Vitamin D Deficiency (VDD) and Benefits of Supplementation in Veterans with IBS-D
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Patient Evaluation
2.3. Data Collection
2.4. Statistical Analysis
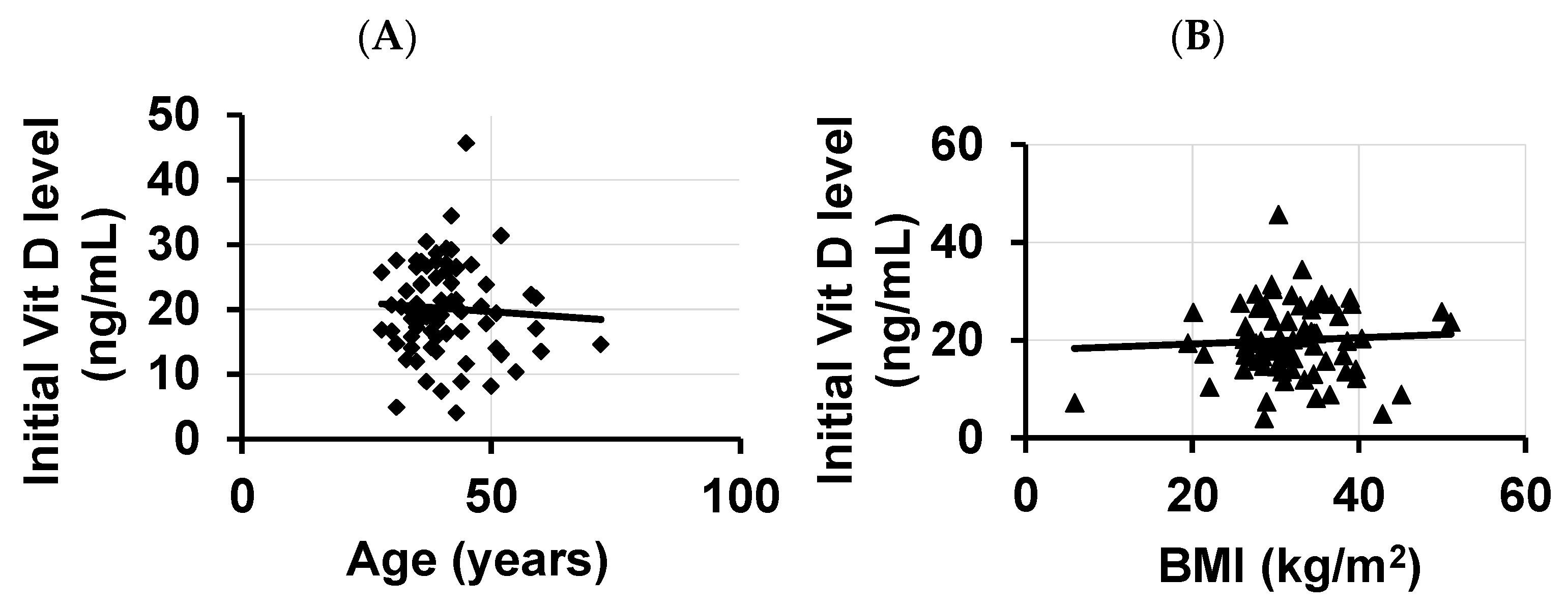
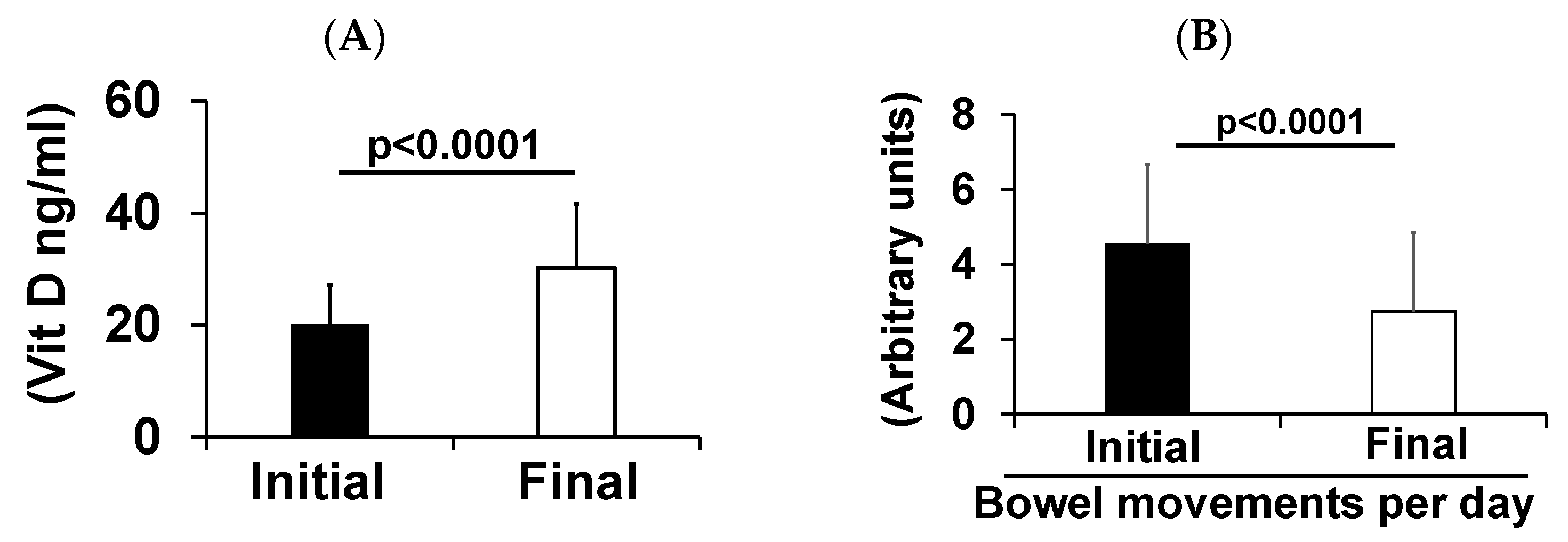
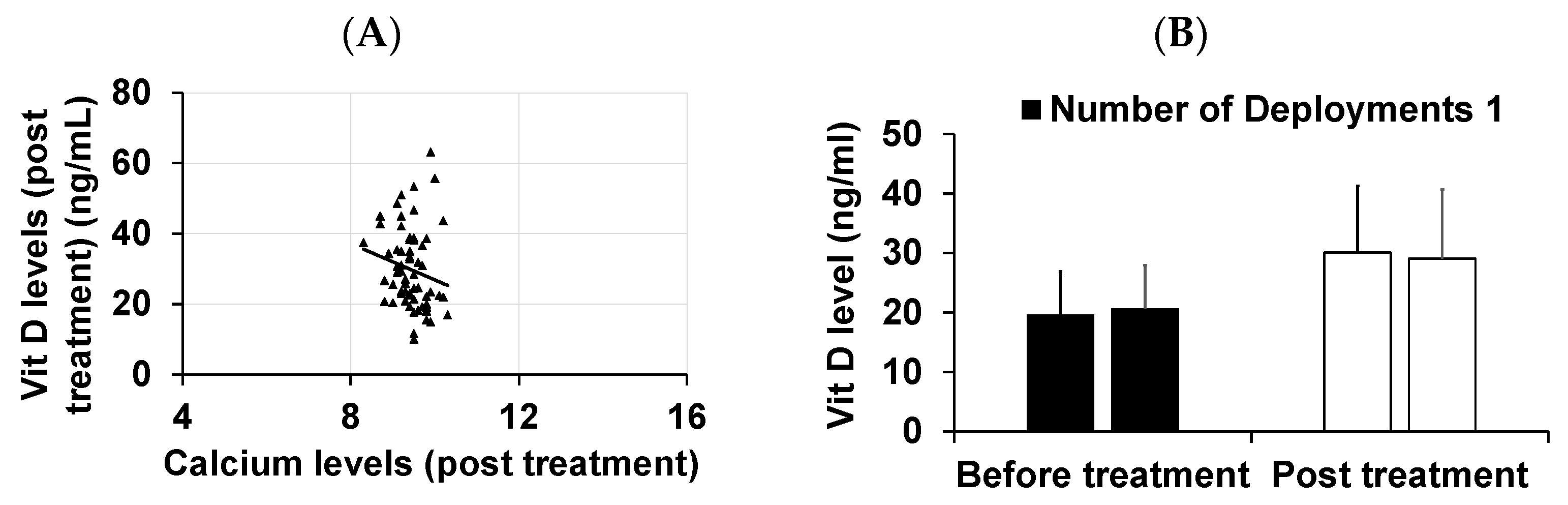
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sartin, J.S. Infectious Diseases During the Civil War: The Triumph of the “Third Army”. Clin. Infect. Dis. 1993, 16, 580–584. [Google Scholar] [CrossRef]
- Tuteja, A.K.; Talley, N.J.; Stoddard, G.J.; Samore, M.H.; Verne, G.N. Risk factors for upper and lower functional gastrointestinal disorders in Persian Gulf War Veterans during and post-deployment. Neurogastroenterol. Motil. 2019, 31, e13533. [Google Scholar] [CrossRef]
- Verne, Z.T.; Fields, J.Z.; Zhang, B.B.; Zhou, Q. Autonomic dysfunction and gastroparesis in Gulf War veterans. J. Investig. Med. 2023, 71, 7–10. [Google Scholar] [CrossRef]
- White, D.L.; Savas, L.S.; Daci, K.; Elserag, R.; Graham, D.P.; Fitzgerald, S.J.; Smith, S.L.; Tan, G.; El-Serag, H.B. Trauma history and risk of the irritable bowel syndrome in women veterans. Aliment. Pharmacol. Ther. 2010, 32, 551–561. [Google Scholar] [CrossRef]
- Riddle, M.S.; Welsh, M.; Porter, C.K.; Nieh, C.; Boyko, E.J.; Gackstetter, G.; Hooper, T.I. The Epidemiology of Irritable Bowel Syndrome in the US Military: Findings from the Millennium Cohort Study. Am. J. Gastroenterol. 2016, 111, 93–104. [Google Scholar] [CrossRef]
- Borges-Vieira, J.G.; Cardoso, C.K.S. Efficacy of B-vitamins and vitamin D therapy in improving depressive and anxiety disorders: A systematic review of randomized controlled trials. Nutr. Neurosci. 2023, 26, 187–207. [Google Scholar] [CrossRef]
- Casseb, G.A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef]
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. [Google Scholar] [CrossRef]
- Terock, J.; Hannemann, A.; Van der Auwera, S.; Janowitz, D.; Spitzer, C.; Bonk, S.; Völzke, H.; Grabe, H.J. Posttraumatic stress disorder is associated with reduced vitamin D levels and functional polymorphisms of the vitamin D binding-protein in a population-based sample. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 96, 109760. [Google Scholar] [CrossRef]
