Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Clinical and Biological Features
3.2. Angiographic Features
3.3. Electrocardiographic and Echocardiographic Characteristics
3.4. The Risk of Thrombotic and Bleeding Events
3.5. Outcome and Prognosis
3.6. Multivariable Logistic Regression Analysis
4. Discussion
4.1. Study Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escaut, L.; Monsuez, J.J.; Chironi, G.; Merad, M.; Teicher, E.; Smadja, D.; Simon, A.; Vittecoq, D. Coronary artery disease in HIV infected patients. Intensive Care Med. 2003, 29, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G. Cardiovascular manifestations of HIV infection. J. R. Soc. Med. 2001, 94, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Boccara, F.; Lang, S.; Meuleman, C.; Ederhy, S.; Mary-Krause, M.; Costagliola, D.; Capeau, J.; Cohen, A. HIV and coronary heart disease: Time for a better understanding. J. Am. Coll. Cardiol. 2013, 61, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Bavinger, C.; Bendavid, E.; Niehaus, K.; Olshen, R.A.; Olkin, I.; Sundaram, V.; Wein, N.; Holodniy, M.; Hou, N.; Owens, D.K.; et al. Risk of Cardiovascular Disease from Antiretroviral Therapy for HIV: A Systematic Review. PLoS ONE 2013, 8, e59551. [Google Scholar] [CrossRef]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef]
- Paisible, A.L.; Chang, C.C.H.; So-Armah, K.A.; Butt, A.A.; Leaf, D.A.; Budoff, M.; Rimland, D.; Bedimo, R.; Goetz, M.B.; Rodriguez-Barradas, M.C.; et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir. Immune. Defic. Syndr. 2015, 68, 209–216. [Google Scholar] [CrossRef]
- Varriale, P.; Saravi, G.; Hernandez, E.; Carbon, F. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am. Heart J. 2004, 147, 55–59. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Giri, K.; Erickson, S.; MacGregor, J.S.; Younes, N.; Shergill, A.; Waters, D.D. Clinical Features of Acute Coronary Syndromes in Patients with Human Immunodeficiency Virus Infection. Circulation 2004, 109, 316–319. [Google Scholar] [CrossRef]
- Boccara, F.; Teiger, E.; Cohen, A.; Ederhy, S.; Janower, S.; Odi, G.; Di Angelantonio, E.; Barbarini, G.; Barbaro, G. Percutaneous coronary intervention in HIV infected patients: Immediate results and long term prognosis. Heart 2006, 92, 543–544. [Google Scholar] [CrossRef]
