SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications
Abstract
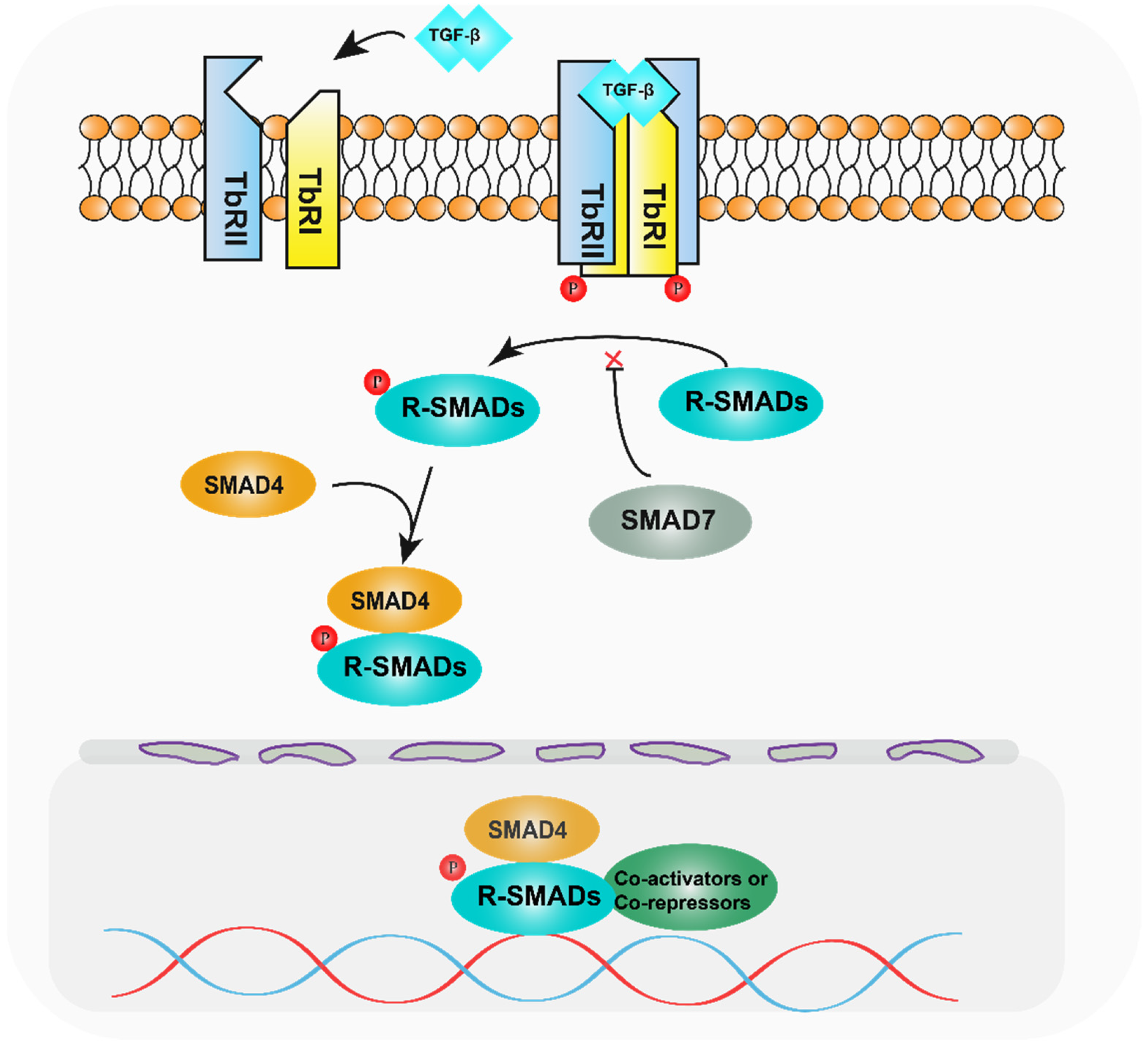
:1. Introduction
1.1. Oesophageal Cancer
1.2. Gastric Cancer
1.3. Colorectal Cancer
1.4. Hepatocellular Carcinoma
1.5. Pancreatic Cancer
1.6. Lung Cancer
1.7. Breast Cancer
1.8. Ovarian and Uterine Tumours
1.9. Other Cancers
| Tumours | Upstream Molecules | Target Molecules | Biological Impact | Ref. |
|---|---|---|---|---|
| Nasopharyngeal Carcinoma | TIM-3 | SMAD2 and SMAD7 | Promoting EMT | [137] |
| HPV-positive head and neck tumours | HPV | SMAD4 | Promoting DNA damage repair Promoting viral replication and tumourigenesis Enhancing resistance to cisplatin | [138] |
| SMAD4-deficient squamous cell carcinoma of the head and neck | Olaparib | Promoting DNA damage and reducing proliferation | [139] | |
| Renal cell carcinoma | Calcitriol | Enhanced interaction of vitamin D receptors with SMAD3 | Inhibiting the migration and invasion | [140] |
| Prostate cancer | HNF1B | SMAD6 | Inhibiting tumour proliferation | [141] |
| SAMD7 | c-JUN and HDAC6 | Promoting aggressive migration of prostate cancer | [142] | |
| PLK1 and PELO | SMAD4 | Promoting proliferation and metastasis | [143] | |
| HOXD13 | SMAD1 | Inhibiting EMT | [144] | |
| SMAD3 | AR | Promoting castration tolerance in prostate cancer | [145] | |
| Sasanquasaponin | SMAD2/3 | Inhibiting the aggression of prostate cancer | [146] |
| Tumours | Upstream Molecules | Downstream Molecules | Target Molecules | Biological Impact | Ref. |
|---|---|---|---|---|---|
| Gastric cancer | MicroRNA-135 | SMAD2 | Suppressing metastasis | [147] | |
| TMEM147-AS1 | miR-326 | SMAD5 | Promoting invasive metastasis | [148] | |
| LncRNA-HCP5 | miR-299-3p | SMAD5 | Inhibiting proliferation, invasion, migration, and promotes apoptosis | [149] | |
| LncFGD5-AS1 | miR-196a-5p | SMAD6 | Suppression of EMT | [150] | |
| Colorectal cancer | CircPTEN1 | Binds SMAD4 competitively with SMAD2/3 | Suppression of EMT | [151] | |
| LINC00657 | SMAD2 | HPSE | Accelerating the CRC invasion | [152] | |
| LINC00941 | SMAD4 | Promoting invasive migration | [153] | ||
| Circ-SMAD2 | Promoting proliferation and invasion | [154] | |||
| miR-186-5p | SMAD6/7 | Inhibiting proliferation and migration | [155] | ||
| MiR-581 | SMAD7 | Promoting CRC metastasis | [156] | ||
| Hepatocellular carcinoma | MiR-1258 | SMAD2/3 | Inhibiting the metastasis of HCC | [157] | |
| LINC00261 | SMAD3 | Suppression of EMT | [158] | ||
| LINC01410 | miR-124-3p | SMAD5 | Promoting invasive migration | [159] | |
| CircFGGY | miR-545-3p | SMAD7 | Inhibits proliferation and invasion of HCC cells | [160] | |
| MiR-21-3p | SMAD7 | YAP1 | Promoting HCC invasive migration | [161] | |
| miR-148a-3p | Argonaute 2 | SMAD2 | Inhibits migration and proliferation of HCC cells | [162] | |
| Pancreatic cancer | MiR-487a-3p | SMAD7 | Suppressing the malignant progression of PC | [163] | |
| Lung Cancer | CircSCAP | SMAD2 | Promoting the metastasis of NSCLC | [164] | |
| OSER1-AS1 | microRNA-433-3p | SMAD2 | Promoting proliferation and invasive migration in NSCLC. | [165] | |
| MiR-361-5p | SMAD2 | Promoting lung adenocarcinoma | [166] | ||
| CLL | miR-26b-5p | SMAD4 | C-Myc | Promoting CLL progress | [167] |
| Adult T-cell lymphoma | LINC00183 | miR-371b-5p | SMAD2 | Promoting chemotherapy resistance | [127] |
| Ovarian cancer | CASC15 | miR-23b-3p/miR-24-3p | SMAD3 | Promoting the development of ovarian cancer | [168] |
| Endometrial cancer | MCTP1-AS1 | miR-650 | SMAD7 | Inhibiting proliferation invasion, migration, and EMT in endometrial cancer | [169] |
| Hsa_cir_0001860 | miR-520 | SMAD7 | Inhibiting cell migration and invasion | [170] | |
| Bladder Cancer | Circ-0002623 | miR-1276 | SMAD2 | Promoting the malignant phenotype of bladder cancer | [171] |
| CircNCOR1 | HNRNPL | SMAD7 | Inhibiting lymph node metastasis from bladder cancer | [172] | |
| MiR-5581-3p | SMAD3 | Inhibiting bladder cancer cell migration and proliferation | [173] | ||
| PlncRNA-1 | miR-136-5p | SMAD3 | Promoting bladder cancer progression | [174] | |
| KCNMB2-AS1 | miR-3194-3p | SMAD5 | Promoting bladder cancer progression | [175] | |
| Prostate cancer | SMAD3 | PCAT7 | MiR-324-5p | Promoting bone metastases from prostate cancer | [176] |
| Breast cancer | MiR-135-5p | SMAD3 | Inhibiting EMT and breast cancer metastasis | [177] | |
| ARHGAP5-AS1 | SMAD7 | Inhibiting stress fibres in breast cancer cells to inhibit cell migration | [178] | ||
| Melanoma | NEAT1 | MiR-200b-3p | SMAD2 | Promoting EMT | [179] |
| Osteosarcoma | MiR-135a | SMAD2 | Promoting proliferative migration | [180] | |
| LINC00266-1 | miR-548c-3p | SMAD2 | Promoting osteosarcoma progression | [181] | |
| MiR-16-5p | SMAD3 | Enhancing cisplatin sensitivity | [182] |
2. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. TGF-β and cancer. Cytokine Growth Factor Rev. 2006, 17, 29–40. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, M.U.; Yatoo, A.M.; Arafah, A.; Khan, A.; Rashid, S.; Majid, S.; Ali, A.; Ali, M.N. TGF-β signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. Eur. J. Pharmacol. 2023, 947, 175678. [Google Scholar] [CrossRef]
- Sekelsky, J.J.; Newfeld, S.J.; Raftery, L.A.; Chartoff, E.H.; Gelbart, W.M. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics 1995, 139, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Gelbart, W.M.; Harland, R.M.; Heldin, C.H.; Kern, S.E.; Massagué, J.; Melton, D.A.; Mlodzik, M.; Padgett, R.W.; Roberts, A.B.; et al. Nomenclature: Vertebrate mediators of TGFβ family signals. Cell 1996, 87, 173. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.A.; Schutte, M.; Hoque, A.T.; Moskaluk, C.A.; da Costa, L.T.; Rozenblum, E.; Weinstein, C.L.; Fischer, A.; Yeo, C.J.; Hruban, R.H.; et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996, 271, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Cook, T.; Urrutia, R. TIEG proteins join the Smads as TGF-β-regulated transcription factors that control pancreatic cell growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G513–G521. [Google Scholar] [CrossRef]