- Kimono, D.A. Gastrointestinal problems, mechanisms and possible therapeutic directions in Gulf war illness: A mini review. Mil. Med. Res. 2021, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; Romine, M.; Monteville, M.R.; Ahmed, S.F.; Rockabrand, D.M.; Frenck, R.W.; Schlett, C.; Sanders, J.W. A Prospective Study of Acute Diarrhea in a Cohort of United States Military Personnel on Deployment to the Multinational Force and Observers, Sinai, Egypt. Am. J. Trop. Med. Hyg. 2011, 84, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Yates, J. Traveler’s diarrhea. Am. Fam. Physician 2005, 71, 2095–2100. [Google Scholar] [PubMed]
- Wang, W.-F.; Guo, X.-X.; Yang, Y.-S. Gastrointestinal problems in modern wars: Clinical features and possible mechanisms. Mil. Med. Res. 2015, 2, 15. [Google Scholar] [CrossRef][Green Version]
- Parry, S.; Forgacs, I. Intestinal infection and irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2005, 17, 5–9. [Google Scholar] [CrossRef]
- Thabane, M.; Kottachchi, D.T.; Marshall, J.K. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007, 26, 535–544. [Google Scholar] [CrossRef]
- Hyams, K.C.; Malone, J.D.; Kapikian, A.Z.; Estes, M.K.; Jiang, X.; Bourgeois, A.L.; Paparello, S.; Hawkins, R.E.; Green, K.Y. Norwalk Virus Infection among Desert Storm Troops. J. Infect. Dis. 1993, 167, 986–987. [Google Scholar] [CrossRef]
- Porter, C.K.; Gloor, K.; Cash, B.D.; Riddle, M.S. Risk of Functional Gastrointestinal Disorders in U.S. Military Following Self-Reported Diarrhea and Vomiting During Deployment. Dig. Dis. Sci. 2011, 56, 3262–3269. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective Study of Predictors of Vitamin D Status and Cancer Incidence and Mortality in Men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef]