- Boccara, F.; Mary-Krause, M.; Potard, V.; Teiger, E.; Lang, S.; Hammoudi, N.; Chauvet, M.; Ederhy, S.; Dufour-Soulat, L.; Ancedy, Y.; et al. Hiv infection and long-term residual cardiovascular risk after acute coronary syndrome. J. Am. Heart Assoc. 2020, 9, e017578. [Google Scholar] [CrossRef]
- Boccara, F.; Mary-Krause, M.; Teiger, E.; Lang, S.; Lim, P.; Wahbi, K.; Beygui, F.; Milleron, O.; Steg, P.G.; Funck-Brentano, C.; et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: Characteristics and 1 year prognosis. Eur. Heart J. 2011, 32, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Cottin, Y.; Potard, V.; Mary-Krause, M.; Lang, S.; Teiger, E.; Collet, J.P.; Chauvet-Droit, M.; Ederhy, S.; Soulat-Dufour, L.; et al. Coronary Angiographic Features and Major Adverse Cardiac or Cerebrovascular Events in People Living with Human Immunodeficiency Virus Presenting with Acute Coronary Syndrome. Circ. Cardiovasc. Interv. 2022, 15, e011945. [Google Scholar] [CrossRef]
- Perelló, R.; Calvo, M.; Miró, O.; Castañeda, M.; Saubí, N.; Camón, S.; Foix, A.; Gatell, J.M.; Masotti, M.; Mallolas, J.; et al. Clinical presentation of acute coronary syndrome in HIV infected adults: A retrospective analysis of a prospectively collected cohort. Eur. J. Intern. Med. 2011, 22, 485–488. [Google Scholar] [CrossRef]
- Bajdechi, M.; Gurghean, A.; Bataila, V.; Scafa-udriste, A.; Radoi, R.; Oprea, A.C.; Marinescu, A.; Ion, S.; Chioncel, V.; Nicula, A.; et al. Cardiovascular Risk Factors, Angiographical Features and Short-Term Prognosis of Acute Coronary Syndrome in People Living with Human Immunodeficiency Virus: Results of a Retrospective Observational Multicentric Romanian Study. Diagnostics 2023, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Goetz, M.B.; Leaf, D.; Oursler, K.A.; et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef]
- Danel, C.; Moh, R.; Gabillard, D.; Badje, A.; Le Carrou, J.; Ouassa, T.; Ouattara, E.; Anzian, A.; Ntakpé, J.B.; Minga, A. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N. Engl. J. Med. 2015, 373, 808–822. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Babiker, A.G.; Gordin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; Llibre, J.M. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef]