- Itoh, S.; Landstrom, M.; Hermansson, A.; Itoh, F.; Heldin, C.H.; Heldin, N.E.; Dijke, P.T. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J. Biol. Chem. 1998, 273, 29195–29201. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Attisano, L.; Wieser, R.; Ventura, F.; Massagué, J. Mechanism of activation of the TGF-β receptor. Nature 1994, 370, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGF-β signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Lo, R.S.; Wotton, D.; Lagna, G.; Massagué, J. Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature 1997, 388, 82–87. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhang, Y.; Wu, R.Y.; Derynck, R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998, 12, 2153–2163. [Google Scholar] [CrossRef]
- Janknecht, R.; Wells, N.J.; Hunter, T. TGF-β-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998, 12, 2114–2119. [Google Scholar] [CrossRef]
- Kahata, K.; Hayashi, M.; Asaka, M.; Hellman, U.; Kitagawa, H.; Yanagisawa, J.; Kato, S.; Imamura, T.; Miyazono, K. Regulation of transforming growth factor-β and bone morphogenetic protein signalling by transcriptional coactivator GCN5. Genes Cells 2004, 9, 143–151. [Google Scholar] [CrossRef]
- Wotton, D.; Lo, R.S.; Lee, S.; Massague, J. A Smad transcriptional corepressor. Cell 1999, 97, 29–39. [Google Scholar] [CrossRef]
- Akiyoshi, S.; Inoue, H.; Hanai, J.; Kusanagi, K.; Nemoto, N.; Miyazono, K.; Kawabata, M. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with smads. J. Biol. Chem. 1999, 274, 35269–35277. [Google Scholar] [CrossRef]
- Luo, K.; Stroschein, S.L.; Wang, W.; Chen, D.; Martens, E.; Zhou, S.; Zhou, Q. The Ski oncoprotein interacts with the Smad proteins to repress TGFβ signaling. Genes Dev. 1999, 13, 2196–2206. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Eaton, E.N.; Lane, W.S.; Lodish, H.F.; Weinberg, R.A. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signaling. Mol. Cell 1999, 4, 499–509. [Google Scholar] [CrossRef]
- Itoh, F.; Asao, H.; Sugamura, K.; Heldin, C.H.; Dijke, P.T.; Itoh, S. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 2001, 20, 4132–4142. [Google Scholar] [CrossRef]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Horitani, S.; Fukui, T.; Tanimura, Y.; Matsumoto, Y.; Miyamoto, S.; Tanaka, T.; Tomiyama, T.; Ikeura, T.; Ando, Y.; Nishio, A.; et al. Specific Smad2/3 Linker Phosphorylation Indicates Esophageal Non-neoplastic and Neoplastic Stem-Like Cells and Neoplastic Development. Dig. Dis. Sci. 2021, 66, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Onwuegbusi, B.A.; Aitchison, A.; Chin, S.F.; Kranjac, T.; Mills, I.; Huang, Y.; Lao-Sirieix, P.; Caldas, C.; Fitzgerald, R.C. Impaired transforming growth factor β signalling in Barrett’s carcinogenesis due to frequent SMAD4 inactivation. Gut 2006, 55, 764–774. [Google Scholar] [CrossRef]
- Gotovac, J.R.; Kader, T.; Milne, J.V.; Fujihara, K.M.; Lara-Gonzalez, L.E.; Gorringe, K.L.; Kalimuthu, S.N.; Jayawardana, M.W.; Duong, C.P.; Phillips, W.A.; et al. Loss of SMAD4 Is Sufficient to Promote Tumorigenesis in a Model of Dysplastic Barrett’s Esophagus. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Watanabe, T.; Heitmiller, R.; Zahurak, M.; Forastiere, A.A.; Hamilton, S.R. Genetic alterations in Barrett esophagus and adenocarcinomas of the esophagus and esophagogastric junction region. Am. J. Pathol. 1998, 153, 287–294. [Google Scholar] [CrossRef]
- Barrett, M.T.; Schutte, M.; Kern, S.E.; Reid, B.J. Allelic loss and mutational analysis of the DPC4 gene in esophageal adenocarcinoma. Cancer Res. 1996, 56, 4351–4353. [Google Scholar]
- Feng, S.; Jia, J.; Lv, G.; Wang, Y. Knockdown of ABCB7 inhibits esophageal cancer progression by inhibiting the TGF-β/Smad signaling. Arch. Biochem. Biophys. 2023, 742, 109620. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Niu, Y.; Lu, J.; Yan, Z.; Xu, T.; Guo, Y.; Dong, Z.; Guo, W. FOXD3 suppresses epithelial-mesenchymal transition through direct transcriptional promotion of SMAD7 in esophageal squamous cell carcinoma. Mol. Carcinog. 2021, 60, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Mori, G.; Nonaka, S.; Yoshinaga, S.; Saito, Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, W.; Yuan, B.; Zhang, S.; Wang, K.; Du, T. TRIM22 inhibits the proliferation of gastric cancer cells through the Smad2 protein. Cell Death Discov. 2021, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Dou, N.; Hu, Q.; Li, L.; Wu, Q.; Li, Y.; Gao, Y. USP32 promotes tumorigenesis and chemoresistance in gastric carcinoma via upregulation of SMAD2. Int. J. Biol. Sci. 2020, 16, 1648–1657. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Liu, W.; Xiao, H.; Qi, Y.; Li, J.; Luo, B. Latent membrane protein 2A inhibits expression level of Smad2 through regulating miR-155-5p in EBV-associated gastric cancer cell lines. J. Med. Virol. 2020, 92, 96–106. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Guo, Y.; Sun, L.-X.; Ni, F.-B.; Xu, K. Prognostic value of small mother against decapentaplegic expression in human gastric cancer. Bioengineered 2021, 12, 2534–2549. [Google Scholar] [CrossRef]
- Chen, W.; Hu, J.; He, Y.; Yu, L.; Liu, Y.; Cheng, Y.; Jia, B.; Li, X.; Yu, G.; Wang, Y. The Interaction Between SMAD1 and YAP1 Is Correlated with Increased Resistance of Gastric Cancer Cells to Cisplatin. Appl. Biochem. Biotechnol. 2022, 194, 1–18. [Google Scholar] [CrossRef]
- An, H.-W.; Seok, S.H.; Kwon, J.-W.; Choudhury, A.D.; Oh, J.-S.; Voon, D.C.; Kim, D.-Y.; Park, J.W. The loss of epithelial Smad4 drives immune evasion via CXCL1 while displaying vulnerability to combinatorial immunotherapy in gastric cancer. Cell Rep. 2022, 41, 111878. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, H.S.; Lee, H.J.; Hur, K.; Kim, W.H.; Bang, Y.J.; Kim, S.J.; Lee, K.U.; Choe, K.J.; Yang, H.K. Prognostic significance of the expression of Smad4 and Smad7 in human gastric carcinomas. Ann. Oncol. 2004, 15, 574–580. [Google Scholar] [CrossRef]
- Zhu, Y.; Richardson, J.A.; Parada, L.F.; Graff, J.M. Smad3 mutant mice develop metastatic colorectal cancer. Cell 1998, 94, 703–714. [Google Scholar] [CrossRef]
- Gu, S.; Zaidi, S.; Hassan, M.I.; Mohammad, T.; Malta, T.M.; Noushmehr, H.; Nguyen, B.; Crandall, K.A.; Srivastav, J.; Obias, V.; et al. Mutated CEACAMs Disrupt Transforming Growth Factor Beta Signaling and Alter the Intestinal Microbiome to Promote Colorectal Carcinogenesis. Gastroenterology 2020, 158, 238–252. [Google Scholar] [CrossRef]
