Ultrasound and Clinical Alterations in the Foot of Children with Obesity and Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Anthropometry
2.3. Clinical Measurements of the Foot
2.4. Ultrasound Examination of the Foot
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Relationship between Clinical Measurements of the Foot and Anthropometric Parameters
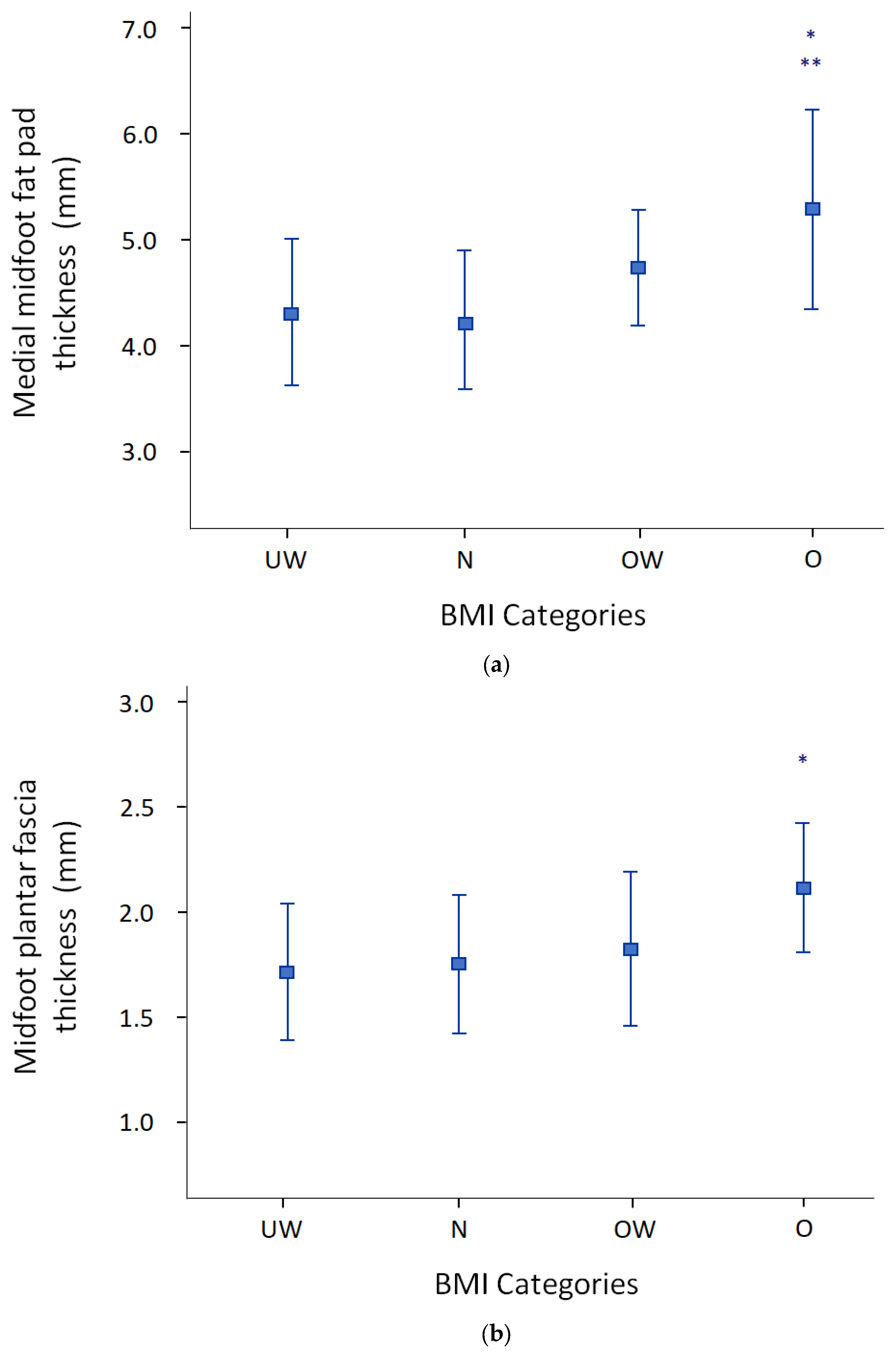
3.3. Relationship between Ultrasound of the Foot and Anthropometric Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riddiford-Harland, D.L.; Steele, J.R.; Storlien, L.H. Does obesity influence foot structure in prepubescent children? Int. J. Obes. Relat. Metab. Disord. 2000, 24, 541–544. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D. Measuring growth and obesity across childhood and adolescence. Proc. Nutr. Soc. 2014, 73, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Lu, D.; Cai, G.; Zhang, J.; Wang, X.; Suzuki, K. Accelerated skeletal maturation is associated with overweight and obesity as early as preschool age: A cross-sectional study. BMC Pediatr. 2020, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.M.; Steele, J.R.; Baur, L.A. Does obesity influence foot structure and plantar pressure patterns in prepubescent children? Int. J. Obes. Relat. Metab. Disord. 2001, 25, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Riddiford-Harland, D.L.; Steele, J.R.; Baur, L.A. Medial midfoot fat pad thickness and plantar pressures: Are these related in children? Int. J. Pediatr. Obes. 2011, 6, 261–266. [Google Scholar] [CrossRef]
- Riddiford-Harland, D.L.; Steele, J.R.; Baur, L.A. Are the feet of obese children fat or flat? Revisiting the debate. Int. J. Obes. 2011, 35, 115–120. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef]
- Tüzün, B.; Tüzün, Y.; Dinççağ, N.; Minareci, O.; Oztürk, S.; Yilmaz, M.T.; Satman, I.; Yazici, H. Diabetic sclerodactyly. Diabetes Res. Clin. Pract. 1995, 27, 153–157. [Google Scholar] [CrossRef]
- Abouaesha, F.; van Schie, C.; Griffiths, G.; Young, R.; Boulton, A. Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care 2001, 24, 1270–1274. [Google Scholar] [CrossRef][Green Version]
