Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Specimen Handling
2.3. Specimen Testing
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Bacterial and Yeast Identification
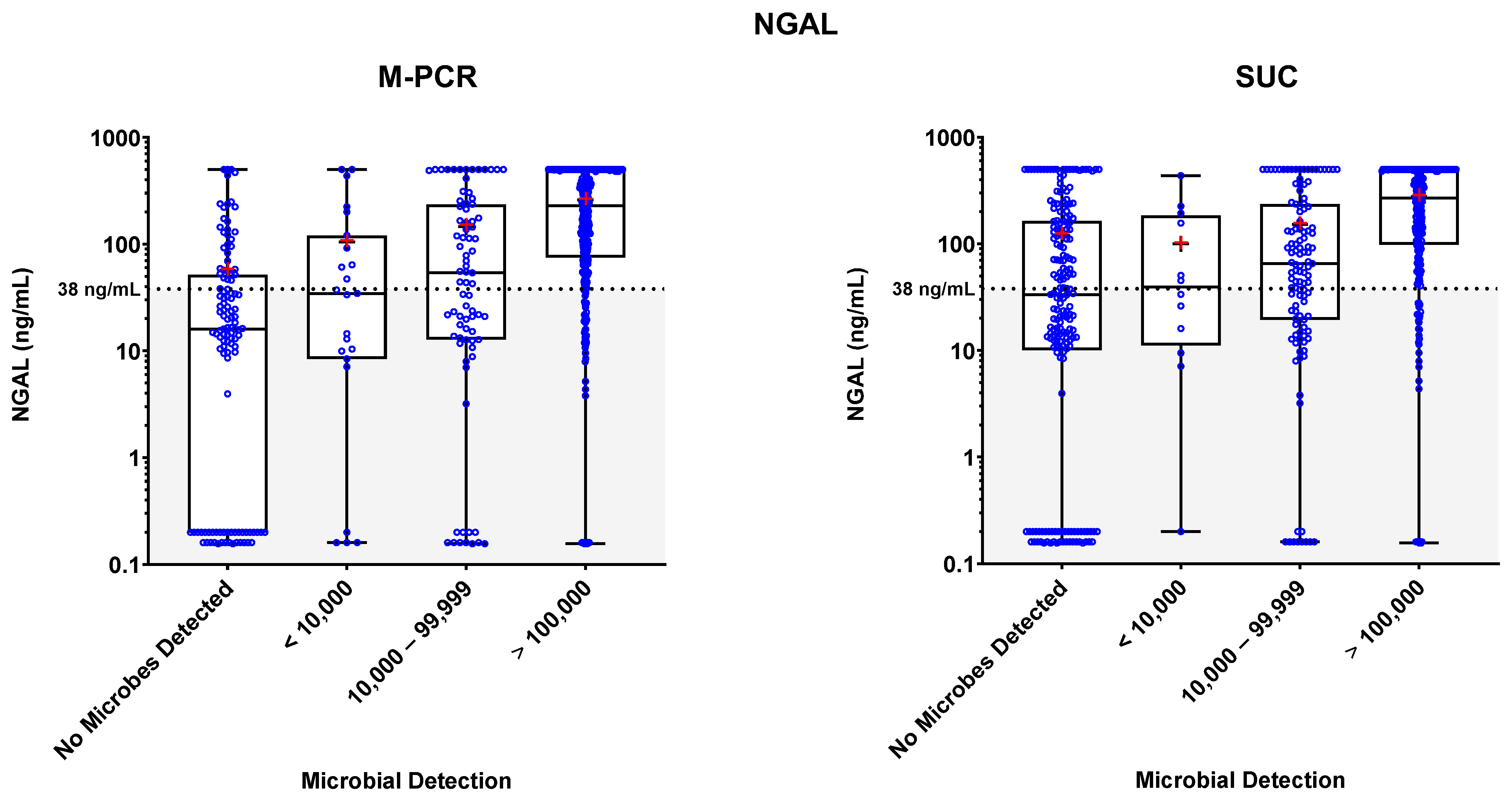
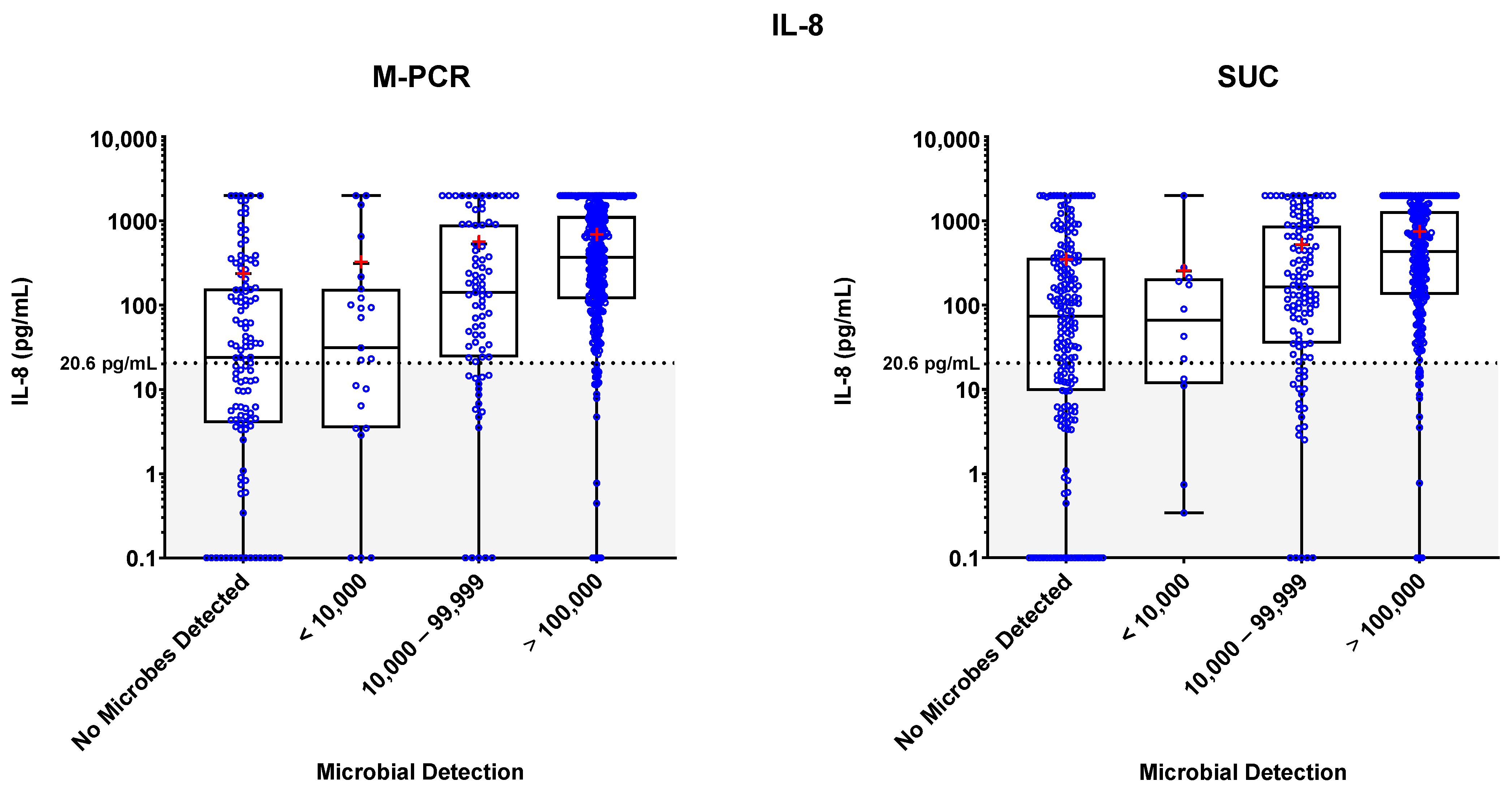
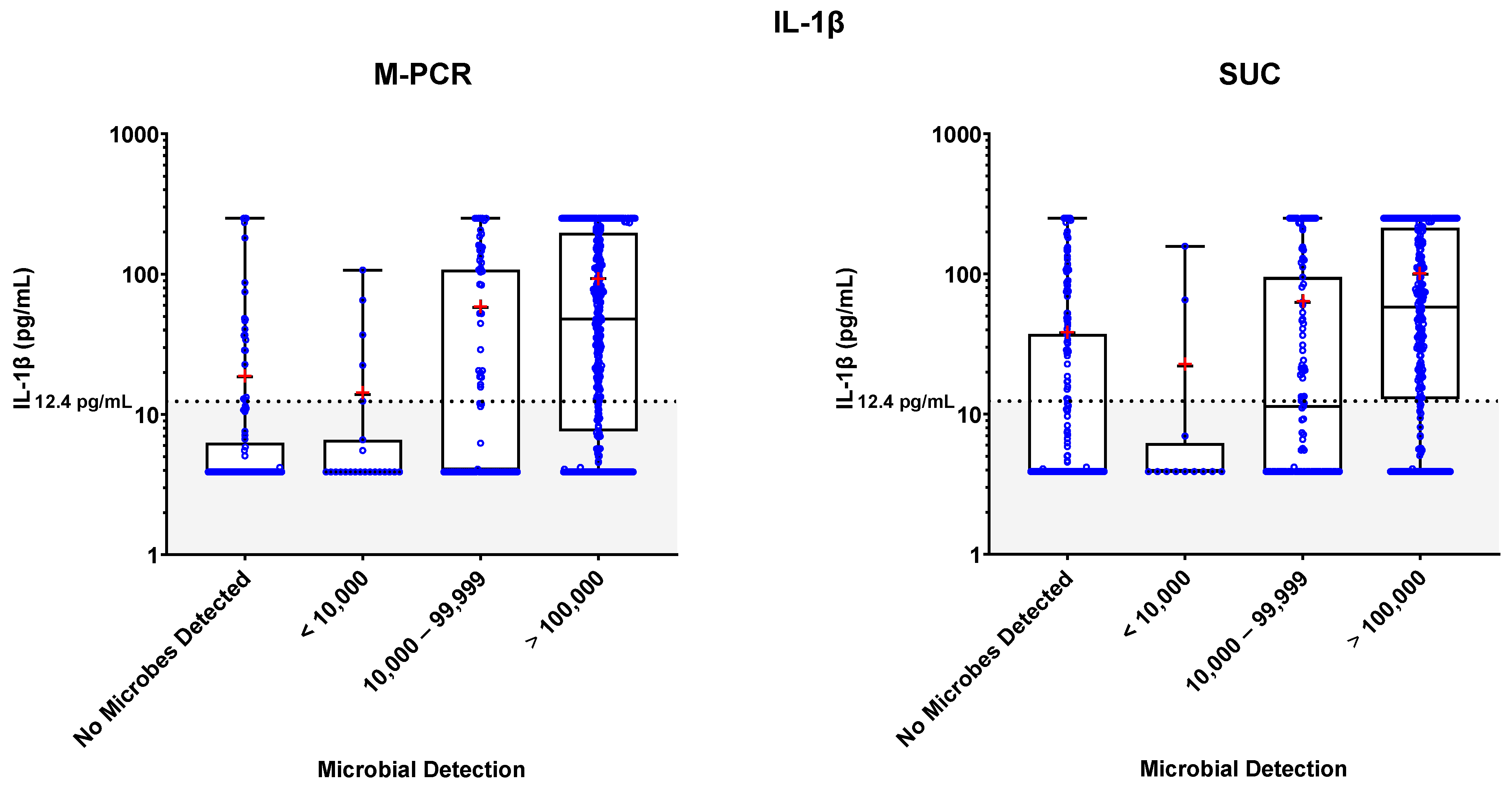