- Tong, K.; Kothari, O.A.; Haro, K.S.; Panda, A.; Bandari, M.M.; Carrick, J.N.; Hur, J.J.; Zhang, L.; Chan, C.S.; Xing, J.; et al. SMAD4 is critical in suppression of BRAF-V600E serrated tumorigenesis. Oncogene 2021, 40, 6034–6048. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Stephens, B.; Von Hoff, D.D.; Han, H. Identification and characterization of a novel anticancer agent with selectivity against deleted in pancreatic cancer locus 4 (DPC4)-deficient pancreatic and colon cancer cells. Pancreas 2009, 38, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; Neikes, H.K.; Rezaeifard, S.; Ma, X.; Voest, E.E.; Tauriello, D.V.F.; Vermeulen, M. Multiomics of Colorectal Cancer Organoids Reveals Putative Mediators of Cancer Progression Resulting from SMAD4 Inactivation. J. Proteome Res. 2023, 22, 138–151. [Google Scholar] [CrossRef]
- Liang, Q.; Tang, C.; Tang, M.; Zhang, Q.; Gao, Y.; Ge, Z. TRIM47 is up-regulated in colorectal cancer, promoting ubiquitination and degradation of SMAD4. J. Exp. Clin. Cancer Res. 2019, 38, 159. [Google Scholar] [CrossRef]
- Loevenich, L.P.; Tschurtschenthaler, M.; Rokavec, M.; Silva, M.G.; Jesinghaus, M.; Kirchner, T.; Klauschen, F.; Saur, D.; Neumann, J.; Hermeking, H.; et al. SMAD4 Loss Induces c-MYC-Mediated NLE1 Upregulation to Support Protein Biosynthesis, Colorectal Cancer Growth, and Metastasis. Cancer Res. 2022, 82, 4604–4623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, X.; Li, Z.; Li, C.; Li, X.; Xiao, J.; Liu, B.; Yang, H.; Zhang, Y. Long Noncoding RNA ASLNC07322 Functions in VEGF-C Expression Regulated by Smad4 during Colon Cancer Metastasis. Mol. Ther. Nucleic Acids 2019, 18, 851–862. [Google Scholar] [CrossRef]
- Hanna, D.N.; Smith, P.M.; Novitskiy, S.V.; Washington, M.K.; Zi, J.; Weaver, C.J.; Hamaamen, J.A.; Lewis, K.B.; Zhu, J.; Yang, J.; et al. SMAD4 Suppresses Colitis-associated Carcinoma Through Inhibition of CCL20/CCR6-mediated Inflammation. Gastroenterology 2022, 163, 1334–1350.e14. [Google Scholar] [CrossRef]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Recruiting Tumor-Associated Neutrophils via the CXCL1/8-CXCR2 Axis. Clin. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef]
- Wasserman, I.; Lee, L.H.; Ogino, S.; Marco, M.R.; Wu, C.; Chen, X.; Datta, J.; Sadot, E.; Szeglin, B.; Guillem, J.G.; et al. SMAD4 Loss in Colorectal Cancer Patients Correlates with Recurrence, Loss of Immune Infiltrate, and Chemoresistance. Clin. Cancer Res. 2019, 25, 1948–1956. [Google Scholar] [CrossRef]
- Lahoz, S.; Rodríguez, A.; Fernández, L.; Gorría, T.; Moreno, R.; Esposito, F.; Oliveres, H.; Albiol, S.; Saurí, T.; Pesantez, D.; et al. Mutational Status of SMAD4 and FBXW7 Affects Clinical Outcome in TP53-Mutated Metastatic Colorectal Cancer. Cancers 2022, 14, 5921. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kopetz, S.; Newhook, T.E.; De Bellis, M.; Chun, Y.S.; Tzeng, C.-W.D.; Aloia, T.A.; Vauthey, J.-N. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin. Cancer Res. 2019, 25, 5843–5851. [Google Scholar] [CrossRef]
- Ansar, M.; Wang, C.-J.; Wang, Y.-H.; Shen, T.-H.; Hung, C.-S.; Chang, S.-C.; Lin, R.-K. SMAD3 Hypomethylation as a Biomarker for Early Prediction of Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 7395. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.G.; Mohammed, S.R.; Mohammed, A.M.; Mahmoud, Z. Impact of microRNA-375 and its target gene SMAD-7 polymorphism on susceptibility of colorectal cancer. J. Clin. Lab. Anal. 2018, 32, e22215. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Canzonieri, V.; Polesel, J.; Mezzalira, S.; Fratte, C.D.; Dreussi, E.; Roncato, R.; Bignucolo, A.; Innocente, R.; Belluco, C.; et al. SMAD3 Host and Tumor Profiling to Identify Locally Advanced Rectal Cancer Patients at High Risk of Poor Response to Neoadjuvant Chemoradiotherapy. Front. Pharmacol. 2021, 12, 778781. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Polesel, J.; Roncato, R.; Labriet, A.; Bignucolo, A.; Gagno, S.; Buonadonna, A.; D’Andrea, M.; Lévesque, E.; Jonker, D.; et al. IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm. Cancers 2021, 13, 1705. [Google Scholar] [CrossRef]
- Halder, S.K.; Beauchamp, R.D.; Datta, P.K. Smad7 induces tumorigenicity by blocking TGF-β-induced growth inhibition and apoptosis. Exp. Cell Res. 2005, 307, 231–246. [Google Scholar] [CrossRef]
- Maresca, C.; Di Maggio, G.; Stolfi, C.; Laudisi, F.; Colella, M.; Pacifico, T.; Di Grazia, A.; Di Fusco, D.; Congiu, D.; Guida, A.M.; et al. Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells. Cancers 2022, 14, 4993. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Mi, S.; Shao, L.; Xu, Z.; Xue, M. ALKBH1-mediated m1 A demethylation of METTL3 mRNA promotes the metastasis of colorectal cancer by downregulating SMAD7 expression. Mol. Oncol. 2023, 17, 344–364. [Google Scholar] [CrossRef]
- Baek, H.J.; Lim, S.C.; Kitisin, K.; Jogunoori, W.; Tang, Y.; Marshall, M.B.; Mishra, B.; Kim, T.H.; Cho, K.H.; Kim, S.S.; et al. Hepatocellular cancer arises from loss of transforming growth factor β signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology 2008, 48, 1128–1137. [Google Scholar] [CrossRef]
- Chen, C.-L.; Tsukamoto, H.; Liu, J.-C.; Kashiwabara, C.; Feldman, D.; Sher, L.; Dooley, S.; French, S.W.; Mishra, L.; Petrovic, L.; et al. Reciprocal regulation by TLR4 and TGF-β in tumor-initiating stem-like cells. J. Clin. Investig. 2013, 123, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Z.; Yuan, Y.-C.; Huang, B.-Y.; Chen, J.-X.; Li, B.-K.; Fang, J.-H.; Zhuang, S.-M. Identification of a TGF-β/SMAD/lnc-UTGF positive feedback loop and its role in hepatoma metastasis. Signal Transduct. Target. Ther. 2021, 6, 395. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.; Yang, H.; Zhang, J.; Wang, J.; Luo, T.; Jiang, Y.; Hua, H. SEPHS1 promotes SMAD2/3/4 expression and hepatocellular carcinoma cells invasion. Exp. Hematol. Oncol. 2021, 10, 17. [Google Scholar] [CrossRef]
- Zhong, F.-J.; Sun, B.; Cao, M.-M.; Xu, C.; Li, Y.-M.; Yang, L.-Y. STMN2 mediates nuclear translocation of Smad2/3 and enhances TGFβ signaling by destabilizing microtubules to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2021, 506, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Pan, Y.; Chen, X.-Y.; Hou, P.-F. BUB1 promotes proliferation of liver cancer cells by activating SMAD2 phosphorylation. Oncol. Lett. 2020, 19, 3506–3512. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, L.; Zhang, X.; Zhang, H.; Tan, X.; Dong, K.; Lu, X.; Zhu, H.; Liu, Q.; Zhang, Z.; et al. PTPRε Acts as a Metastatic Promoter in Hepatocellular Carcinoma by Facilitating Recruitment of SMAD3 to TGF-β Receptor 1. Hepatology 2020, 72, 997–1012. [Google Scholar] [CrossRef]
