Bogdan-Mircea Mihai, and Laurențiu Șorodoc. 2023. "Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up" Diagnostics 13, no. 17: 2817. https://doi.org/10.3390/diagnostics13172817
APA StyleGrigorescu, E.-D., Lăcătușu, C.-M., Floria, M., Cazac, G.-D., Onofriescu, A., Sauciuc, L.-A., Ceasovschih, A., Crețu, I., Mihai, B.-M., & Șorodoc, L. (2023). Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up. Diagnostics, 13(17), 2817. https://doi.org/10.3390/diagnostics13172817