- Dobnig, H.; Pilz, S.; Scharnagl, H.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Kinkeldei, J.; Boehm, B.O.; Weihrauch, G.; Maerz, W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008, 168, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Ranjan, P. Post-infectious irritable bowel syndrome: The past, the present and the future. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S3), 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Guo, X.; Yang, Y.; Peng, L.; Mao, G.; Qurratulain, H.; Wang, W.; Sun, G. The Prevalence of Functional Gastrointestinal Disorders in the Chinese Air Force Population. Gastroenterol. Res. Pract. 2013, 2013, 497585. [Google Scholar] [CrossRef] [PubMed]
- Dierkes-Globisch, A.; Fallen, H.; Hans-Heinrich, M. Functional Gastrointestinal Disorders among Soldiers in Peacetime Versus Out-of-Area Missions. Mil. Med. 2001, 166, 223–225. [Google Scholar] [CrossRef]
- Koch, T.R.; Emory, T.S. Evaluation of Chronic Gastrointestinal Symptoms following Persian Gulf War Exposure. Mil. Med. 2005, 170, 696–700. [Google Scholar] [CrossRef][Green Version]
- Camilleri, M.; Boeckxstaens, G. Irritable bowel syndrome: Treatment based on pathophysiology and biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Di Rosa, C.; Imperia, E.; Emerenziani, S.; Cicala, M.; Guarino, M.P.L. Diarrhea Predominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2021, 13, 1506. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2020, 116, 17–44. [Google Scholar] [CrossRef]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent advances in the treatment of irritable bowel syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef]
- Brenner, D.M.; Sayuk, G.S. Current US Food and Drug Administration-Approved Pharmacologic Therapies for the Treatment of Irritable Bowel Syndrome with Diarrhea. Adv. Ther. 2020, 37, 83–96. [Google Scholar] [CrossRef]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Hungin, A.P.S.; Chang, L.; Locke, G.R.; Dennis, E.H.; Barghout, V. Irritable bowel syndrome in the United States: Prevalence, symptom patterns and impact. Aliment. Pharmacol. Ther. 2005, 21, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Nellesen, D.; Yee, K.; Chawla, A.; Lewis, B.E.; Carson, R.T. A Systematic Review of the Economic and Humanistic Burden of Illness in Irritable Bowel Syndrome and Chronic Constipation. J. Manag. Care Pharm. 