- Siedner, M.J. START or SMART? Timing of Antiretroviral Therapy Initiation and Cardiovascular Risk for People with Human Immunodeficiency Virus Infection. Open Forum Infect. Dis. 2016, 3, ofw032. [Google Scholar] [CrossRef]
- El-Sadr, W.M.; Lundgren, J.; Neaton, J.D.; Gordin, F.; Abrams, D.; Arduino, R.C.; Babiker, A.; Burman, W.; Clumeck, N.; Cohen, C.J.; et al. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006, 355, 2283–2296. [Google Scholar] [CrossRef]
- Marso, S.P. Revascularization Approaches. In Chronic Coronary Artery Disease: A Companion to Braunwald’s Heart Disease; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367, Erratum in Eur. Heart J. 2021, 42, 1908. Erratum in Eur. Heart J. 2021, 42, 1925. Erratum in Eur. Heart J. 2021, 42, 2298. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Duncan, M.S.; Alcorn, C.W.; So-Armah, K.; Butt, A.A.; Goetz, M.B.; Tindle, H.A.; Sico, J.J.; Tracy, R.P.; Justice, A.C.; et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation 2018, 138, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, K.A.; Armon, C.; Buchacz, K.; Chmiel, J.S.; Buckner, K.; Tedaldi, E.M.; Wood, K.; Holmberg, S.D.; Brooks, J.T. Low CD4+ T Cell Count Is a Risk Factor for Cardiovascular Disease Events in the HIV Outpatient Study. Clin. Infect. Dis. 2010, 51, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Matetzky, S.; Domingo, M.; Kar, S.; Noc, M.; Shah, P.K.; Kaul, S.; Daar, E. Acute myocardial infarction in human immunodeficiency virus-infected patients. Arch. Intern. Med. 2003, 163, 457–460. [Google Scholar] [CrossRef]
- Lewden, C.; May, T.; Rosenthal, E.; Burty, C.; Bonnet, F.; Costagliola, D.; Jougla, E.; Semaille, C.; Morlat, P.; Salmon, D.; et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “mortalité 2000 and 2005” surveys (ANRS EN19 and mortavic). J. Acquir. Immune. Defic. Syndr. 2008, 48, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Peyracchia, M.; De Lio, G.; Montrucchio, C.; Omedè, P.; d’Ettore, G.; Calcagno, A.; Vullo, V.; Cerrato, E.; Pennacchi, M.; Sardella, G.; et al. Evaluation of coronary features of HIV patients presenting with ACS: The CUORE, a multicenter study. Atherosclerosis 2018, 274, 218–226. [Google Scholar] [CrossRef]