- Rosenbloom, A.; Silverstein, J.; Lezotte, D.; Richardson, K.; McCallum, M. Limited joint mobility indicates increased risk of microvascular disease. N. Engl. J. Med. 1981, 305, 191–194. [Google Scholar] [CrossRef]
- Duffin, A.C.; Lam, A.; Kidd, R.; Chan, F.K.A.; Donaghue, K.C. Ultrasonography of plantar soft tissues thickness in young people with diabetes. Diabet. Med. 2002, 19, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Truby, H.; Abbott, R.A.; Davies, P.S.W. Validating the waist-height ratio and developing centiles for use amongst children and adolescents. Acta Paediatr. 2009, 98, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.C.; Ferrari, J. Inter-rater reliability of the Foot Posture Index (FPI-6) in the assessment of the paediatric foot. J. Foot Ankle Res. 2009, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.M. Techniques in the evaluation and treatment of the injured runner. Orthop. Clin. N. Am. 1982, 13, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Zuil-Escobar, J.C.; Martinez-Cepa, C.B.; Martin-Urrialde, J.A. Evaluating the Medial Longitudinal Arch of the Foot: Correlations, Reliability, and Accuracy in People with a Low Arch. Phys. Ther. 2019, 99, 364–372. [Google Scholar] [CrossRef]
- Riddiford-Harland, D.L.; Steele, J.R.; Baur, L.A. The use of ultrasound imaging to measure midfoot plantar fat pad thickness in children. J. Orthop. Sports Phys. Ther. 2007, 37, 644–647. [Google Scholar] [CrossRef][Green Version]
- McNally, E.G.; Shetty, S. Plantar fascia: Imaging diagnosis and guided treatment. Semin. Musculoskelet. Radiol. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wong, T.Y.; Wong, S.M.; Wong, M.W.; Metreweli, C. Sonography of plantar fibromatosis. AJR Am. J. Roentgenol. 2002, 179, 1167–1172. [Google Scholar] [CrossRef]
- Boules, M.; Batayyah, E.; Froylich, D.; Zelisko, A.; O’Rourke, C.; Brethauer, S.; El-Hayek, K.; Boike, A.; Strong, A.T.; Kroh, M. Effect of Surgical Weight Loss on Plantar Fasciitis and Health-Care Use. J. Am. Podiatr. Med. Assoc. 2018, 108, 442–448. [Google Scholar] [CrossRef]