2013, 19, 755–764. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Burchette, R.J. Family practitioners’ attitudes and knowledge about irritable bowel syndrome: Effect of a trial of physician education. Fam. Pract. 2003, 20, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Heaton, K.W.; Smyth, G.T.; Smyth, C. Irritable bowel syndrome: The view from general practice. Eur. J. Gastroenterol. Hepatol. 1997, 9, 689–692. [Google Scholar] [CrossRef]
- Wilson, S.; Roberts, L.; Roalfe, A.; Bridge, P.; Singh, S. Prevalence of irritable bowel syndrome: A community survey. Br. J. Gen. Pract. 2004, 54, 495–502. [Google Scholar]
- Russo, M.W.; Gaynes, B.N.; Drossman, D.A. A National Survey of Practice Patterns of Gastroenterologists With Comparison to the Past Two Decades. J. Clin. Gastroenterol. 1999, 29, 339–343. [Google Scholar] [CrossRef]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef]
- Jung, E.; Ro, Y.S.; Park, J.H.; Moon, S.B.; Lee, S.G.W.; Park, G.J.; Ryu, H.H.; Shin, S.D.; The Pan-Asia Trauma Outcomes Study for Traumatic Brain Injury (PATOS-TBI) Research Network. Vitamin D Deficiency and Prognosis after Traumatic Brain Injury with Intracranial Injury: A Multi-Center Observational Study. J. Neurotrauma 2022, 39, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.; Amani, R.; Hasanvand, A.; Rad, E.Y.; Alipour, M.; Saboori, S.; Choghakhori, R. Association of Serum Vitamin D Concentration With Clinical Symptoms and Quality of Life in Patients With Irritable Bowel Syndrome. J. Am. Coll. Nutr. 2019, 38, 327–333. [Google Scholar] [CrossRef]
- Gutiérrez, O.I.; Polo, J.D.; Zambrano, M.J.; Molina, D.C. Meta-analysis and Scientific Mapping of Well-being and Job Performance. Span. J. Psychol. 2020, 23, e43. [Google Scholar] [CrossRef]
- Khayyat, Y.; Attar, S. Vitamin D Deficiency in Patients with Irritable Bowel Syndrome: Does it Exist? Oman Med. J. 2015, 30, 115–118. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Aluisio, A.R.; Maroof, Z.; Chandramohan, D.; Bruce, J.; Mughal, M.Z.; Bhutta, Z.; Walraven, G.; Masher, M.I.; Ensink, J.H.; Manaseki-Holland, S. Vitamin D3 Supplementation and Childhood Diarrhea: A Randomized Controlled Trial. Pediatrics 2013, 132, e832–e840. [Google Scholar] [CrossRef]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The efficacy of vitamin D supplementation for irritable bowel syndrome: A systematic review with meta-analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Bhandari, A.; Chaudhary, A. Low Vitamin D Levels in Patients with Irritable Bowel Syndrome of a Tertiary Care Hospital: A Descriptive Cross-sectional Study. J. Nepal Med. Assoc. 2021, 59, 894–898. [Google Scholar] [CrossRef]
- Zhang, L.; Han, C.; Zhang, S.; Duan, C.; Shang, H.; Bai, T.; Hou, X. Diarrhea and altered inflammatory cytokine pattern in severe coronavirus disease 2019: Impact on disease course and in-hospital mortality. J. Gastroenterol. Hepatol. 2021, 36, 421–429. [Google Scholar] [CrossRef] [PubMed]
| N | Mean | SD | SEM | |
|---|---|---|---|---|
| Vitamin D initial level | 69 | 20.06 | 7.10 | 0.86 |
| Test value = 30.0 | ||||
| Vitamin D initial level | t | df | Sig. (2-tailed) | |
| −11.62 | 68 | 0.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesavan, C.; Das, A.; Goyal, P.; Jackson, C.S.; Strong, D.D.; Strong, R.M. Vitamin D Deficiency (VDD) and Benefits of Supplementation in Veterans with IBS-D. Diagnostics 2023, 13, 2807. https://doi.org/10.3390/diagnostics13172807
Kesavan C, Das A, Goyal P, Jackson CS, Strong DD, Strong RM. Vitamin D Deficiency (VDD) and Benefits of Supplementation in Veterans with IBS-D. Diagnostics. 2023; 13(17):2807. https://doi.org/10.3390/diagnostics13172807
Chicago/Turabian StyleKesavan, Chandrasekhar, Anjali Das, Preeya Goyal, Christian S. Jackson, Donna D. Strong, and Richard M. Strong. 2023. "Vitamin D Deficiency (VDD) and Benefits of Supplementation in Veterans with IBS-D" Diagnostics 13, no. 17: 2807. https://doi.org/10.3390/diagnostics13172807
APA StyleKesavan, C., Das, A., Goyal, P., Jackson, C. S., Strong, D. D., & Strong, R. M. (2023). Vitamin D Deficiency (VDD) and Benefits of Supplementation in Veterans with IBS-D. Diagnostics, 13(17), 2807. https://doi.org/10.3390/diagnostics13172807