- Tarr, P.E.; Ledergerber, B.; Calmy, A.; Doco-Lecompte, T.; Marzel, A.; Weber, R.; Kaufmann, P.A.; Nkoulou, R.; Buechel, R.R.; Kovari, H.; et al. Subclinical coronary artery disease in Swiss HIV-positive and HIV-negative persons. Eur. Heart J. 2018, 39, 2147–2154. [Google Scholar] [CrossRef]
- Bajdechi, M.; Scafa-Udriste, A.; Ploscaru, V.; Calmac, L.; Bajeu, T.; Gurghean, A.; Rugina, S. Porcelain Aorta in a Young Person Living with HIV Who Presented with Angina. Diagnostics 2022, 12, 3147. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bezsonov, E.E.; Borisov, E.E.; Grechko, A.V.; Kartuesov, A.G.; Orekhov, A.N. Atherosclerosis in HIV Patients: What Do We Know so Far? Int. J. Mol. Sci. 2022, 23, 2504. [Google Scholar] [CrossRef]
- Triant, V.A. Cardiovascular disease and HIV infection. Curr. HIV/AIDS Rep. 2013, 10, 199–206. [Google Scholar] [CrossRef]
- Triant, V.A.; Regan, S.; Lee, H.; Sax, P.E.; Meigs, J.B.; Grinspoon, S.K. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J. Acquir. Immune. Defic. Syndr. 2010, 55, 615–619. [Google Scholar] [CrossRef]
- Ho, J.E.; Scherzer, R.; Hecht, F.M.; Maka, K.; Selby, V.; Martin, J.N.; Ganz, P.; Deeks, S.G.; Hsue, P.Y. The association of CD4 + T-cell counts and cardiovascular risk in treated HIV disease. AIDS 2012, 26, 1115–1120. [Google Scholar] [CrossRef]
- Duarte, H.; Matta, J.R.; Muldoon, N.; Masur, H.; Hadigan, C.; Gharib, A.M. Non-calcified coronary plaque volume inversely related to CD4 + T-cell count in HIV infection. Antivir. Ther. 2012, 17, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Van Lelyveld, S.F.L.; Gras, L.; Kesselring, A.; Zhang, S.; De Wolf, F.; Wensing, A.M.J.; Hoepelman, A.I.M. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS 2012, 26, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Seecheran, R.; Kawall, T.; Seecheran, V.; Persad, S.; Kanhai, J.; Jagdeo, C.L.; Giddings, S.; Raza, S.; Seecheran, N.A. Chronic total occlusion of the left main coronary artery in an hiv-infected patient. Int. Med. Case Rep. J. 2020, 13, 623–629. [Google Scholar] [CrossRef]
- Bajdechi, M.; Mihai, C.; Scafa-Udriste, A.; Cherry, A.; Zamfir, D.; Dumitru, I.; Cernat, R.; Cernat, R. Severe coronary artery disease in a person living with hiv. Medicina 2021, 57, 595. [Google Scholar] [CrossRef]
