Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
2.2. EUS-FNB/FNA Procedure
2.3. Immunohistochemistry
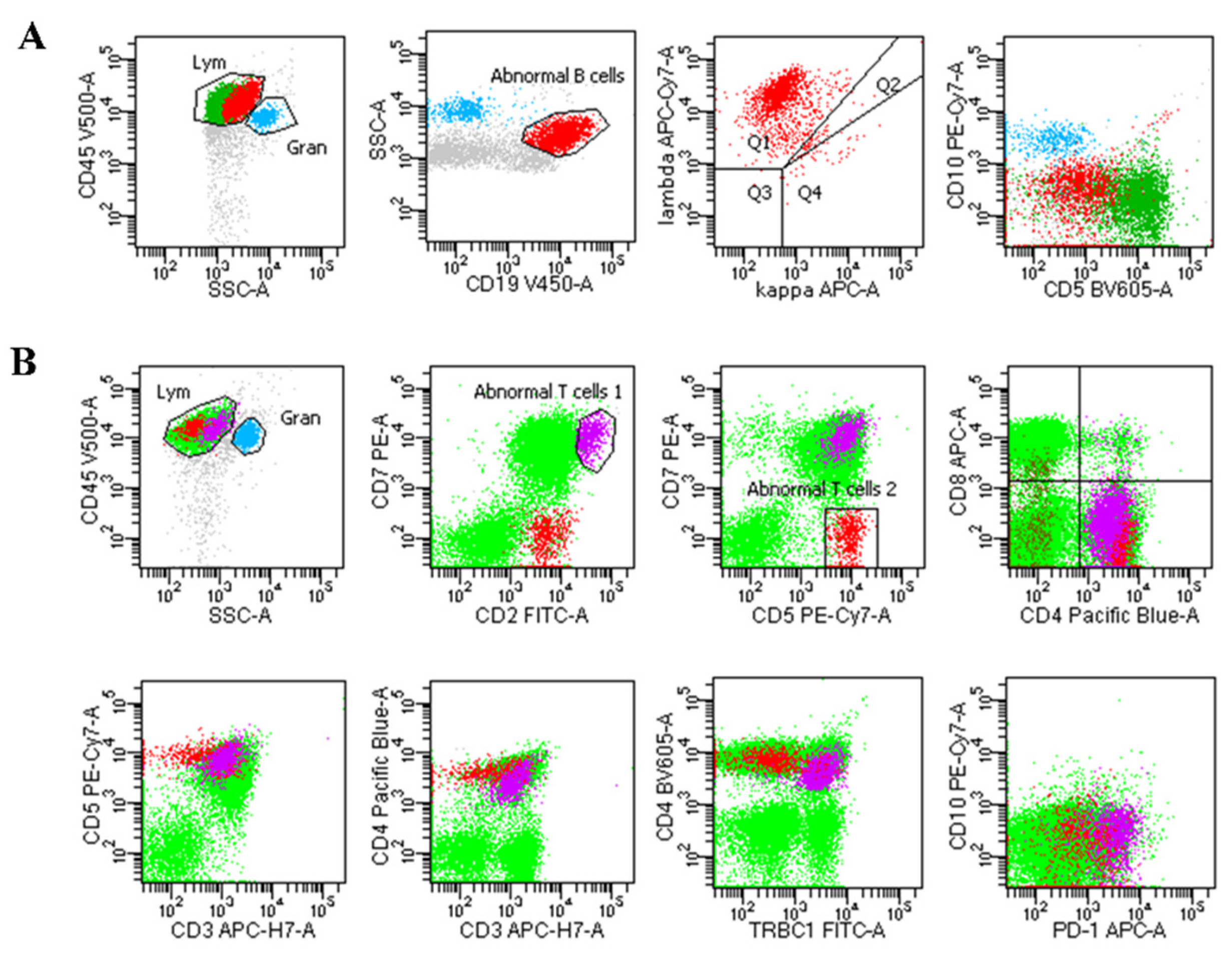
2.4. Flow Cytometry
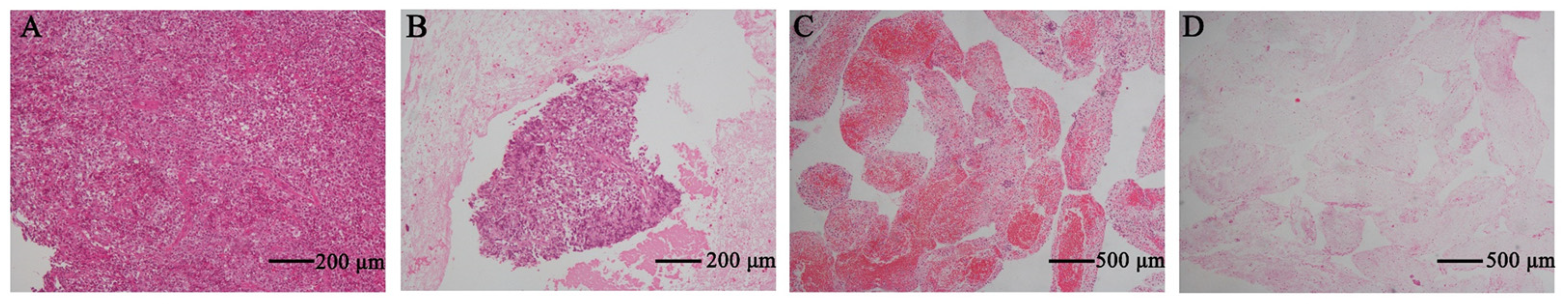
2.5. Specimen Evaluation
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Baseline Data and Lesion Characteristics of Patients
3.2. Characteristics of Needle and Sampling
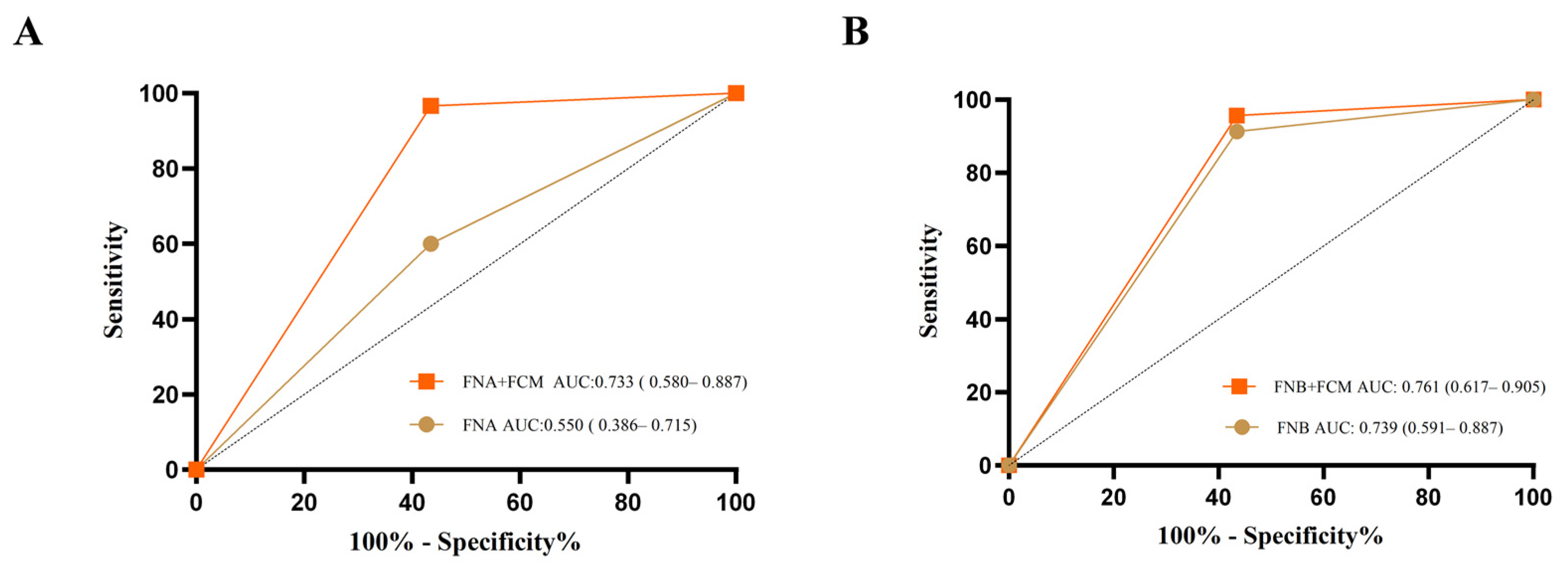
3.3. Diagnostic Accuracy
3.4. Multivariate Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.K.; Eloubeidi, M.A. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: A prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am. J. Gastroenterol. 2004, 99, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.L.; Jhala, N.C.; Eloubeidi, M.A.; Chhieng, D.C.; Eltoum, I.A.; Crowe, D.R.; Varadarajulu, S.; Jhala, D.N. Diagnosis of deep-seated lymphoma and leukemia by endoscopic ultrasound-guided fine-needle aspiration biopsy. Am. J. Clin. Pathol. 2006, 125, 703–709. [Google Scholar] [CrossRef]
- Ribeiro, A.; Pereira, D.; Escalón, M.P.; Goodman, M.; Byrne, G.E., Jr. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest. Endosc. 2010, 71, 851–855. [Google Scholar] [CrossRef]
- Poincloux, L.; André, M.; Darcha, C.; Goutte, M.; Dapoigny, M.; Bommelaer, G.; Abergel, A.; Tournilhac, O. Usefulness of EUS-guided fine needle aspiration biopsy in the diagnosis of suspected or recurring lymphoproliferative disorders. Surg. Oncol. 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Tejedor-Tejada, J.; Chavarría, C.; Burgueño-Gómez, B.; Fanjul, I.; García-Alonso, F.J.; Torres, M.Á.; Madrigal, B.; Pérez-Miranda, M.; De la Serna-Higuera, C. Role of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and classification of lymphomas. Rev. Esp. De Enfermedades Dig. 2021, 113, 404–410. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Gao, X.; Lin, S.; He, L.; Luo, G.; Li, J.; Huang, C.; Wang, G.; Yang, Q.; et al. High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 2763–2775. [Google Scholar] [CrossRef]