- Taş, S.; Bek, N.; Ruhi Onur, M.; Korkusuz, F. Effects of Body Mass Index on Mechanical Properties of the Plantar Fascia and Heel Pad in Asymptomatic Participants. Foot Ankle Int. 2017, 38, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Hasanabadi, H.; Ashrafzadeh, F.; Sarvari, S.; Rahimi, H.; Hashemian, S. Peripheral Neuropathy in Children and Adolescents with Insulin-dependent Diabetes Mellitus. Iran. J. Child Neurol. 2018, 12, 83–90. [Google Scholar] [PubMed]
- Terada, M.; Wittwer, A.M.; Gribble, P.A. Intra-rater and inter-rater reliability of the five image-based criteria of the foot posture index-6. Int. J. Sports Phys. Ther. 2014, 9, 187–194. [Google Scholar] [PubMed]
- Hegazy, F.A.; Aboelnasr, E.A.; Salem, Y.; Zaghloul, A.A. Validity and diagnostic accuracy of foot posture Index-6 using radiographic findings as the gold standard to determine paediatric flexible flatfoot between ages of 6–18 years: A cross-sectional study. Musculoskelet. Sci. Pract. 2020, 46, 102107. [Google Scholar] [CrossRef]
- Evans, A.M.; Karimi, L. The relationship between paediatric foot posture and body mass index: Do heavier children really have flatter feet? J. Foot Ankle Res 2015, 8, 46. [Google Scholar] [CrossRef]
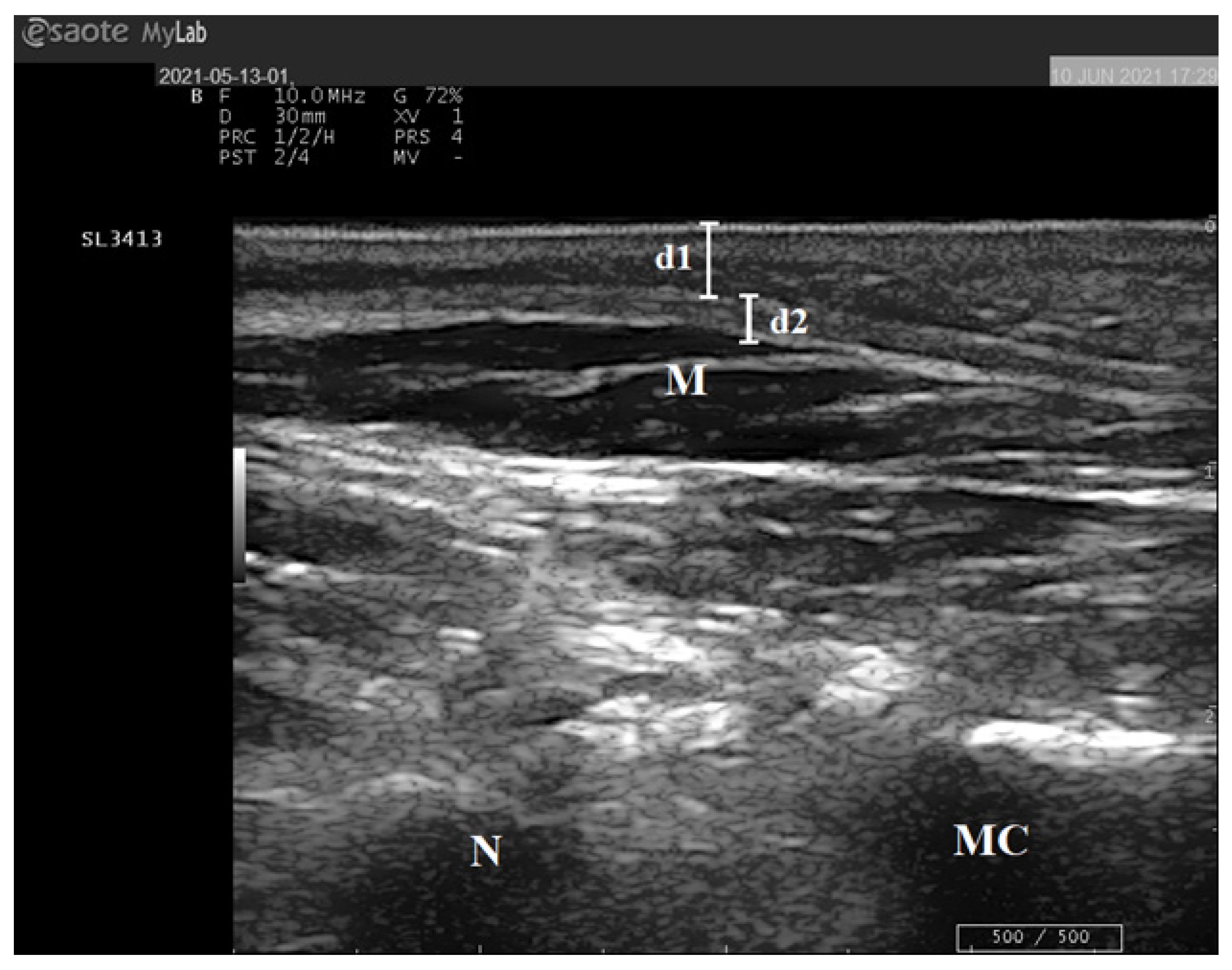
| Characteristics | Total (n = 43) | T1D (n = 29) | Controls (n = 14) | p-Value |
|---|---|---|---|---|
| Age (years) | 12.9 (2.6) | 12.86 (2.35) | 12.93 (2.74) | 0.931 |
| Male, n (%) | 20 (46.5) | 16 (55) | 7 (50) | 0.75 |
| Height (cm) | 157.7 (15) | 158 (15) | 155 (16) | 0.487 |
| Weight (kg) | 64.2 (27.6) | 68.73 (29) | 54.93 (22.64) | 0.126 |
| WC (cm) | 86.4 (19.1) | 89.74 (20.39) | 79.36 (14.13) | 0.094 |
| WHtR (cm) | 0.5 (0.1) | 0.56 (0.11) | 0.51 (0.06) | 0.087 |
| BMI (kg/m2) | 24.9 (7.6) | 26.48 (8.12) | 21.76 (5.49) | 0.057 |
| Underweight, n (%) | 11 (25.6) | 5 (17) | 6 (43) | 0.24 |
| Normal weight, n (%) | 10 (23.3) | 7 (24) | 3 (21) | |
| Overweight, n (%) | 9 (20.9) | 6 (21) | 3 (21) | |
| Obese, n (%) | 13 (30.2) | 11 (38) | 2 (14) | |
| r-NDT (mm) | 11.4 (3.4) | 11.72 (3.62) | 10.86 (2.77) | 0.435 |
| l-NDT (mm) | 12.0 (2.9) | 12.24 (3.16) | 11.50 (2.35) | 0.441 |
| r-FPI6 | 6.9 (3.4) | 7.24 (3.63) | 6.14 (2.98) | 0.332 |
| l-FPI6 | 7.4 (3.5) | 7.93 (3.61) | 6.21 (2.94) | 0.130 |
| r-MMFP (mm) | 4.8 (0.9) | 4.90 (1.02) | 4.52 (0.70) | 0.222 |
| l-MMFP (mm) | 4.6 (0.9) | 4.69 (0.93) | 4.28 (0.76) | 0.158 |