- Belgrave, K.; Shaikh, K.; Budoff, M.J. Risk of peripheral artery disease in human immunodeficiency virus infected individuals. Ann. Transl. Med. 2018, 6, S46. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.F.; Poretsky, L.; Seres, D.S.; Zumoff, B. Hyporeninemic hypoaldosteronism associated with acquired immune deficiency syndrome. Am. J. Med. 1987, 82, 1035–1038. [Google Scholar] [CrossRef]
- Choi, M.J.; Fernandez, P.C.; Patnaik, A.; Coupaye-Gerard, B.; D’Andrea, D.; Szerlip, H.; Kleyman, T.R. Trimethoprim-Induced Hyperkalemia in a Patient with, A.I.D.S. N. Engl. J. Med. 1993, 328, 703–706. [Google Scholar] [CrossRef]
- Odeniyi, I.; Fasanmade, O.; Ajala, M.; Ohwovoriole, A. CD4 count as a predictor of adrenocortical insufficiency in persons with human immunodeficiency virus infection: How useful? Indian J. Endocrinol. Metab. 2013, 17, 1012–1017. [Google Scholar] [CrossRef]
- Mehta, N.J.; Khan, I.A. HIV-associated coronary artery disease. Angiology 2003, 54, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.L.; Hurley, L.B.; Prasad, A.; Zaroff, J.; Klein, D.B.; Horberg, M.A.; Go, A.S.; DeLorenze, G.N.; Quesenberry, C.P., Jr.; Sidney, S.; et al. Recurrence after hospitalization for acute coronary syndrome among HIV-infected and HIV-uninfected individuals. HIV Med. 2019, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
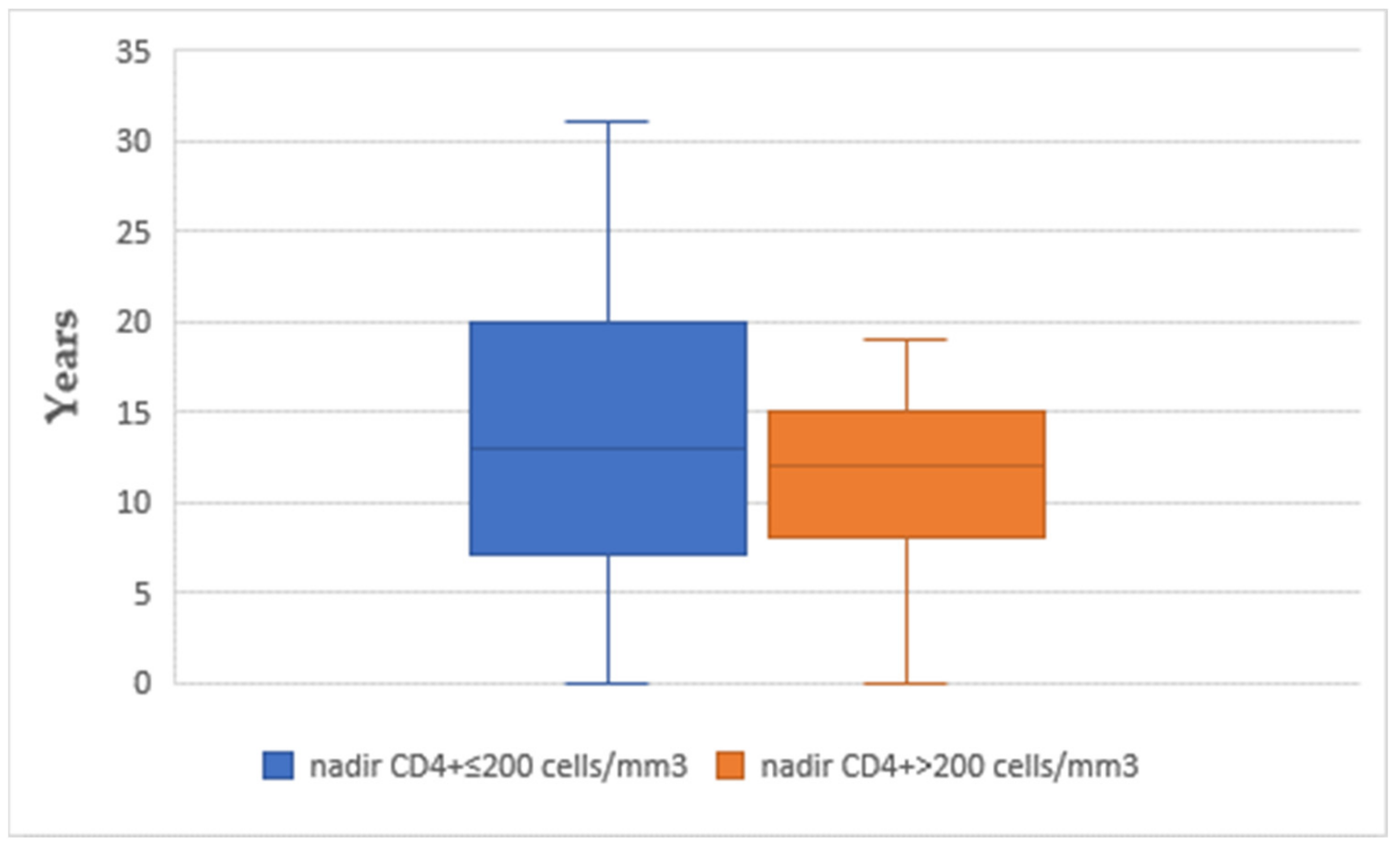


| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| Males, n% | 25 (92.6%) | 18 (94.7%) | 0.774 |
| Detectable HIV viral load, n% | 4 (13.79%) | 6 (35.29%) | 0.179 |
| Smoking, n% | 16 (61.5%) | 12 (63.2%) | 0.911 |
| Hypertension, n% | 17 (65.4%) | 11 (57.9%) | 0.608 |
| Dyslipidemia, n% | 20 (76.9%) | 15 (78.9%) | 0.871 |
| Treated dyslipidemia, n% | 12 (60%) | 10 (66.6%) | 0.847 |
| Diabetes mellitus, n% | 4 (15.4%) | 5 (26.3%) | 0.365 |
| Obesity, n% | 1 (3.8%) | 3 (15.8%) | 0.164 |
| CAD history, n% | 8 (29.6%) | 6 (31.6%) | 0.887 |
| Atypical angina, n% | 7 (28%) | 3 (16.7%) | 0.480 |
| Peripheral artery disease, n% | 9 (36%) | 3 (16%) | 0.191 |
| Chronic kidney disease, n% | 6 (23.1%) | 2 (10.5%) | 0.435 |
| Anemia, n% | 9 (33.3%) | 4 (21.1%) | 0.510 |
| Macrocytosis, n% | 9 (33.3%) | 10 (52.6%) | 0.233 |
| Creatinine clearance < 60 mL/min/m2 (CKD-EPI), n% | 6 (22.2%) | 1 (5.6%) | 0.215 |
| Hyponatremia, n% | 4 (14.8%) | 3 (15.8%) | 0.927 |
| Hypernatremia, n% | 1 (3.7%) | 0 | 1 |
| Hypokalemia, n% | 3 (11.1%) | 3 (15.8%) | 0.642 |