- Chaomin, W.; Wenhao, N.; Jialei, H.; Ting, Z.; Honglei, F.; Zhuang, H.; Yichao, W.; Changsen, B.; Yueguo, L. Spatiotemporal modulation of SMAD4 by HBx is required for cellular proliferation in hepatitis B-related liver cancer. Cell. Oncol. 2022, 45, 573–589. [Google Scholar] [CrossRef]
- Dooley, S.; Hamzavi, J.; Ciuclan, L.; Godoy, P.; Ilkavets, I.; Ehnert, S.; Ueberham, E.; Gebhardt, R.; Kanzler, S.; Geier, A.; et al. Hepatocyte-specific Smad7 expression attenuates TGF-β-mediated fibrogenesis and protects against liver damage. Gastroenterology 2008, 135, 642–659.e46. [Google Scholar] [CrossRef]
- Feng, T.; Dzieran, J.; Yuan, X.; Dropmann, A.; Maass, T.; Teufel, A.; Marhenke, S.; Gaiser, T.; Rückert, F.; Kleiter, I.; et al. Hepatocyte-specific Smad7 deletion accelerates DEN-induced HCC via activation of STAT3 signaling in mice. Oncogenesis 2017, 6, e294. [Google Scholar] [CrossRef]
- Sun, N.; Zhong, X.; Wang, S.; Zeng, K.; Sun, H.; Sun, G.; Zou, R.; Liu, W.; Liu, W.; Lin, L.; et al. ATXN7L3 positively regulates SMAD7 transcription in hepatocellular carcinoma with growth inhibitory function. EBioMedicine 2020, 62, 103108. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Li, L.; Yang, J.; Ding, H.; Zhang, C.; Wen, G.; Wu, C.; Fang, Z.; Hou, S.; et al. Smad3 C-terminal phosphorylation site mutation attenuates the hepatoprotective effect of salvianolic acid B against hepatocarcinogenesis. Food Chem. Toxicol. 2021, 147, 111912. [Google Scholar] [CrossRef]
- Shu, G.; Dai, C.; Yusuf, A.; Sun, H.; Deng, X. Limonin relieves TGF-β-induced hepatocyte EMT and hepatic stellate cell activation in vitro and CCl4-induced liver fibrosis in mice via upregulating Smad7 and subsequent suppression of TGF-β/Smad cascade. J. Nutr. Biochem. 2022, 107, 109039. [Google Scholar] [CrossRef]
- Liu, L.-H.; Fang, C.-K.; Ge, F.-C.; Wang, J.-N.; Zhang, X.-B.; Luo, R.; Zhang, Y.; Feng, K.-L.; Qiu, Z.-W.; Zhong, C. JPHYD Inhibits miR-21-5p/Smad7-Mediated Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma Cells. J. Oncol. 2022, 2022, 7823433. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, W.; Liu, C.; Jiang, Z.; Gu, P.; Li, J.; Wang, W.; You, R.; Ba, Q.; et al. Rebalancing TGF-β/Smad7 signaling via Compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis. Clin. Transl. Med. 2021, 11, e410. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, G.; Li, K.; Liu, Z.; Wang, Y.; Chen, T.; Mu, W.; Yang, X.; Li, X.; Shi, A.; et al. The Smad4-MYO18A-PP1A complex regulates β-catenin phosphorylation and pemigatinib resistance by inhibiting PAK1 in cholangiocarcinoma. Cell Death Differ. 2022, 29, 818–831. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Huang, A.; Gao, Y.; Peng, B.; Li, Z.; Ma, W.; Songyang, Z.; Zhang, S.; He, M.; et al. GOLPH3 Promotes Cancer Growth by Interacting With STIP1 and Regulating Telomerase Activity in Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 575358. [Google Scholar] [CrossRef]
- Wilentz, R.E.; Iacobuzio-Donahue, C.A.; Argani, P.; McCarthy, D.M.; Parsons, J.L.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000, 60, 2002–2006. [Google Scholar]
- Bardeesy, N.; Cheng, K.-H.; Berger, J.H.; Chu, G.C.; Pahler, J.; Olson, P.; Hezel, A.F.; Horner, J.; Lauwers, G.Y.; Hanahan, D.; et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006, 20, 3130–3146. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ehdaie, B.; Ohara, N.; Yoshino, T.; Deng, C.X. Synergistic action of Smad4 and Pten in suppressing pancreatic ductal adenocarcinoma formation in mice. Oncogene 2010, 29, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shi, S.; Qin, Y.; Meng, Q.; Hua, J.; Hu, Q.; Ji, S.; Zhang, B.; Xu, J.; Yu, X.-J. Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut 2020, 69, 888–900. [Google Scholar] [CrossRef]
- Dai, C.; Rennhack, J.P.; Arnoff, T.E.; Thaker, M.; Younger, S.T.; Doench, J.G.; Huang, A.Y.; Yang, A.; Aguirre, A.J.; Wang, B.; et al. SMAD4 represses FOSL1 expression and pancreatic cancer metastatic colonization. Cell Rep. 2021, 36, 109443. [Google Scholar] [CrossRef]
- Bertrand-Chapel, A.; Caligaris, C.; Fenouil, T.; Savary, C.; Aires, S.; Martel, S.; Huchedé, P.; Chassot, C.; Chauvet, V.; Cardot-Ruffino, V.; et al. SMAD2/3 mediate oncogenic effects of TGF-β in the absence of SMAD4. Commun. Biol. 2022, 5, 1068. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Zhu, R.; Liang, R.; Wang, W.; Li, J.; Zhang, Y.; Guo, C.; Han, X.; Sun, Y. Multi-omics landscape and clinical significance of a SMAD4-driven immune signature: Implications for risk stratification and frontline therapies in pancreatic cancer. Comput. Struct. Biotechnol. J. 2022, 20, 1154–1167. [Google Scholar] [CrossRef]
- Liang, C.; Xu, J.; Meng, Q.; Zhang, B.; Liu, J.; Hua, J.; Zhang, Y.; Shi, S.; Yu, X. TGFB1-induced autophagy affects the pattern of pancreatic cancer progression in distinct ways depending on SMAD4 status. Autophagy 2020, 16, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Navarro-Serer, B.; Jeong, Y.J.; Chianchiano, P.; Xia, L.; Luchini, C.; Veronese, N.; Dowiak, C.; Ng, T.; Trujillo, M.A.; et al. Pattern of Invasion in Human Pancreatic Cancer Organoids Is Associated with Loss of SMAD4 and Clinical Outcome. Cancer Res. 2020, 80, 2804–2817. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, W.; Cao, R.; Ye, C.; Zhang, L.; Chen, H.; Wang, J.; Shi, Y.; Li, R.; Li, Y.; et al. An EGFR/HER2-targeted conjugate sensitizes gemcitabine-sensitive and resistant pancreatic cancer through different SMAD4-mediated mechanisms. Nat. Commun. 2022, 13, 5506. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Liao, Q.; Dai, M.; Guo, J.; Yang, X.; Tan, W.; Lin, D.; Wu, C.; Zhao, Y. Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell 2021, 12, 128–144. [Google Scholar] [CrossRef]
- Ezrova, Z.; Nahacka, Z.; Stursa, J.; Werner, L.; Vlcak, E.; Viziova, P.K.; Berridge, M.V.; Sedlacek, R.; Zobalova, R.; Rohlena, J.; et al. SMAD4 loss limits the vulnerability of pancreatic cancer cells to complex I inhibition via promotion of mitophagy. Oncogene 2021, 40, 2539–2552. [Google Scholar] [CrossRef]
- Cai, H.; Guo, F.; Wen, S.; Jin, X.; Wu, H.; Ren, D. Overexpressed integrin alpha 2 inhibits the activation of the transforming growth factor β pathway in pancreatic cancer via the TFCP2-SMAD2 axis. J. Exp. Clin. Cancer Res. 2022, 41, 73. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Zheng, Z.; Lu, J.-D.; Duan, S.-Q.; Zhang, J.; Zhang, H.-Q.; Ding, Y.-X.; Yin, J.; Cao, F.; Zhang, J.; et al. KLF16 promotes pancreatic adenocarcinoma cell proliferation and migration by positively regulating SMAD6. World J. Gastrointest. Oncol. 2022, 14, 2157–2169. [Google Scholar] [CrossRef]