| MMFP total (mm) | 4.7 (0.9) | 4.80 (0.92) | 4.40 (0.64) | 0.159 |
| r-MPF (mm) | 1.8 (0.4) | 1.85 (0.41) | 1.76 (0.32) | 0.507 |
| l-MPF (mm) | 1.9 (0.4) | 1.94 (0.44) | 1.81 (0.38) | 0.361 |
| MPF total (mm) | 1.9 (0.4) | 1.90 (0.39) | 1.79 (0.31) | 0.400 |
| Factor | Statistics | Age | Weight | Height | BMI | WC | WHtR | r-NDT | l-NDT | r-FPI6 | l-FPI6 | MMFP Tot | r-MMFP | l-MMFP | MPF Tot | r-MPF | l-MPF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Pearson’s r | 1 | 0.562 ** | 0.865 ** | 0.297 | 0.362 * | −0.031 | −0.178 | −0.142 | −0.138 | −0.107 | 0.278 | 0.238 | 0.284 | 0.084 | 0.070 | 0.057 |
| p-value | <0.001 | <0.001 | 0.053 | 0.017 | 0.842 | 0.252 | 0.363 | 0.379 | 0.493 | 0.071 | 0.125 | 0.065 | 0.591 | 0.665 | 0.718 | ||
| Weight | Pearson’s r | 0.562 ** | 1 | 0.743 ** | 0.928 ** | 0.929 ** | 0.704 ** | 0.144 | 0.143 | 0.098 | 0.073 | 0.668 ** | 0.608 ** | 0.643 ** | 0.357 * | 0.376 * | 0.278 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.356 | 0.360 | 0.530 | 0.642 | <0.001 | <0.001 | <0.001 | 0.019 | 0.015 | 0.075 | ||
| Height | Pearson’s r | 0.865 ** | 0.743 ** | 1 | 0.465 ** | 0.541 ** | 0.122 | −0.132 | −0.067 | −0.124 | −0.059 | 0.383 * | 0.313 * | 0.406 ** | 0.197 | 0.225 | 0.121 |
| p-value | <0.001 | <0.001 | 0.002 | <0.001 | 0.435 | 0.397 | 0.670 | 0.428 | 0.707 | 0.011 | 0.041 | 0.007 | 0.205 | 0.157 | 0.444 | ||
| BMI | Pearson’s r | 0.297 | 0.928 ** | 0.465 ** | 1 | 0.953 ** | 0.883 ** | 0.258 | 0.255 | 0.245 | 0.164 | 0.659 ** | 0.629 ** | 0.603 ** | 0.377 * | 0.376 * | 0.316 * |
| p-value | 0.053 | <0.001 | 0.002 | <0.001 | <0.001 | 0.094 | 0.099 | 0.113 | 0.293 | <0.001 | <0.001 | <0.001 | 0.013 | 0.016 | 0.042 | ||
| WC | Pearson’s r | 0.362 * | 0.929 ** | 0.541 ** | 0.953 ** | 1 | 0.898 ** | 0.225 | 0.304 * | 0.145 | 0.183 | 0.681 ** | 0.665 ** | 0.606 ** | 0.376 * | 0.390 * | 0.300 |
| p-value | 0.017 | <0.001 | <0.001 | <0.001 | <0.001 | 0.148 | 0.048 | 0.352 | 0.240 | <0.001 | <0.001 | <0.001 | 0.013 | 0.012 | 0.054 | ||
| WHtR | Pearson’s r | −0.031 | 0.704 ** | 0.122 | 0.883 ** | 0.898 ** | 1 | 0.317 * | 0.392 ** | 0.248 | 0.258 | 0.587 ** | 0.610 ** | 0.484 ** | 0.339 * | 0.337 * | 0.288 |
| p-value | 0.842 | <0.001 | 0.435 | <0.001 | <0.001 | 0.038 | 0.009 | 0.109 | 0.094 | <0.001 | <0.001 | 0.001 | 0.026 | 0.031 | 0.064 | ||