| Hyperkalemia, n% | 4 (22.2%) | 0 (0%) | 0.021 |
| Elevated myocardial necrosis markers, n% | 24 (88.9%) | 15 (78.9%) | 0.424 |
| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| STEMI, n% | 11 (40.7%) | 8 (42.1%) | 0.914 |
| NSTEMI, n% | 6 (22.3%) | 5 (26.3%) | |
| Unstable angina, n% | 10 (37%) | 6 (31.6%) | |
| Killip I class, n% | 13 (76.5%) | 12 (92.3%) | 0.464 |
| Killip II class, n% | 0 (0%) | 0 (0%) | |
| Killip III class, n% | 1 (5.9%) | 0 (0%) | |
| Killip IV class, n% | 3 (17.6%) | 1 (7.7%) |
| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| Culprit lesion LAD, n% | 10 (43.5%) | 9 (56.2%) | 0.504 |
| Culprit lesion LCx, n% | 3 (13%) | 4 (25%) | |
| Culprit lesion RCA, n% | 5 (21.7%) | 2 (12.5%) | |
| Culprit lesion LM, n% | 2 (8.7%) | 0 (0%) | |
| Non-culprit lesion present, n% | 3 (13%) | 1 (6.2%) | |
| Associated lesion LAD, n% | 9 (37.5%) | 3 (20%) | 0.215 |
| Associated lesion LCx, n% | 6 (25%) | 4 (26.7%) | 1 |
| Associated lesion RCA, n% | 9 (37.5%) | 7 (49.7%) | 0.740 |
| Associated lesion LM, n% | 4 (16.7%) | 1 (6.7%) | 0.631 |
| Other associated lesions, n% | 5 (20.8%) | 4 (26.7%) | 0.711 |
| Single vessel disease, n% | 10 (45.45%) | 5 (33.33%) | 0.704 |
| Two-vessel disease, n% | 5 (27.73%) | 5 (33.33%) | |
| Three-vessel disease, n% | 7 (31.18%) | 5 (33.33%) | |
| SYNTAX I score ≥ 23 p, n% | 9 (40.9%) | 0 (0%) | 0.013 |
| Indication for coronary artery bypass graft surgery, n% | 4 (16.7%) | 1 (6.2%) | 0.205 |
| Performed coronary artery bypass graft surgery, n% | 3 (12.5%) | 0 (0%) | |
| Intrastent restenosis/venous graft stenosis, n% | 4 (15.4%) | 3 (16.7%) | 1 |
| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| Ischaemic changes on ECG, n% | 18 (69.2%) | 15 (83.3%) | 0.480 |
| Supraventricular arrhythmia, n% | 5 (19.2%) | 2 (10.5%) | 0.681 |
| Ventricular arrhythmia, n% | 5 (19.2%) | 3 (15.8%) | 1 |
| LVEF ≤ 40%, n% | 12 (44%) | 7 (36.8%) | 0.763 |
| LVEF 41–49%, n% | 5 (18.5%) | 2 (10.5%) | 0.682 |
| LVEF ≥ 50%, n% | 9 (34.6%) | 8 (47.1%) | 0.528 |
| LV diastolic dysfunction, n% | 11 (45.8%) | 5 (29.4%) | 0.344 |
| Significant valvulopathy, n% | 7 (29.2%) | 3 (16.7%) | 0.473 |
| LV aneurysm, n% | 2 (8.3%) | 2 (11.1%) | 1 |
| Pericardial effusion, n% | 1 (4.2%) | 1 (5.6%) | 1 |
| Improbable PH, n% | 20 (83.3%) | 16 (94.1%) | 0.525 |