- Park, J.H.; Ameri, A.H.; Dempsey, K.E.; Conrad, D.N.; Kem, M.; Mino-Kenudson, M.; Demehri, S. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. 2021, 40, e106151. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gong, X.; Zi, L.; Li, G.; Dong, S.; Chen, X.; Li, Y. Silencing of DLEU2 suppresses pancreatic cancer cell proliferation and invasion by upregulating microRNA-455. Cancer Sci. 2019, 110, 1676–1685. [Google Scholar] [CrossRef]
- Wang, P.; Fan, J.; Chen, Z.; Meng, Z.-Q.; Luo, J.-M.; Lin, J.-H.; Zhou, Z.-H.; Chen, H.; Wang, K.; Xu, Z.-D.; et al. Low-level expression of Smad7 correlates with lymph node metastasis and poor prognosis in patients with pancreatic cancer. Ann. Surg. Oncol. 2009, 16, 826–835. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Cheng, A.-C.; Tang, H.-C.; Huang, G.-C.; Cai, L.; Lin, T.-H.; Wu, K.-J.; Tseng, P.-H.; Wang, G.G.; Chen, W.-Y. USP7 facilitates SMAD3 autoregulation to repress cancer progression in p53-deficient lung cancer. Cell Death Dis. 2021, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Wang, S.; Liu, Q.; Feng, X.; Wang, W.; Huang, Z.; Huang, Y.; Wu, J.; Cai, M.; et al. ATOH8 binds SMAD3 to induce cellular senescence and prevent Ras-driven malignant transformation. Proc. Natl. Acad. Sci. USA 2023, 120, e2208927120. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, T.Y.; Jung, E.; Han, T.-S.; Lee, J.; Kim, S.-K.; Roh, Y.N.; Lee, M.-S.; Jung, C.-R.; Lim, J.H.; et al. Epigenetic regulation of SMAD3 by histone methyltransferase SMYD2 promotes lung cancer metastasis. Exp. Mol. Med. 2023, 55, 952–964. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Xu, Z.; He, Y.; Guo, C.; He, L.; Huan, C.; Cai, C.; Huang, J.; Zhang, J.; et al. PDLIM5 inhibits STUB1-mediated degradation of SMAD3 and promotes the migration and invasion of lung cancer cells. J. Biol. Chem. 2020, 295, 13798–13811. [Google Scholar] [CrossRef]
- Sun, Z.; Su, Z.; Zhou, Z.; Wang, S.; Wang, Z.; Tong, X.; Li, C.; Wang, Y.; Chen, X.; Lei, Z.; et al. RNA demethylase ALKBH5 inhibits TGF-β-induced EMT by regulating TGF-β/SMAD signaling in non-small cell lung cancer. FASEB J. 2022, 36, e22283. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Lei, Z.; Li, C.; Zhang, C.; Su, Z.; Liu, X.; Zhao, J.; Zhang, H.-T. MYOCD and SMAD3/SMAD4 form a positive feedback loop and drive TGF-β-induced epithelial-mesenchymal transition in non-small cell lung cancer. Oncogene 2020, 39, 2890–2904. [Google Scholar] [CrossRef]
- Ren, H.; Yang, B.; Li, M.; Lu, C.; Li, X. RAB26 contributes to the progression of non-small cell lung cancer after being transcriptionally activated by SMAD3. Bioengineered 2022, 13, 8064–8075. [Google Scholar] [CrossRef]
- Niu, H.; Huang, Y.; Yan, L.; Zhang, L.; Zhao, M.; Lu, T.; Yang, X.; Chen, Z.; Zhan, C.; Shi, Y.; et al. Knockdown of SMAD3 inhibits the growth and enhances the radiosensitivity of lung adenocarcinoma via p21 in vitro and in vivo. Int. J. Biol. Sci. 2020, 16, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.-T.; Chung, J.Y.-F.; Xue, V.W.-W.; Xiao, J.; Meng, X.-M.; Huang, X.-R.; Zhou, S.; Chan, A.S.-W.; Tsang, A.C.-M.; Cheng, A.S.-L.; et al. Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition. Adv. Sci. 2022, 9, e2101235. [Google Scholar] [CrossRef] [PubMed]
- Ikemori, R.; Gabasa, M.; Duch, P.; Vizoso, M.; Bragado, P.; Arshakyan, M.; Luis, I.-C.; Marín, A.; Morán, S.; Castro, M.; et al. Epigenetic SMAD3 Repression in Tumor-Associated Fibroblasts Impairs Fibrosis and Response to the Antifibrotic Drug Nintedanib in Lung Squamous Cell Carcinoma. Cancer Res. 2020, 80, 276–290. [Google Scholar] [CrossRef]
- Chung, J.Y.-F.; Tang, P.C.-T.; Chan, M.K.-K.; Xue, V.W.; Huang, X.-R.; Ng, C.S.-H.; Zhang, D.; Leung, K.-T.; Wong, C.-K.; Lee, T.-L.; et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat. Commun. 2023, 14, 1794. [Google Scholar] [CrossRef]
- Marwitz, S.; Ballesteros-Merino, C.; Jensen, S.M.; Reck, M.; Kugler, C.; Perner, S.; Drömann, D.; Goldmann, T.; Fox, B.A. Phosphorylation of SMAD3 in immune cells predicts survival of patients with early stage non-small cell lung cancer. J. Immunother. Cancer 2021, 9, e001469. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tong, L.; Li, L.; Xu, J.; Xie, S.; Ji, L.; Fu, J.; Liu, Q.; Shen, S.; Liu, Y.; et al. Loss of Smad4 promotes aggressive lung cancer metastasis by de-repression of PAK3 via miRNA regulation. Nat. Commun. 2021, 12, 4853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Wang, S.; Sun, Z.; Lei, Z.; Zhang, H.-T.; Huang, J. RGS6 suppresses TGF-β-induced epithelial-mesenchymal transition in non-small cell lung cancers via a novel mechanism dependent on its interaction with SMAD4. Cell Death Dis. 2022, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Q.; Zheng, Q.; Jin, Y.; Shen, X.; Yang, M.; Zhou, X.; Li, Y. SMAD4 mutation correlates with poor prognosis in non-small cell lung cancer. Lab. Investig. 2021, 101, 463–476. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, L.; Liu, Y.; Zhang, S.; Li, S.; Wang, G.; Wang, P.; Chen, L. SMAD7 and SERPINE1 as novel dynamic network biomarkers detect and regulate the tipping point of TGF-β induced EMT. Sci. Bull. 2020, 65, 842–853. [Google Scholar] [CrossRef]
- Tong, L.; Shen, S.; Huang, Q.; Fu, J.; Wang, T.; Pan, L.; Zhang, P.; Chen, G.; Huang, T.; Li, K.; et al. Proteasome-dependent degradation of Smad7 is critical for lung cancer metastasis. Cell Death Differ. 2020, 27, 1795–1806. [Google Scholar] [CrossRef]
- Cai, C.; Gu, S.; Yu, Y.; Zhu, Y.; Zhang, H.; Yuan, B.; Shen, L.; Yang, B.; Feng, X.-H. PRMT5 Enables Robust STAT3 Activation via Arginine Symmetric Dimethylation of SMAD7. Adv. Sci. 2021, 8, 2003047. [Google Scholar] [CrossRef]
- Yu, B.; Luo, F.; Sun, B.; Liu, W.; Shi, Q.; Cheng, S.-Y.; Chen, C.; Chen, G.; Li, Y.; Feng, H. KAT6A Acetylation of SMAD3 Regulates Myeloid-Derived Suppressor Cell Recruitment, Metastasis, and Immunotherapy in Triple-Negative Breast Cancer. Adv. Sci. 2022, 9, e2105793. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, F.; Song, D.; Sun, X.; Liu, A.; Wu, Q.; She, X.; Chen, Y.; Chen, L.; Hu, F.; et al. EZH2-triggered methylation of SMAD3 promotes its activation and tumor metastasis. J. Clin. Investig. 2022, 132, e152394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Qin, J.; Wu, J.; Dai, X.; Xu, J. OSR1 phosphorylates the Smad2/3 linker region and induces TGF-β1 autocrine to promote EMT and metastasis in breast cancer. Oncogene 2021, 40, 68–84. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, L.; Huang, J.; Zhao, X.; Li, Y.; Hu, Y.; Lei, Y. GDF10 inhibits proliferation and epithelial-mesenchymal transition in triple-negative breast cancer via upregulation of Smad7. Aging 2019, 11, 3298–3314. [Google Scholar] [CrossRef]