| r-NDT | Pearson’s r | −0.178 | 0.144 | −0.132 | 0.258 | 0.225 | 0.317 * | 1 | 0.805 ** | 0.606 ** | 0.467 ** | 0.179 | 0.023 | 0.319 * | 0.175 | 0.211 | 0.115 |
| p-value | 0.252 | 0.356 | 0.397 | 0.094 | 0.148 | 0.038 | <0.001 | <0.001 | 0.002 | 0.251 | 0.882 | 0.037 | 0.262 | 0.186 | 0.469 | ||
| l-NDT | Pearson’s r | −0.142 | 0.143 | −0.067 | 0.255 | 0.304 * | 0.392 ** | 0.805 ** | 1 | 0.657 ** | 0.726 ** | 0.243 | 0.142 | 0.318 * | 0.165 | 0.213 | 0.087 |
| p-value | 0.363 | 0.360 | 0.670 | 0.099 | 0.048 | 0.009 | <0.001 | <0.001 | <0.001 | 0.116 | 0.363 | 0.038 | 0.291 | 0.180 | 0.585 | ||
| r-FPI6 | Pearson’s r | −0.138 | 0.098 | −0.124 | 0.245 | 0.145 | 0.248 | 0.606 ** | 0.657 ** | 1 | 0.754 ** | 0.116 | 0.008 | 0.216 | 0.161 | 0.233 | 0.082 |
| p-value | 0.379 | 0.530 | 0.428 | 0.113 | 0.352 | 0.109 | <0.001 | <0.001 | <0.001 | 0.457 | 0.962 | 0.165 | 0.303 | 0.143 | 0.605 | ||
| l-FPI6 | Pearson’s r | −0.107 | 0.073 | −0.059 | 0.164 | 0.183 | 0.258 | 0.467 ** | 0.726 ** | 0.754 ** | 1 | 0.220 | 0.164 | 0.250 | 0.120 | 0.158 | 0.063 |
| p-value | 0.493 | 0.642 | 0.707 | 0.293 | 0.240 | 0.094 | 0.002 | <0.001 | <0.001 | 0.156 | 0.294 | 0.106 | 0.445 | 0.324 | 0.690 | ||
| MMFP tot | Pearson’s r | 0.278 | 0.668 ** | 0.383 * | 0.659 ** | 0.681 ** | 0.587 ** | 0.179 | 0.243 | 0.116 | 0.220 | 1 | 0.939 ** | 0.931 ** | 0.337 * | 0.416 ** | 0.214 |
| p-value | 0.071 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 | 0.251 | 0.116 | 0.457 | 0.156 | <0.001 | <0.001 | 0.027 | 0.007 | 0.173 | ||
| r-MMFP | Pearson’s r | 0.238 | 0.608 ** | 0.313 * | 0.629 ** | 0.665 ** | 0.610 ** | 0.023 | 0.142 | 0.008 | 0.164 | 0.939 ** | 1 | 0.748 ** | 0.356 * | 0.396 * | 0.264 |
| p-value | 0.125 | <0.001 | 0.041 | <0.001 | <0.001 | <0.001 | 0.882 | 0.363 | 0.962 | 0.294 | <0.001 | <0.001 | 0.019 | 0.010 | 0.091 | ||
| l-MMFP | Pearson’s r | 0.284 | 0.643 ** | 0.406 ** | 0.603 ** | 0.606 ** | 0.484 ** | 0.319 * | 0.318 * | 0.216 | 0.250 | 0.931 ** | 0.748 ** | 1 | 0.271 | 0.371 * | 0.132 |
| p-value | 0.065 | <0.001 | 0.007 | <0.001 | <0.001 | 0.001 | 0.037 | 0.038 | 0.165 | 0.106 | <0.001 | <0.001 | 0.079 | 0.017 | 0.404 | ||