3.3. Biomarker Detections
3.4. Biomarker Patterns: Microbial Density and Technique Differences
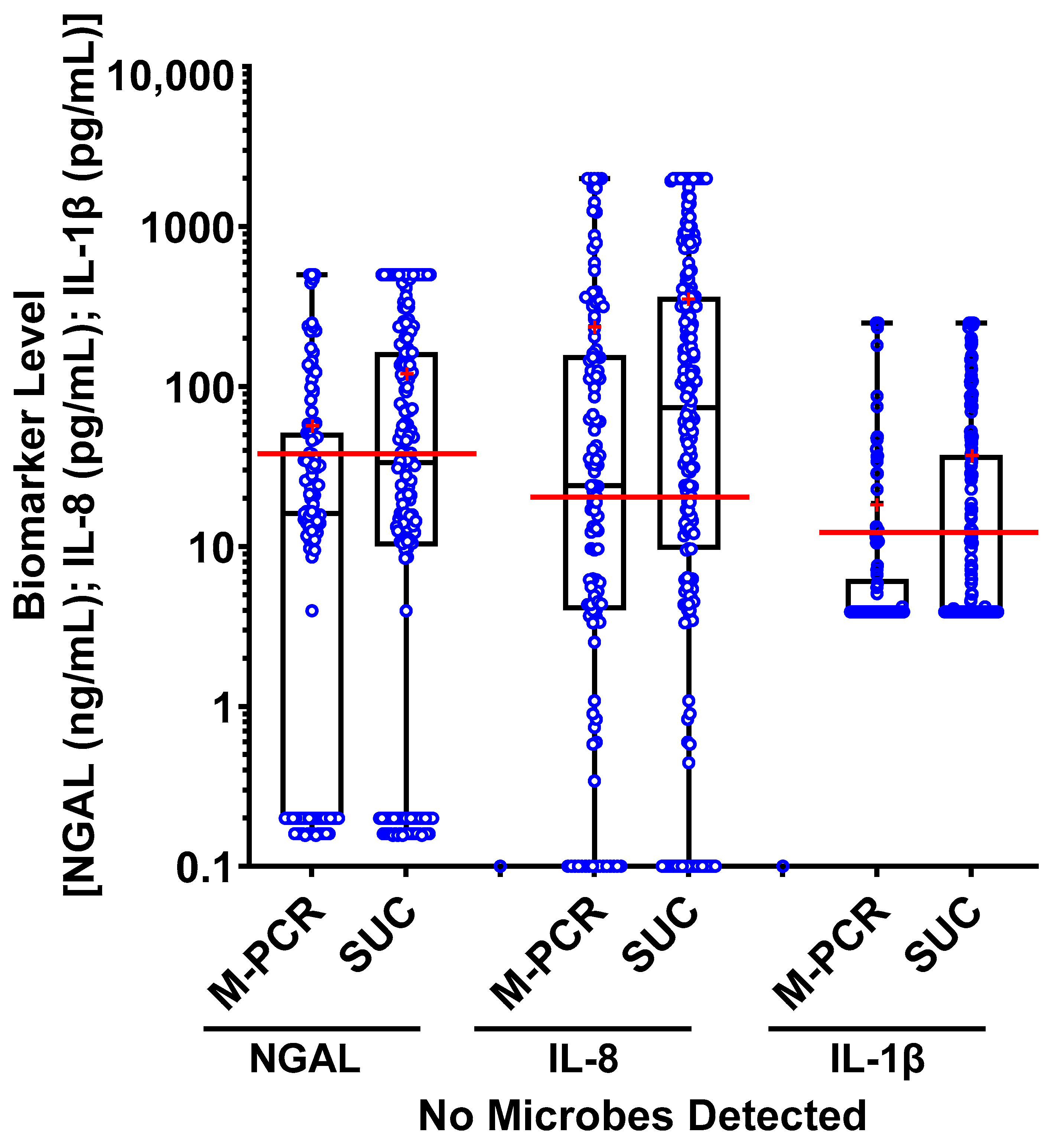
3.5. Biomarker Consensus by Microbial Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, R.H.; Shapiro, E.D.; Andriole, V.T.; Davis, R.J.; Stamm, W.E. Evaluation of New Anti-Infective Drugs for the Treatment of Urinary Tract Infection. Clin. Infect. Dis. 1992, 15, S216–S227. [Google Scholar] [CrossRef] [PubMed]
- Hovelius, B.; Mårdh, P.-A.; Bygren, P. Urinary Tract Infections Caused by Staphylococcus Saprophyticus: Recurrences and Complications. J. Urol. 1979, 122, 645–647. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C. PHE/NHS Diagnosis of Urinary Tract Infections. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/927195/UTI_diagnostic_flowchart_NICE-October_2020-FINAL.pdfov.uk (accessed on 15 February 2023).