- Frederiksen, J.K.; Sharma, M.; Casulo, C.; Burack, W.R. Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch. Pathol. Lab. Med. 2015, 139, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhang, Y.; Chen, Q.; Sun, B.; Deng, Z.; Shan, H.; Dou, L.; Wang, J.; Li, Y.; Yang, X.; et al. Analysis of Fine-Needle Biopsy vs Fine-Needle Aspiration in Diagnosis of Pancreatic and Abdominal Masses: A Prospective, Multicenter, Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, Y.; Itoi, T.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Nagai, K.; Yamamoto, K.; Asai, Y.; et al. Objective evaluation of the resistance forces of 22-gauge EUS-FNA and fine-needle biopsy needles. Endosc. Ultrasound 2023, 12, 251–258. [Google Scholar]
- Zeppa, P.; Marino, G.; Troncone, G.; Fulciniti, F.; De Renzo, A.; Picardi, M.; Benincasa, G.; Rotoli, B.; Vetrani, A.; Palombini, L. Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: A critical review of 307 cases with technical suggestions. Cancer 2004, 102, 55–65. [Google Scholar] [CrossRef]
- Yasuda, I.; Goto, N.; Tsurumi, H.; Nakashima, M.; Doi, S.; Iwashita, T.; Kanemura, N.; Kasahara, S.; Adachi, S.; Hara, T.; et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am. J. Gastroenterol. 2012, 107, 397–404. [Google Scholar] [CrossRef]
- Barroca, H.; Marques, C. A Basic Approach to Lymph Node and Flow Cytometry Fine-Needle Cytology. Acta Cytol. 2016, 60, 284–301. [Google Scholar] [CrossRef]
- Cozzolino, I.; Rocco, M.; Villani, G.; Picardi, M. Lymph Node Fine-Needle Cytology of Non-Hodgkin Lymphoma: Diagnosis and Classification by Flow Cytometry. Acta Cytol. 2016, 60, 302–314. [Google Scholar] [CrossRef]
- Griesel, C.; Desmirean, M.; Esterhuizen, T.; Pasca, S.; Petrushev, B.; Selicean, C.; Roman, A.; Fetica, B.; Teodorescu, P.; Swanepoel, C.; et al. Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases. J. Clin. Med. 2020, 9, 283. [Google Scholar] [CrossRef]