- Huang, X.; Jia, Z.; Li, X.; Hu, Z.; Yu, X.; Xia, J. Asiaticoside hampers epithelial-mesenchymal transition by promoting PPARG expression and suppressing P2RX7-mediated TGF-β/Smad signaling in triple-negative breast cancer. Phytother. Res. 2023, 37, 1771–1786. [Google Scholar] [CrossRef]
- Zhao, B.M.; Hoffmann, F.M. Inhibition of transforming growth factor-β1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol. Biol. Cell 2006, 17, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, M.; Wang, S.; Wang, Q.; Lu, H.; Wang, F.; Wang, L.; Peng, D.; Chen, W. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int. J. Pharm. 2022, 623, 121953. [Google Scholar] [CrossRef]
- Miah, S.; Banks, C.A.S.; Ogunbolude, Y.; Bagu, E.T.; Berg, J.M.; Saraf, A.; Tettey, T.T.; Hattem, G.; Dayebgadoh, G.; Kempf, C.G.; et al. BRK phosphorylates SMAD4 for proteasomal degradation and inhibits tumor suppressor FRK to control SNAIL, SLUG, and metastatic potential. Sci. Adv. 2019, 5, eaaw3113. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Kim, K.; Kim, S.-K.; Oh, J.-H.; Min, J.-K.; Jung, C.-R.; Son, M.-Y.; Kim, D.-S.; Cho, H.-S. SETDB1 regulates SMAD7 expression for breast cancer metastasis. BMB Rep. 2019, 52, 139–144. [Google Scholar] [CrossRef]
- Eckhardt, B.L.; Cao, Y.; Redfern, A.D.; Chi, L.H.; Burrows, A.D.; Roslan, S.; Sloan, E.K.; Parker, B.S.; Loi, S.; Ueno, N.T.; et al. Activation of Canonical BMP4-SMAD7 Signaling Suppresses Breast Cancer Metastasis. Cancer Res. 2020, 80, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Shonibare, Z.; Monavarian, M.; O’Connell, K.; Altomare, D.; Shelton, A.; Mehta, S.; Jaskula-Sztul, R.; Phaeton, R.; Starr, M.D.; Whitaker, R.; et al. Reciprocal SOX2 regulation by SMAD1-SMAD3 is critical for anoikis resistance and metastasis in cancer. Cell Rep. 2022, 40, 111066. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, J.; Liu, M.; Huang, Y.; Yin, L. SMAD3 inducing the transcription of STYK1 to promote the EMT process and improve the tolerance of ovarian carcinoma cells to paclitaxel. J. Cell. Biochem. 2019, 120, 10796–10811. [Google Scholar] [CrossRef]
- Weis-Banke, S.E.; Lerdrup, M.; Kleine-Kohlbrecher, D.; Mohammad, F.; Sidoli, S.; Jensen, O.N.; Yanase, T.; Nakamura, T.; Iwase, A.; Stylianou, A.; et al. Mutant FOXL2C134W Hijacks SMAD4 and SMAD2/3 to Drive Adult Granulosa Cell Tumors. Cancer Res. 2020, 80, 3466–3479. [Google Scholar] [CrossRef]
- Kriseman, M.; Monsivais, D.; Agno, J.; Masand, R.P.; Creighton, C.J.; Matzuk, M.M. Uterine double-conditional inactivation of Smad2 and Smad3 in mice causes endometrial dysregulation, infertility, and uterine cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3873–3882. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Guo, S.-B.; Shi, H.; Li, X.-L.; Zhu, Y.; Li, M.; Song, L.-Y.; Yu, R.-M.; Cai, Q.-Q.; Tian, X.-P. The β-catenin-LINC00183-miR-371b-5p-Smad2/LEF1 axis promotes adult T-cell lymphoblastic lymphoma progression and chemoresistance. J. Exp. Clin. Cancer Res. 2023, 42, 105. [Google Scholar] [CrossRef]
- Eritja, N.; Navaridas, R.; Ruiz-Mitjana, A.; Vidal-Sabanés, M.; Egea, J.; Encinas, M.; Matias-Guiu, X.; Dolcet, X. Endometrial PTEN Deficiency Leads to SMAD2/3 Nuclear Translocation. Cancers 2021, 13, 4990. [Google Scholar] [CrossRef]
- Haque, P.S.; Apu, M.N.H.; Nahid, N.A.; Islam, F.; Islam, M.R.; Hasnat, A.; Islam, M.S. SMAD2 rs4940086 heterozygosity increases the risk of cervical cancer development among the women in Bangladesh. Mol. Biol. Rep. 2020, 47, 5033–5040. [Google Scholar] [CrossRef]
- Li, B.; An, W.; Wang, H.; Baslan, T.; Mowla, S.; Krishnan, A.; Xiao, W.; Koche, R.P.; Liu, Y.; Cai, S.F.; et al. BMP2/SMAD pathway activation in JAK2/p53-mutant megakaryocyte/erythroid progenitors promotes leukemic transformation. Blood 2022, 139, 3630–3646. [Google Scholar] [CrossRef]
- Witkowska, M.; Majchrzak, A.; Cebula-Obrzut, B.; Wawrzyniak, E.; Robak, T.; Smolewski, P. The distribution and potential prognostic value of SMAD protein expression in chronic lymphocytic leukemia. Tumour Biol. 2017, 39, 1010428317694551. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Cui, H.; Zhang, X.; Zhang, G.; Yang, X.; Yang, S.; Zhang, Z.; Wang, J.; Hu, K.; et al. High expression levels of SMAD3 and SMAD7 at diagnosis predict poor prognosis in acute myeloid leukemia patients undergoing chemotherapy. Cancer Gene Ther. 2019, 26, 119–127. [Google Scholar] [CrossRef]
- Wang, L.; Gu, S.; Chen, F.; Yu, Y.; Cao, J.; Li, X.; Gao, C.; Chen, Y.; Yuan, S.; Liu, X.; et al. Imatinib blocks tyrosine phosphorylation of Smad4 and restores TGF-β growth-suppressive signaling in BCR-ABL1-positive leukemia. Signal Transduct. Target. Ther. 2023, 8, 120. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Biecek, P.; Donizy, P.; Pieniazek, M.; Matkowski, R.; Halon, A. SMAD7 is a novel independent predictor of survival in patients with cutaneous melanoma. Transl. Res. 2019, 204, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Iwakami, Y.; Hang, Z.; Kin, R.; Zhou, Y.; Yasuta, Y.; Takahashi, A.; Hayakawa, Y.; Sakurai, H. Targeting PSMD14 inhibits melanoma growth through SMAD3 stabilization. Sci. Rep. 2020, 10, 19214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Tian, Y.; Zhu, Q.; Ge, Y.; Yu, H.; Huang, J.; Li, H.; Zhang, J.; Zhang, L.; et al. MED1 Downregulation Contributes to TGFβ-Induced Metastasis by Inhibiting SMAD2 Ubiquitination Degradation in Cutaneous Melanoma. J. Investig. Dermatol. 2022, 142, 2229–2237.e4. [Google Scholar] [CrossRef]
- Xiao, Y.; Qing, J.; Li, B.; Chen, L.; Nong, S.; Yang, W.; Tang, X.; Chen, Z. TIM-3 Participates in the Invasion and Metastasis of Nasopharyngeal Carcinoma via SMAD7/SMAD2/SNAIL1 Axis-Mediated Epithelial-Mesenchymal Transition. Onco Targets Ther. 2020, 13, 1993–2006. [Google Scholar] [CrossRef]