- Kouri, T.; Fogazzi, G.; Gant, V.; Hallander, H.; Hofmann, W.; Guder, W.G. European Urinalysis Guidelines. Scand. J. Clin. Lab. Investig. 2000, 60, 1–96. [Google Scholar] [CrossRef]
- Roberts, F.J. Quantitative Urine Culture in Patients with Urinary Tract Infection and Bacteremia. Am. J. Clin. Pathol. 1986, 85, 616–618. [Google Scholar] [CrossRef]
- Kunin, M. Urinary Tract Infections: Detection, Prevention, and Management; Kunin, M., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1997. [Google Scholar]
- Choe, H.; Lee, S.; Yang, S.S.; Hamasuna, R.; Yamamoto, S.; Cho, Y.; Matsumoto, T.; on behalf of the Committee for Development of the UAA-AAUS Guidelines for UTI and STI. Summary of the UAA-AAUS Guidelines for Urinary Tract Infections. Int. J. Urol. 2018, 25, 175–185. [Google Scholar] [CrossRef]
- De Cueto, M.; Aliaga, L.; Alós, J.-I.; Canut, A.; Los-Arcos, I.; Martínez, J.A.; Mensa, J.; Pintado, V.; Rodriguez-Pardo, D.; Yuste, J.R.; et al. Executive Summary of the Diagnosis and Treatment of Urinary Tract Infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enfermedades Infecc. Microbiol. Clínica 2017, 35, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Bergman, B.; Bishop, M.C.; Bjerklund-Johansen, T.E.; Botto, H.; Lobel, B.; Cruz, F.J.; Selvaggi, F.P. EAU Guidelines for the Management of Urinary and Male Genital Tract Infections: Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur. Urol. 2001, 40, 576–588. [Google Scholar] [CrossRef]
- Patel, R.; Polage, C.R.; Bard, J.D.; May, L.; Lee, F.M.; Fabre, V.; Hayden, M.K.; Doernberg, S.D.B.; Haake, D.A.; Trautner, B.W.; et al. Envisioning Future UTI Diagnostics. Clin. Infect. Dis. 2021, 74, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Johansen, T.E.B.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, Definition and Treatment of Complicated Urinary Tract Infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- Hernández-Hernández, D.; Padilla-Fernández, B.; Ortega-González, M.Y.; Castro-Díaz, D.M. Recurrent Urinary Tract Infections and Asymptomatic Bacteriuria in Adults. Curr. Bladder Dysfunct. Rep. 2021, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Hilt, E.E.; Dune, T.J.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Urine Trouble: Should We Think Differently about UTI? Int. Urogynecol. J. 2018, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive Immune Mechanisms: Mediators of Host Defence and Immune Regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef]
- Mariano, L.L.; Ingersoll, M.A. The Immune Response to Infection in the Bladder. Nat. Rev. Urol. 2020, 17, 439–458. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yang, J.; Xie, X.; Chen, H. The Immune Responses to Different Uropathogens Call Individual Interventions for Bladder Infection. Front. Immunol. 2022, 13, 953354. [Google Scholar] [CrossRef]
- Abraham, S.N.; Miao, Y. The Nature of Immune Responses to Urinary Tract Infections. Nat. Rev. Immunol. 2015, 15, 655–663. [Google Scholar] [CrossRef]