- Chi, P.D.; Liu, Y.J.; Huang, Y.H.; Mao, M.J.; Wang, Y.; Li, Z.M.; Li, J. Rinsing sampling of core needle biopsy for flow cytometric analysis: A favorable method for lymphoma diagnosis. Cancer Med. 2020, 9, 9336–9345. [Google Scholar] [CrossRef]
- Gao, H.; Cai, F.; Liu, L.; Shen, H. Flow cytometry assessment of reactive T-cells distinguishes classic Hodgkin lymphoma from benign lymphadenopathy in children. J. Clin. Lab. Anal. 2022, 36, e24661. [Google Scholar] [CrossRef]
- Ribeiro, A.; Vazquez-Sequeiros, E.; Wiersema, L.M.; Wang, K.K.; Clain, J.E.; Wiersema, M.J. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest. Endosc. 2001, 53, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.; Tamhane, A.; Eloubeidi, M.A. EUS-guided FNA combined with flow cytometry in the diagnoses of suspected or recurrent intrathoracic or retroperitoneal lymphoma. Gastrointest. Endosc. 2005, 62, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, M.; Savabi, M.S.; Sherman, S.; McHenry, L.; Leblanc, J.; Cramer, H.; Emerson, R.; O’Neil, J.; Khashab, M.; Dewitt, J. Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: A single center experience. J. Gastroenterol. Hepatol. 2009, 24, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wakely, P.E., Jr. Endoscopic/Endobronchial Ultrasound-Guided Fine Needle Aspiration and Ancillary Techniques, Particularly Flow Cytometry, in Diagnosing Deep-Seated Lymphomas. Acta Cytol. 2016, 60, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Xavier, A.C.; Armenian, S.; Audino, A.N.; Blazin, L.; Bloom, D.; Chung, J.; Davies, K.; Ding, H.; Ford, J.B.; et al. Pediatric Aggressive Mature B-Cell Lymphomas, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 1267–1275. [Google Scholar] [CrossRef]
- Van Riet, P.A.; Larghi, A.; Attili, F.; Rindi, G.; Nguyen, N.Q.; Ruszkiewicz, A.; Kitano, M.; Chikugo, T.; Aslanian, H.; Farrell, J.; et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest. Endosc. 2019, 89, 329–339. [Google Scholar] [CrossRef]
- De Moura DT, H.; Mccarty, T.R.; Jirapinyo, P.; Ribeiro, I.B.; Farias, G.F.A.; Ryou, M.; Lee, L.S.; Thompson, C.C. Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis. Clin. Endosc. 2020, 53, 600–610. [Google Scholar] [CrossRef]