- Citro, S.; Miccolo, C.; Medda, A.; Ghiani, L.; Tagliabue, M.; Ansarin, M.; Chiocca, S. HPV-mediated regulation of SMAD4 modulates the DNA damage response in head and neck cancer. J. Exp. Clin. Cancer Res. 2022, 41, 59. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.L.; Young, C.D.; Bian, L.; Weigel, K.; Nolan, K.; Frederick, B.; Han, G.; He, G.; Trahan, G.D.; Rudolph, M.C.; et al. PARP Inhibition Enhances Radiotherapy of SMAD4-Deficient Human Head and Neck Squamous Cell Carcinomas in Experimental Models. Clin. Cancer Res. 2020, 26, 3058–3070. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.-H.; Fu, L.; Song, J.; Xie, D.-D.; Yu, D.-X.; Xu, D.-X.; Sun, G.-P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and β-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020, 111, 59–71. [Google Scholar] [CrossRef]
- Lu, W.; Sun, J.; Zhou, H.; Wang, F.; Zhao, C.; Li, K.; Fan, C.; Ding, G.; Wang, J. HNF1B inhibits cell proliferation via repression of SMAD6 expression in prostate cancer. J. Cell Mol. Med. 2020, 24, 14539–14548. [Google Scholar] [CrossRef]
- Thakur, N.; Hamidi, A.; Song, J.; Itoh, S.; Bergh, A.; Heldin, C.-H.; Landström, M. Smad7 Enhances TGF-β-Induced Transcription of c-Jun and HDAC6 Promoting Invasion of Prostate Cancer Cells. iScience 2020, 23, 101470. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Hao, J.-L.; Xie, Q.-W.; Han, G.-Q.; Xu, B.-B.; Hu, H.; Sa, N.-E.; Du, X.-W.; Tang, H.-L.; Yan, J.; et al. PELO facilitates PLK1-induced the ubiquitination and degradation of Smad4 and promotes the progression of prostate cancer. Oncogene 2022, 41, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shangguan, X.; Pan, J.; Yue, Z.; Shen, K.; Ji, Y.; Zhang, W.; Zhu, Y.; Sha, J.; Wang, Y.; et al. HOXD13 suppresses prostate cancer metastasis and BMP4-induced epithelial-mesenchymal transition by inhibiting SMAD1. Int. J. Cancer 2021, 148, 3060–3070. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-Y.; Pornour, M.; Ryu, H.; Khadka, S.; Xu, R.; Jang, J.; Li, D.; Chen, H.; Hussain, A.; Fazli, L.; et al. SMAD3 promotes expression and activity of the androgen receptor in prostate cancer. Nucleic Acids Res. 2023, 51, 2655–2670. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, Y.; Hua, B.; Gu, X.; Lu, C.; Xu, B.; Pan, W. Sasanquasaponin inhibited epithelial to mesenchymal transition in prostate cancer by regulating the PI3K/Akt/mTOR and Smad pathways. Pharm. Biol. 2022, 60, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, L.; Dai, Y.; Li, J.; Liu, S. MicroRNA-135 inhibits gastric cancer metastasis by targeting SMAD2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9436–9444. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Z.; Zhu, Y.; Xue, H.; Wei, C. Long noncoding RNA TMEM147-AS1 serves as a microRNA-326 sponge to aggravate the malignancy of gastric cancer by upregulating SMAD5. Oncol. Res. 2021, 29, 263–273. [Google Scholar] [CrossRef]
- Yin, D.; Lu, X. Silencing of long non-coding RNA HCP5 inhibits proliferation, invasion, migration, and promotes apoptosis via regulation of miR-299-3p/SMAD5 axis in gastric cancer cells. Bioengineered 2021, 12, 225–239. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Wang, J.; Liu, X.; Qu, H.; Zhang, G.; Liang, T.; Wang, J.; Zhang, J. A high level of lncFGD5-AS1 inhibits epithelial-to-Mesenchymal transition by regulating the miR-196a-5p/SMAD6/BMP axis in gastric Cancer. BMC Cancer 2021, 21, 453. [Google Scholar] [CrossRef]
- Zheng, L.; Liang, H.; Zhang, Q.; Shen, Z.; Sun, Y.; Zhao, X.; Gong, J.; Hou, Z.; Jiang, K.; Wang, Q.; et al. circPTEN1, a circular RNA generated from PTEN, suppresses cancer progression through inhibition of TGF-β/Smad signaling. Mol. Cancer 2022, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Yang, F.; Song, Y.; Wang, X.; Yang, Y. LINC00657 regulate colorectal carcinoma invasion and migration by enhancing heparanase expression through recruiting SMAD family member 2. Anticancer Drugs 2022, 33, 803–814. [Google Scholar] [CrossRef]
- Wu, N.; Jiang, M.; Liu, H.; Chu, Y.; Wang, D.; Cao, J.; Wang, Z.; Xie, X.; Han, Y.; Xu, B. LINC00941 promotes CRC metastasis through preventing SMAD4 protein degradation and activating the TGF-β/SMAD2/3 signaling pathway. Cell Death Differ. 2021, 28, 219–232. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, G.; Sun, P.; Zhu, Y.; Zhang, H. circ_SMAD2 regulate colorectal cancer cells proliferation through targeting miR-1258/RPN2 signaling pathway. J. Cancer 2021, 12, 1678–1686. [Google Scholar] [CrossRef]
- Bayat, Z.; Ghaemi, Z.; Behmanesh, M.; Soltani, B.M. Hsa-miR-186-5p regulates TGFβ signaling pathway through expression suppression of SMAD6 and SMAD7 genes in colorectal cancer. Biol. Chem. 2021, 402, 469–480. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yan, B.; Yang, J.; Chen, E. MiR-581/SMAD7 Axis Contributes to Colorectal Cancer Metastasis: A Bioinformatic and Experimental Validation-Based Study. Int. J. Mol. Sci. 2020, 21, 6499. [Google Scholar] [CrossRef]
- Huang, W.-J.; Tian, X.-P.; Bi, S.-X.; Zhang, S.-R.; He, T.-S.; Song, L.-Y.; Yun, J.-P.; Zhou, Z.-G.; Yu, R.-M.; Li, M. The β-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes hepatocellular carcinoma metastasis. Oncogene 2020, 39, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiang, L.; Li, L.; Ou, H.; Fang, Y.; Xu, Y.; Liu, Q.; Hu, Z.; Huang, Y.; Li, X.; et al. TGF-β1 induced deficiency of linc00261 promotes epithelial-mesenchymal-transition and stemness of hepatocellular carcinoma via modulating SMAD3. J. Transl. Med. 2022, 20, 75. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, C.; Zhu, Y.; Han, H. LINC01410 promotes cell proliferation and migration of cholangiocarcinoma through modulating miR-124-3p/SMAD5 axis. J. Gene Med. 2020, 22, e3162. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.-L.; Diao, N.; Zhou, Z.-W.; Fang, C.-K.; Wang, J.-N.; Zhang, Y.; Luo, R.; Zhong, C. CircFGGY Inhibits Cell Growth, Invasion and Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma via Regulating the miR-545-3p/Smad7 Axis. Front. Cell Dev. Biol. 2022, 10, 850708. [Google Scholar] [CrossRef]
- Hong, Y.; Ye, M.; Wang, F.; Fang, J.; Wang, C.; Luo, J.; Liu, J.; Liu, J.; Liu, L.; Zhao, Q.; et al. MiR-21-3p Promotes Hepatocellular Carcinoma Progression via SMAD7/YAP1 Regulation. Front. Oncol. 2021, 11, 642030. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, J.; Yu, J.; Liao, J.; Liu, S.; Cai, N.; Liang, H.; Chen, X.; Ding, Z.; Zhang, B. MicroRNA-148a-3p inhibits progression of hepatocelluar carcimoma by repressing SMAD2 expression in an Ago2 dependent manner. J. Exp. Clin. Cancer Res. 2020, 39, 150. [Google Scholar] [CrossRef]