- Horváth, J.; Wullt, B.; Naber, K.G.; Köves, B. Biomarkers in Urinary Tract Infections—Which Ones Are Suitable for Diagnostics and Follow-Up? GMS Infect. Dis. 2020, 8, 68. [Google Scholar] [CrossRef]
- Martino, F.K.; Novara, G. Asymptomatic Bacteriuria or Urinary Tract Infection? New and Old Biomarkers. Int. J. Transl. Med. 2022, 2, 6. [Google Scholar] [CrossRef]
- Edwards, G.; Seeley, A.; Carter, A.; Smith, M.P.; Cross, E.L.; Hughes, K.; den Bruel, A.V.; Llewelyn, M.J.; Verbakel, J.Y.; Hayward, G. What Is the Diagnostic Accuracy of Novel Urine Biomarkers for Urinary Tract Infection? Biomark Insights 2023, 18, 11772719221144460. [Google Scholar] [CrossRef]
- Nanda, N.; Juthani-Mehta, M. Novel Biomarkers for the Diagnosis of Urinary Tract Infection–-A Systematic Review. Biomark Insights 2009, 4, BMI.S3155. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Martin, J.M.; Hoberman, A.; Skae, M.; Milkovich, L.; McElheny, C.; Hickey, R.W.; Gabriel, L.V.; Kearney, D.H.; Majd, M.; et al. Biomarkers That Differentiate False Positive Urinalyses from True Urinary Tract Infection. Pediatr. Nephrol. 2020, 35, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Rodhe, N.; Löfgren, S.; Strindhall, J.; Matussek, A.; Molstad, S. Cytokines in Urine in Elderly Subjects with Acute Cystitis and Asymptomatic Bacteriuria. Scand. J. Prim. Health Care 2009, 27, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Masajtis-Zagajewska, A.; Nowicki, M. New Markers of Urinary Tract Infection. Clin. Chim. Acta 2017, 471, 286–291. [Google Scholar] [CrossRef]
- Yilmaz, A.; Sevketoglu, E.; Gedikbasi, A.; Karyagar, S.; Kiyak, A.; Mulazimoglu, M.; Aydogan, G.; Ozpacaci, T.; Hatipoglu, S. Early Prediction of Urinary Tract Infection with Urinary Neutrophil Gelatinase Associated Lipocalin. Pediatr. Nephrol. 2009, 24, 2387. [Google Scholar] [CrossRef]
- Akhlaghpour, M.; Haley, E.; Parnell, L.; Luke, N.; Mathur, M.; Festa, R.; Percaccio, M.; Magallon, J.; Remedios-Chan, M.; Rosas, A.; et al. Urine Biomarkers Individually and as a Consensus Model Show High Sensitivity and Specificity for Detecting UTIs. Diagnostics 2023. preprint. [Google Scholar] [CrossRef]
- Tullus, K. Low Urinary Bacterial Counts: Do They Count? Pediatr. Nephrol. 2016, 31, 171–174. [Google Scholar] [CrossRef]
- Scott, V.; Haake, D.A.; Churchill, B.M.; Justice, S.S.; Kim, J.-H. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology 2015, 86, 425–431. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Festa, R.A.; Opel, M.; Mathur, M.; Luke, N.; Parnell, L.K.S.; Wang, D.; Zhao, X.; Magallon, J.; Percaccio, M.; Baunoch, D. Quantitative Multiplex PCR in Copies Ml–1 Linearly Correlates with Standard Urine Culture in Colonies Ml−1 for Urinary Tract Infection (UTI) Pathogens. Lett. Appl. Microbiol. 2023, ovad085. [Google Scholar] [CrossRef]
- Clinic, M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) 2008. Available online: https://www.mayocliniclabs.com/test-notifications/attachment.php?id=11315 (accessed on 28 April 2023).