- Gerke, H.; Rizk, M.K.; Vanderheyden, A.D.; Jensen, C.S. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2010, 21, 44–51. [Google Scholar] [CrossRef]
- Erickson, R.A. EUS-guided FNA. Gastrointest. Endosc. 2004, 60, 267–279. [Google Scholar] [CrossRef]
- Gkolfakis, P.; Crinò, S.F.; Tziatzios, G.; Ramai, D.; Papaefthymiou, A.; Papanikolaou, I.S.; Triantafyllou, K.; Arvanitakis, M.; Lisotti, A.; Fusaroli, P.; et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2022, 95, 1067–1077.e15. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.H.; Ding, Z.; Tan, S.Y.; Chen, Q.; Duan, Y.Q.; Zhu, L.R.; Cao, J.W.; Wang, J.; Shi, G.; et al. Wet- versus dry-suction techniques for endoscopic ultrasound-guided fine-needle aspiration of solid lesions: A multicenter randomized controlled trial. Endoscopy 2020, 52, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, Y.; Wang, Y.; Zhao, Z.; Chen, Q.; Duan, Y.; Xiong, S.; Luan, Z.; Wang, J.; Cheng, B. Diagnostic and Prognostic Values of KRAS Mutations on EUS-FNA Specimens and Circulating Tumor DNA in Patients with Pancreatic Cancer. Clin. Transl. Gastroenterol. 2022, 13, e00487. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, G.; Feichter, G.; Hagmar, B.; Kapila, K.; Kardum-Skelin, I.; Kloboves, V.; Kobayashi, T.K.; Koutselini, H.; Majak, B.; Schenck, U.; et al. Fine needle aspiration cytology: A survey of current European practice. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2006, 17, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Brammer, J.; Clemens, M.W.; Dogan, A.; Foss, F.; Ghione, P.; et al. T-Cell Lymphomas, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 285–308. [Google Scholar] [CrossRef]
- Aadam, A.A.; Wani, S.; Amick, A.; Shah, J.N.; Bhat, Y.M.; Hamerski, C.M.; Klapman, J.B.; Muthusamy, V.R.; Watson, R.R.; Rademaker, A.W.; et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc. Int. Open 2016, 4, E497–E505. [Google Scholar] [CrossRef]