| Intermediate probability of PH, n% | 3 (12.5%) | 1 (5.9%) | |
| High probability of PH, n% | 1 (4.2%) | 0 (0%) |
| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| High DAPT score (≥2 points), n% | 5 (29.41%) | 3 (20%) | 0.539 |
| PRECISE-DAPT score ≥ 25 points, n% | 6 (30%) | 1 (5.88%) | 0.061 |
| CD4+ Nadir ≤ 200 Cells/mm3 | CD4+ Nadir > 200 Cells/mm3 | p Value | |
|---|---|---|---|
| In-hospital mortality, n% | 4 (14.81%) | 1 (5.26%) | 0.305 |
| Recurrent ACS at 30 days, n% | 2 (8.69%) | 1 (5.55%) | 0.701 |
| HF requiring hospitalization at 30 days, n% | 2 (8.69%) | 1 (5.55%) | 0.701 |
| Cardiovascular death at 30 days, n% | 1 (4.34%) | 0 (0%) | 1 |
| Stroke at 30 days, n% | 1 (4.34%) | 0 (0%) | 1 |
| Cumulative MACCE at 30 days, n% | 6 (26.08%) | 2 (11.11%) | 0.229 |
| Recurrent ACS at 360 days, n% | 2 (11.8%) | 2 (13.3%) | 0.893 |
| HF requiring hospitalization at 360 days, n% | 0 (0%) | 0 (0%) | - |
| Cardiovascular death at 360 days, n% | 2 (11.8%) | 2 (13.3%) | 0.893 |
| Stroke at 360 days, n% | 0 (0%) | 0 (0%) | - |
| Cumulative MACCE at 360 days, n% | 4 (23.52%) | 4 (26.66%) | 0.837 |
| Variable | CD4+ Nadir (Cells/mm3) | Number | Mean Value | Standard Deviation | p Value |
|---|---|---|---|---|---|
| Age (years) | ≤200 | 27 | 50.00 | 12.676 | 0.849 |
| >200 | 19 | 49.32 | 10.822 | ||
| CD4+ at the onset of the ACS (cells/mm3) | ≤200 | 27 | 476.74 | 242.531 | 0.001 |
| >200 | 19 | 805.74 | 384.344 | ||
| Hemoglobin (g/dL) | ≤200 | 27 | 13.9000 | 2.02123 | 0.254 |
| >200 | 18 | 14.5600 | 1.62463 | ||
| MCV (fL) | ≤200 | 27 | 94.6481 | 11.25920 | 0.454 |
| >200 | 18 | 97.0150 | 8.63796 | ||
| MCHC (g/dL) | ≤200 | 27 | 32.1185 | 3.47198 | 0.091 |
| >200 | 18 | 33.6256 | 1.51113 | ||
| Leucocytes (/mm3) | ≤200 | 27 | 12,574.44 | 10,366.140 | 0.490 |
| >200 | 18 | 10,833.33 | 2497.156 | ||
| Thrombocytes (/mm3) | ≤200 | 27 | 226,037.04 | 114,442.959 | 0.603 |
| >200 | 18 | 246,877.78 | 152,396.339 | ||
| Serum creatinine (mg/dL) | ≤200 | 27 | 1.4207 | 1.22942 | 0.160 |
| >200 | 18 | 0.9767 | 0.56335 | ||
| Creatinine clearance (CKD-EPI) (mL/min/m2) | ≤200 | 27 | 77.796 | 32.6624 | 0.059 |
| >200 | 18 | 97.083 | 32.5647 | ||
| Natremia (mmol/L) | ≤200 | 25 | 139.996 | 3.4388 | 0.177 |
| >200 | 18 | 138.500 | 3.6340 | ||
| Kalemia (mmol/L) | ≤200 | 25 | 4.1048 | 0.43413 | 0.153 |