- Yuan, Q.; Huang, R.; Tang, L.; Yuan, L.; Gao, L.; Liu, Y.; Cao, Y. Screening Biomarkers and Constructing a Predictive Model for Symptomatic Urinary Tract Infection and Asymptomatic Bacteriuria in Patients Undergoing Cutaneous Ureterostomy: A Metagenomic Next-Generation Sequencing Study. Dis. Markers 2022, 2022, 7056517. [Google Scholar] [CrossRef] [PubMed]
- Mysorekar, I.; National, H.S. Mechanisms of Uropathogenic Escherichia Coli Persistence and Eradication from the Urinary Tract. Proc. Natl. Acad. Sci. USA 2006, 103, 14170–14175. [Google Scholar] [CrossRef]
- Holá, V.; Opazo-Capurro, A.; Scavone, P. Editorial: The Biofilm Lifestyle of Uropathogens. Front. Cell. Infect. Microbiol. 2021, 11, 763415. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease Burden and Long-Term Trends of Urinary Tract Infections: A Worldwide Report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- Baunoch, D.; Luke, N.; Wang, D.; Vollstedt, A.; Zhao, X.; Ko, D.S.C.; Huang, S.; Cacdac, P.; Sirls, L.T. Concordance between Antibiotic Resistance Genes and Susceptibility in Symptomatic Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 3275–3286. [Google Scholar]
- Vollstedt, A.; Baunoch, D.; Wojno, K.; Luke, N.; Cline, K.; Belkoff, L.; Milbank, A.; Sherman, N.; Haverkorn, R.M.; Gaines, N.; et al. Multisite Prospective Comparison of Multiplex Polymerase Chain Reaction Testing with Urine Culture for Diagnosis of Urinary Tract Infections in Symptomatic Patients. J. Surg. Urol. 2020, 2020, JSU-102. [Google Scholar]
- Harding, C.; Rantell, A.; Cardozo, L.; Jacobson, S.K.; Anding, R.; Kirschner-Hermanns, R.; Greenwell, T.; Swamy, S.; Malde, S.; Abrams, P. How Can We Improve Investigation, Prevention and Treatment for Recurrent Urinary Tract Infections—ICI-RS 2018. Neurourol. Urodyn. 2019, 38, S90–S97. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-Analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4, 459–502. [Google Scholar] [CrossRef]
- Lotte, R.; Lotte, L.; Ruimy, R. Actinotignum Schaalii (Formerly Actinobaculum Schaalii): A Newly Recognized Pathogen—Review of the Literature. Clin. Microbiol. Infect. 2016, 22, 28–36. [Google Scholar] [CrossRef]
- Kaido, M.; Yasuda, M.; Komeda, H.; Okano, M.; Ito, Y.; Ohashi, H.; Ohta, H.; Akai, Y. Prediction of Presence of Fastidious Bacteria by the Fully Automated Urine Particle Analyzer UF-1000i in the Case of Ineffective Antimicrobial Therapy for Urinary Tract Infection. J. Infect. Chemother. 2023, 29, 443–452. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Stapleton, A.E. Asymptomatic Bacteriuria and Pyuria in Premenopausal Women. Clin. Infect. Dis. 2020, 72, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W. Urinary Tract Infection as a Continuum—Implications for Diagnostic and Antibiotic Stewardship. Clin. Infect. Dis. 2020, 72, 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Grant, V.; Kelly, R.W. Urinalysis for Interleukin-8 in the Non-Invasive Diagnosis of Acute and Chronic Inflammatory Diseases. Postgrad. Med. J 2003, 79, 159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devarajan, P. Neutrophil Gelatinase-associated Lipocalin (NGAL): A New Marker of Kidney Disease. Scand. J. Clin. Lab. Investig. 2008, 68, 89–94. [Google Scholar] [CrossRef]