- Van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef]
- Oppong, K.W.; Bekkali, N.L.H.; Leeds, J.S.; Johnson, S.J.; Nayar, M.K.; Darné, A.; Egan, M.; Bassett, P.; Haugk, B. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized crossover study. Endoscopy 2020, 52, 454–461. [Google Scholar] [CrossRef]
- Tanisaka, Y.; Mizuide, M.; Fujita, A.; Ogawa, T.; Araki, R.; Suzuki, M.; Katsuda, H.; Saito, Y.; Miyaguchi, K.; Tashima, T.; et al. Comparison of Endoscopic Ultrasound-Guided Fine-Needle Aspiration and Biopsy Device for Lymphadenopathy. Gastroenterol. Res. Pract. 2021, 2021, 6640862. [Google Scholar] [CrossRef]
| Variables | FNB (n = 23) | FNA (n = 30) | p-Value |
|---|---|---|---|
| Age, years (SD) | 54.70 (17.47) | 51.87 (16.62) | 0.479 |
| Sex, male/female | 12/11 | 13/17 | 0.359 |
| Puncture site, n (%) | 0.604 | ||
| Mediastinum | 1 (4.35%) | 2 (6.70%) | |
| Retroperitoneum | 20 (86.96%) | 23 (76.7%) | |
| Pancreas | 2 (8.70%) | 3 (10.00%) | |
| GI tract | 0 (0.00%) | 2 (6.70%) | |
| Lesion size r, mm (SD) | 38.12 (20.45) | 30.90 (17.75) | 0.176 |
| Needle size, n (%) | <0.001 *** | ||
| 20 gauge | 18 (78.26%) | 0 (0.00%) | |
| 22 gauge | 5 (21.74%) | 29 (96.70%) | |
| 25 gauge | 0 (0.00%) | 1 (2.00%) | |
| No. passes, median (quartile) | 4 (3, 5) | 4 (3, 6) | 0.365 |
| Subtype | 1.000 | ||
| NHL | 22 (95.65%) | 29 (96.70%) | |
| B cell lymphoma | 20 (86.96%) | 25 (83.40%) | |
| Diffuse large B-cell lymphoma | 14 (60.87%) | 15 (50.00%) | |
| Follicular lymphoma | 2 (18.70%) | 2 (6.70%) | |
| Mantle cell lymphoma | 1 (4.35%) | 0 (0.00%) | |
| Marginal zone lymphoma | 1 (4.35%) | 3 (10.00%) | |
| Unclassifiable | 2 (8.70%) | 5 (16.70%) | |
| T cell lymphoma | 2 (8.70%) | 4 (13.20%) | |
| Anaplastic large cell lymphoma | 1 (4.35%) | 1 (3.30%) | |
| γδT-cell lymphoma | 0 (0.00%) | 1 (3.30%) | |
| Small lymphocytic Lymphoma | 0 (0.00%) | 1 (3.30%) | |
| peripheral T cell lymphomas | 1 (4.35%) | 1 (3.30%) | |
| HL | 1 (4.35%) | 1 (3.30%) |
| FNB (n = 23) | FNA (n = 30) | p-Value | |