| MPF tot | Pearson’s r | 0.084 | 0.357 * | 0.197 | 0.377 * | 0.376 * | 0.339 * | 0.175 | 0.165 | 0.161 | 0.120 | 0.337 * | 0.356 * | 0.271 | 1 | 0.922 ** | 0.936 ** |
| p-value | 0.591 | 0.019 | 0.205 | 0.013 | 0.013 | 0.026 | 0.262 | 0.291 | 0.303 | 0.445 | 0.027 | 0.019 | 0.079 | <0.001 | <0.001 | ||
| r-MPF | Pearson’s r | 0.070 | 0.376 * | 0.225 | 0.376 * | 0.390 * | 0.337 * | 0.211 | 0.213 | 0.233 | 0.158 | 0.416 ** | 0.396 * | 0.371 * | 0.922 ** | 1 | 0.727 ** |
| p-value | 0.665 | 0.015 | 0.157 | 0.016 | 0.012 | 0.031 | 0.186 | 0.180 | 0.143 | 0.324 | 0.007 | 0.010 | 0.017 | <0.001 | <0.001 | ||
| l-MPF | Pearson’s r | 0.057 | 0.278 | 0.121 | 0.316 * | 0.300 | 0.288 | 0.115 | 0.087 | 0.082 | 0.063 | 0.214 | 0.264 | 0.132 | 0.936 ** | 0.727 ** | 1 |
| p-value | 0.718 | 0.075 | 0.444 | 0.042 | 0.054 | 0.064 | 0.469 | 0.585 | 0.605 | 0.690 | 0.173 | 0.091 | 0.404 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappalardo, M.; Gori, L.; Randazzo, E.; Morganti, R.; Scaglione, M.; Valiani, M.; Beni, A.; Di Cicco, M.; Peroni, D.G.; Franzoni, F.; et al. Ultrasound and Clinical Alterations in the Foot of Children with Obesity and Diabetes. Diagnostics 2023, 13, 2781. https://doi.org/10.3390/diagnostics13172781
Pappalardo M, Gori L, Randazzo E, Morganti R, Scaglione M, Valiani M, Beni A, Di Cicco M, Peroni DG, Franzoni F, et al. Ultrasound and Clinical Alterations in the Foot of Children with Obesity and Diabetes. Diagnostics. 2023; 13(17):2781. https://doi.org/10.3390/diagnostics13172781
Chicago/Turabian StylePappalardo, Martina, Laura Gori, Emioli Randazzo, Riccardo Morganti, Michelangelo Scaglione, Margherita Valiani, Alessandra Beni, Maria Di Cicco, Diego G. Peroni, Ferdinando Franzoni, and et al. 2023. "Ultrasound and Clinical Alterations in the Foot of Children with Obesity and Diabetes" Diagnostics 13, no. 17: 2781. https://doi.org/10.3390/diagnostics13172781
APA StylePappalardo, M., Gori, L., Randazzo, E., Morganti, R., Scaglione, M., Valiani, M., Beni, A., Di Cicco, M., Peroni, D. G., Franzoni, F., & Comberiati, P. (2023). Ultrasound and Clinical Alterations in the Foot of Children with Obesity and Diabetes. Diagnostics, 13(17), 2781. https://doi.org/10.3390/diagnostics13172781