- Zhou, J.; Qie, S.; Fang, H.; Xi, J. MiR-487a-3p suppresses the malignant development of pancreatic cancer by targeting SMAD7. Exp. Mol. Pathol. 2020, 116, 104489. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, W.; Wu, Y. EIF4A3-induced circular RNA SCAP facilitates tumorigenesis and progression of non-small-cell lung cancer via miR-7/SMAD2 signaling. Environ. Sci. Pollut. Res. Int. 2023, 30, 65237–65249. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Guan, Y.; Zhang, Q. Long noncoding RNA OSER1-AS1 promotes the malignant properties of non-small cell lung cancer by sponging microRNA-433-3p and thereby increasing Smad2 expression. Oncol. Rep. 2020, 44, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Nagoor, N.H. Overexpression of miR-361-5p plays an oncogenic role in human lung adenocarcinoma through the regulation of SMAD2. Int. J. Oncol. 2019, 54, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Marquez, M.E.; Sernbo, S.; Payque, E.; Uria, R.; Tosar, J.P.; Querol, J.; Berca, C.; Uriepero, A.; Prieto, D.; Alvarez-Saravia, D.; et al. TGF-β/SMAD Pathway Is Modulated by miR-26b-5p: Another Piece in the Puzzle of Chronic Lymphocytic Leukemia Progression. Cancers 2022, 14, 1676. [Google Scholar] [CrossRef]
- Lin, H.; Xu, X.; Chen, K.; Fu, Z.; Wang, S.; Chen, Y.; Zhang, H.; Niu, Y.; Chen, H.; Yu, H.; et al. LncRNA CASC15, MiR-23b Cluster and SMAD3 form a Novel Positive Feedback Loop to promote Epithelial-Mesenchymal Transition and Metastasis in Ovarian Cancer. Int. J. Biol. Sci. 2022, 18, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Huang, Q.; Li, F.; Luo, F. LncRNA MCTP1-AS1 Regulates EMT Process in Endometrial Cancer by Targeting the miR-650/SMAD7 Axis. Onco Targets Ther. 2021, 14, 751–761. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, P.; Sun, X.; Zeng, J.; Cao, W.; Gao, W.; Wang, Y.; Wang, L. Hsa_Circ_0001860 Promotes Smad7 to Enhance MPA Resistance in Endometrial Cancer via miR-520h. Front. Cell Dev. Biol. 2021, 9, 738189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Zhou, G.; Han, R.; Sun, Z.; Zhong, M.; Jiang, X. Circ_0002623 promotes bladder cancer progression by regulating the miR-1276/SMAD2 axis. Cancer Sci. 2022, 113, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Zheng, H.; Huang, J.; Lin, Y.; Luo, Y.; Kong, Y.; Pang, M.; Zhang, D.; Yang, J.; Chen, J.; et al. Aberrant Nuclear Export of circNCOR1 Underlies SMAD7-Mediated Lymph Node Metastasis of Bladder Cancer. Cancer Res. 2022, 82, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, X.; Ying, Y.; Wang, W.; Shen, H.; Wang, S.; Xie, H.; Yi, J.; Zhan, W.; Li, J.; et al. SMAD3 and FTO are involved in miR-5581-3p-mediated inhibition of cell migration and proliferation in bladder cancer. Cell Death Discov. 2022, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Wang, Q.; Dai, Y.; Wang, H.; Wang, M.; Wang, J.; Zhang, D.; Sun, P.; Qi, T.; Jin, X.; et al. Hypomethylation of PlncRNA-1 promoter enhances bladder cancer progression through the miR-136-5p/Smad3 axis. Cell Death Dis. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Xu, Y.-P.; Liu, W.-H.; Li, D.-C.; Wang, H.; Li, C.-F. Long Noncoding RNA KCNMB2-AS1 Promotes SMAD5 by Targeting miR-3194-3p to Induce Bladder Cancer Progression. Front. Oncol. 2021, 11, 649778. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Dai, Y.; Wu, Z.; Yang, Q.; He, S.; Zhang, X.; Guo, W.; Lai, Y.; Du, H.; Wang, H.; et al. SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-β signaling promotes prostate cancer bone metastasis. Mol. Oncol. 2020, 14, 808–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, W.; Wu, F.; Gao, Y.; Sun, Q.; Hu, N.; Lu, W.; Zhou, J. MiR-135-5p inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by targeting SMAD3 in breast cancer. J. Cancer 2020, 11, 6402–6412. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-L.; Li, J.-C.; Zhou, C.-X.; Ma, C.-N.; Wang, D.-F.; Wo, L.-L.; He, M.; Yin, Q.; He, J.-R.; Zhao, Q. Long non-coding RNA ARHGAP5-AS1 inhibits migration of breast cancer cell via stabilizing SMAD7 protein. Breast Cancer Res. Treat. 2021, 189, 607–619. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, H.-Y.; Zhang, J.; Dai, H.-Y.; Yao, Z.-X.; Zheng, Z.; Meng-Yan, S.; Wu, K. NEAT1/miR-200b-3p/SMAD2 axis promotes progression of melanoma. Aging 2020, 12, 22759–22775. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, B.; Lian, X.; Xu, J.; Zhang, T. miR-135a Targets SMAD2 to Promote Osteosarcoma Proliferation and Migration. J. Oncol. 2022, 2022, 3037348. [Google Scholar] [CrossRef]
- Zheng, S.; Wan, L.; Ge, D.; Jiang, F.; Qian, Z.; Tang, J.; Yang, J.; Yao, Y.; Yan, J.; Zhao, L.; et al. LINC00266-1/miR-548c-3p/SMAD2 feedback loop stimulates the development of osteosarcoma. Cell Death Dis. 2020, 11, 576. [Google Scholar] [CrossRef]
- Gu, Z.; Li, Z.; Xu, R.; Zhu, X.; Hu, R.; Xue, Y.; Xu, W. miR-16-5p Suppresses Progression and Invasion of Osteosarcoma via Targeting at Smad3. Front. Pharmacol. 2020, 11, 1324. [Google Scholar] [CrossRef]
- Hoot, K.E.; Lighthall, J.; Han, G.; Lu, S.-L.; Li, A.; Ju, W.; Kulesz-Martin, M.; Bottinger, E.; Wang, X.-J. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J. Clin. Investig. 2008, 118, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H.; Groth, S.; Sebens, S.; Lehnert, H.; Gieseler, F.; Fändrich, F. Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: Control by Rac1. Mol. Cancer 2011, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Pardali, E.; van der Horst, G.; Cheung, H.; van den Hoogen, C.; van der Pluijm, G.; Dijke, P.T. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2010, 29, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xiong, F.; Wu, G.; Wang, D.; Liu, W.; Chen, J.; Qi, Y.; Wang, B.; Chen, Y. SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications. Diagnostics 2023, 13, 2769. https://doi.org/10.3390/diagnostics13172769
Wang Q, Xiong F, Wu G, Wang D, Liu W, Chen J, Qi Y, Wang B, Chen Y. SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications. Diagnostics. 2023; 13(17):2769. https://doi.org/10.3390/diagnostics13172769
Chicago/Turabian StyleWang, Qi, Fei Xiong, Guanhua Wu, Da Wang, Wenzheng Liu, Junsheng Chen, Yongqiang Qi, Bing Wang, and Yongjun Chen. 2023. "SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications" Diagnostics 13, no. 17: 2769. https://doi.org/10.3390/diagnostics13172769
APA StyleWang, Q., Xiong, F., Wu, G., Wang, D., Liu, W., Chen, J., Qi, Y., Wang, B., & Chen, Y. (2023). SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications. Diagnostics, 13(17), 2769. https://doi.org/10.3390/diagnostics13172769