|---|---|---|---|
| Core tissue length, mm (quartile) | 0.80 (0.55, 1.00) | 0.45 (0.30, 0.50) | 0.009 ** |
| Score of specimen adequacy, median (quartile) | 4 (3.75, 4.00) | 3 (1.00, 4.00) | 0.025 * |
| FNB (n = 23) | FNA (n = 30) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| No. of cases consistent with final diagnosis by IHC, n (%) | 21 (91.30%) | 18 (60.00%) | 7.000 (1.380–35.511) | 0.013 * |
| No. of cases consistent with final diagnosis by IHC+ FCM, n (%) | 22 (95.65%) | 29 (96.70%) | 0.759 (0.045–12.812) | 1.000 |
| Variable | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Exp (b) | OR (95% CI) | p-Value | Exp (b) | OR (95% CI) | p-Value | |
| Needle type (FNB vs. FNA) | 7.000 | 1.630–48.960 | 0.019 * | 1.292 | 1.037–1.609 | 0.023 * |
| Needle size | 0.384 | 0.120–0.855 | 0.042 * | - | - | - |
| Lesion site | 0.746 | 0.283–1.745 | 0.515 | - | - | - |
| Lesion size | 8.750 | 2.101–42.035 | 0.004 ** | 1.518 | 1.161–1.985 | 0.003 ** |
| Endoscopists | 2.917 | 0.821–12.133 | 0.112 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Aruna; Cheng, B.; Xiong, D.; Kuang, D.; Cui, H.; Xiong, S.; Mao, X.; Feng, Y.; Zhao, Y. Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma. Diagnostics 2023, 13, 2777. https://doi.org/10.3390/diagnostics13172777
Yang Y, Aruna, Cheng B, Xiong D, Kuang D, Cui H, Xiong S, Mao X, Feng Y, Zhao Y. Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma. Diagnostics. 2023; 13(17):2777. https://doi.org/10.3390/diagnostics13172777
Chicago/Turabian StyleYang, Yilei, Aruna, Bin Cheng, Dingkun Xiong, Dong Kuang, Haochen Cui, Si Xiong, Xia Mao, Yunlu Feng, and Yuchong Zhao. 2023. "Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma" Diagnostics 13, no. 17: 2777. https://doi.org/10.3390/diagnostics13172777
APA StyleYang, Y., Aruna, Cheng, B., Xiong, D., Kuang, D., Cui, H., Xiong, S., Mao, X., Feng, Y., & Zhao, Y. (2023). Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma. Diagnostics, 13(17), 2777. https://doi.org/10.3390/diagnostics13172777