- Sharif-Askari, F.S.; Sharif-Askari, N.S.; Guella, A.; Alabdullah, A.; Sheleh, H.B.A.; AlRawi, A.M.H.; Haddad, E.S.; Hamid, Q.; Halwani, R.; Hamoudi, R. Blood Neutrophil-to-Lymphocyte Ratio and Urine IL-8 Levels Predict the Type of Bacterial Urinary Tract Infection in Type 2 Diabetes Mellitus Patients. Infect. Drug Resist. 2020, 13, 1961–1970. [Google Scholar] [CrossRef]

| ICD-10-CM Code | Code Description | Frequency | Percent |
|---|---|---|---|
| N39.0 | Urinary tract infection, site not specified | 534 | 81.8 |
| R30.0 | Dysuria | 43 | 6.6 |
| R31.0 | Gross hematuria | 25 | 3.8 |
| Z87.440 | Personal history of diseases of urinary system | 5 | 0.8 |
| R31.9 | Hematuria, unspecified | 4 | 0.6 |
| R82.998 | Other abnormal findings in urine | 4 | 0.6 |
| Others | - | 38 | 12.4 |
| NGAL | M-PCR | SUC | ||||||
|---|---|---|---|---|---|---|---|---|
| Microbial Density Category | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) * |
| No Microbes Detected | 117 (20.0%) | 16.05 | 56.68 | - | 193 (33.1%) | 33.49 | 120.71 | - |
| <10,000 | 23 (3.9%) | 34.55 | 105.03 | 0.2076 | 12 (2.1%) | 39.44 | 100.12 | 0.8056 |
| 10,000–99,999 | 79 (13.6%) | 53.78 | 145.3 | 0.0002 | 115 (19.7%) | 65.49 | 152.44 | 0.0104 |
| ≥100,000 | 364 (62.4%) | 228.87 | 260.27 | <0.0001 | 263 (45.1%) | 268.81 | 278.46 | <0.0001 |
| IL-8 | M-PCR | SUC | ||||||
|---|---|---|---|---|---|---|---|---|
| Microbial Density Category | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) * |
| No Microbes Detected | 117 (20.0%) | 23.95 | 236.57 | - | 193 (33.1%) | 73.84 | 354.64 | - |
| <10,000 | 23 (3.9%) | 31.22 | 312.06 | 0.7018 | 12 (2.1%) | 66.30 | 253.22 | 0.7767 |
| 10,000–99,999 | 79 (13.6%) | 141.84 | 531.96 | <0.0001 | 115 (19.7%) | 164.57 | 523.82 | 0.0014 |
| ≥100,000 | 364 (62.4%) | 371.12 | 685.69 | <0.0001 | 263 (45.1%) | 431.6 | 740.5 | <0.0001 |
| IL-1β | M-PCR | SUC | ||||||
|---|---|---|---|---|---|---|---|---|
| Microbial Density Category | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) | n (% of Total) | Median | Mean | p-Value (Relative to Symptomatic no Microbes Detected) * |
| No Microbes Detected | 117 (20.0%) | 3.9 | 18.57 | - | 193 (33.1%) | 3.9 | 38.17 | - |
| <10,000 | 23 (3.9%) | 3.9 | 13.86 | 0.86 | 12 (2.1%) | 3.9 | 22.09 | 0.2083 |
| 10,000–99,999 | 79 (13.6%) | 4.08 | 57.95 | <0.0001 | 115 (19.7%) | 11.38 | 62.75 | 0.0064 |
| ≥100,000 | 364 (62.4%) | 47.85 | 93.4 | <0.0001 | 263 (45.1%) | 57.88 | 99.69 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnell, L.K.S.; Luke, N.; Mathur, M.; Festa, R.A.; Haley, E.; Wang, J.; Jiang, Y.; Anderson, L.; Baunoch, D. Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs. Diagnostics 2023, 13, 2688. https://doi.org/10.3390/diagnostics13162688
Parnell LKS, Luke N, Mathur M, Festa RA, Haley E, Wang J, Jiang Y, Anderson L, Baunoch D. Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs. Diagnostics. 2023; 13(16):2688. https://doi.org/10.3390/diagnostics13162688
Chicago/Turabian StyleParnell, Laura K. S., Natalie Luke, Mohit Mathur, Richard A. Festa, Emery Haley, Jimin Wang, Yan Jiang, Lori Anderson, and David Baunoch. 2023. "Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs" Diagnostics 13, no. 16: 2688. https://doi.org/10.3390/diagnostics13162688
APA StyleParnell, L. K. S., Luke, N., Mathur, M., Festa, R. A., Haley, E., Wang, J., Jiang, Y., Anderson, L., & Baunoch, D. (2023). Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs. Diagnostics, 13(16), 2688. https://doi.org/10.3390/diagnostics13162688