| >200 | 18 | 4.3389 | 0.62269 | ||
| Total cholesterol (mg/dL) | ≤200 | 26 | 181.35 | 47.935 | 0.413 |
| >200 | 18 | 168.67 | 53.005 | ||
| Triglycerides (mg/dL) | ≤200 | 24 | 202.04 | 135.867 | 0.601 |
| >200 | 17 | 0.18 | 123.408 | ||
| Maximum value of CK (U/L) | ≤200 | 21 | 702.71 | 1153.950 | 0.835 |
| >200 | 13 | 779.31 | 797.249 | ||
| Maximum value of CKMB (U/L) | ≤200 | 26 | 118.81 | 126.024 | 0.361 |
| >200 | 16 | 86.44 | 76.772 | ||
| LVEF (%) | ≤200 | 26 | 41.35 | 13.151 | 0.580 |
| >200 | 17 | 43.59 | 12.435 | ||
| SYNTAX I score (points) | ≤200 | 22 | 17.068 | 13.3865 | 0.022 |
| >200 | 13 | 7.731 | 5.0275 | ||
| SYNTAX II score PCI (points) | ≤200 | 22 | 31.432 | 20.6306 | 0.046 |
| >200 | 13 | 18.977 | 7.9748 | ||
| 4-year mortality after PCI (%) | ≤200 | 22 | 18.618 | 25.0906 | 0.036 |
| >200 | 13 | 3.254 | 2.0915 | ||
| SYNTAX II score after CABG (points) | ≤200 | 22 | 19.818 | 17.2018 | 0.194 |
| >200 | 13 | 13.046 | 8.2357 | ||
| 4-year mortality after CABG (%) | ≤200 | 22 | 8.032 | 16.4931 | 0.194 |
| >200 | 13 | 2.015 | 1.3915 | ||
| EuroScore II (%) | ≤200 | 22 | 3.8295454 | 4.905126062 | 0.056 |
| >200 | 13 | 1.1007692 | 0.7843836384 | ||
| GRACE score (points) | ≤200 | 15 | 84.67 | 36.203 | 0.492 |
| >200 | 9 | 74.22 | 34.003 | ||
| GRACE death probability (admission–6 months) (%) | ≤200 | 15 | 4.440 | 6.0655 | 0.445 |
| >200 | 9 | 2.756 | 2.8386 | ||
| TIMI risk score–mortality at 30 days after STEMI (%) | ≤200 | 12 | 9.708 | 9.9378 | 0.519 |
| >200 | 8 | 7.063 | 6.6565 | ||
| PRECISE-DAPT score | ≤200 | 22 | 18.00 | 13.956 | 0.037 |
| >200 | 15 | 9.73 | 5.548 | ||
| TIMI risk of major and minor bleeding at 12 months | ≤200 | 22 | 1.6536 | 1.51422 | 0.016 |
| >200 | 15 | 0.6467 | 0.32264 | ||
| TIMI risk score of major bleeding at 12 months | ≤200 | 22 | 0.8659 | 0.73168 | 0.013 |
| >200 | 15 | 0.3573 | 0.17119 |
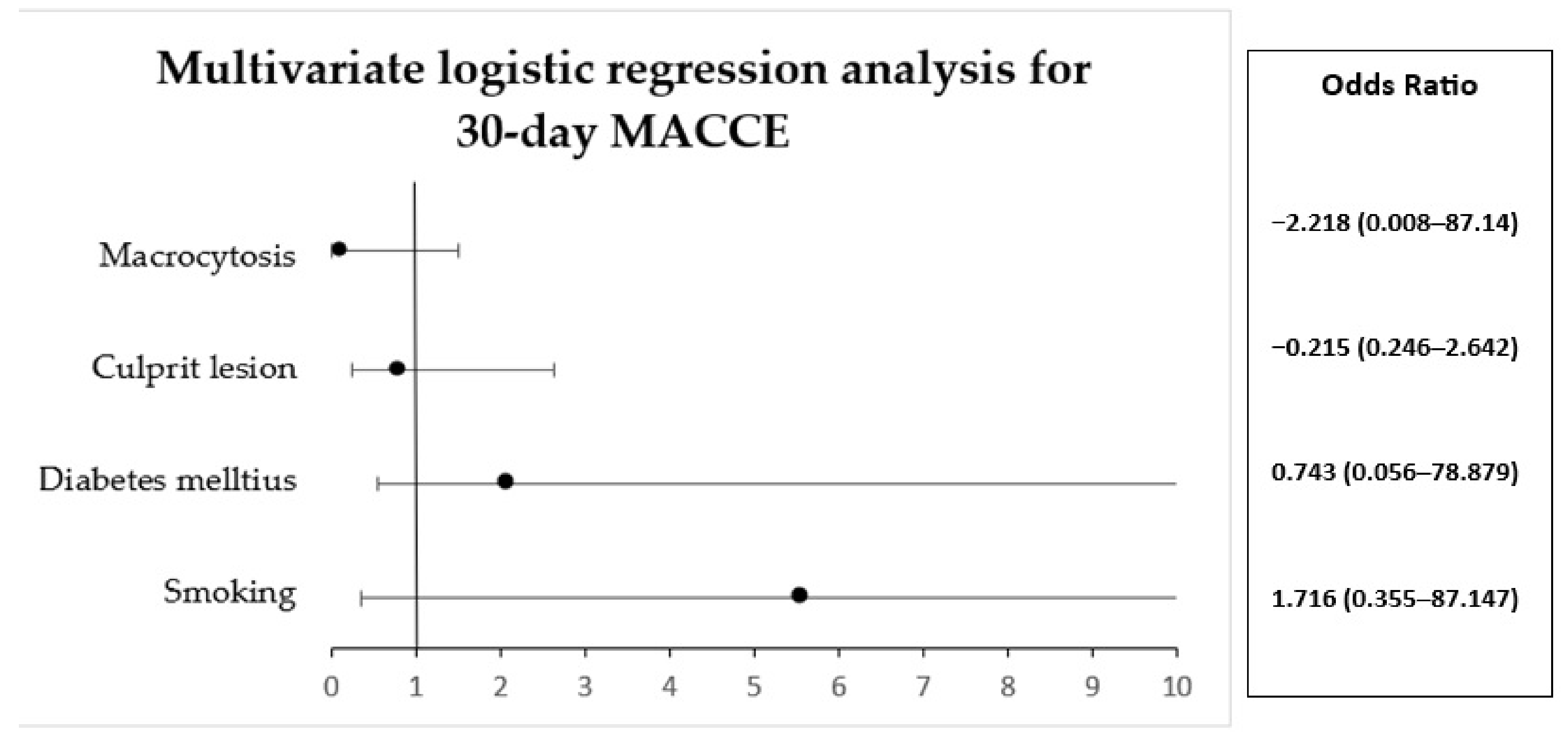
| Parameter | B | S.E. | Wald | Df | Val. P | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Smoking | 1.716 | 1.404 | 1.493 | 1 | 0.222 | 5.56 | 0.355 | 87.147 |
| Diabetes mellitus | 0.743 | 1.849 | 0.161 | 1 | 0.688 | 2.102 | 0.056 | 78.879 |
| Culprit lesion | −0.215 | 0.605 | 0.126 | 1 | 0.723 | 0.807 | 0.246 | 2.642 |
| Macrocytosis | −2.218 | 1.341 | 2.736 | 1 | 0.098 | 0.109 | 0.008 | 1.507 |
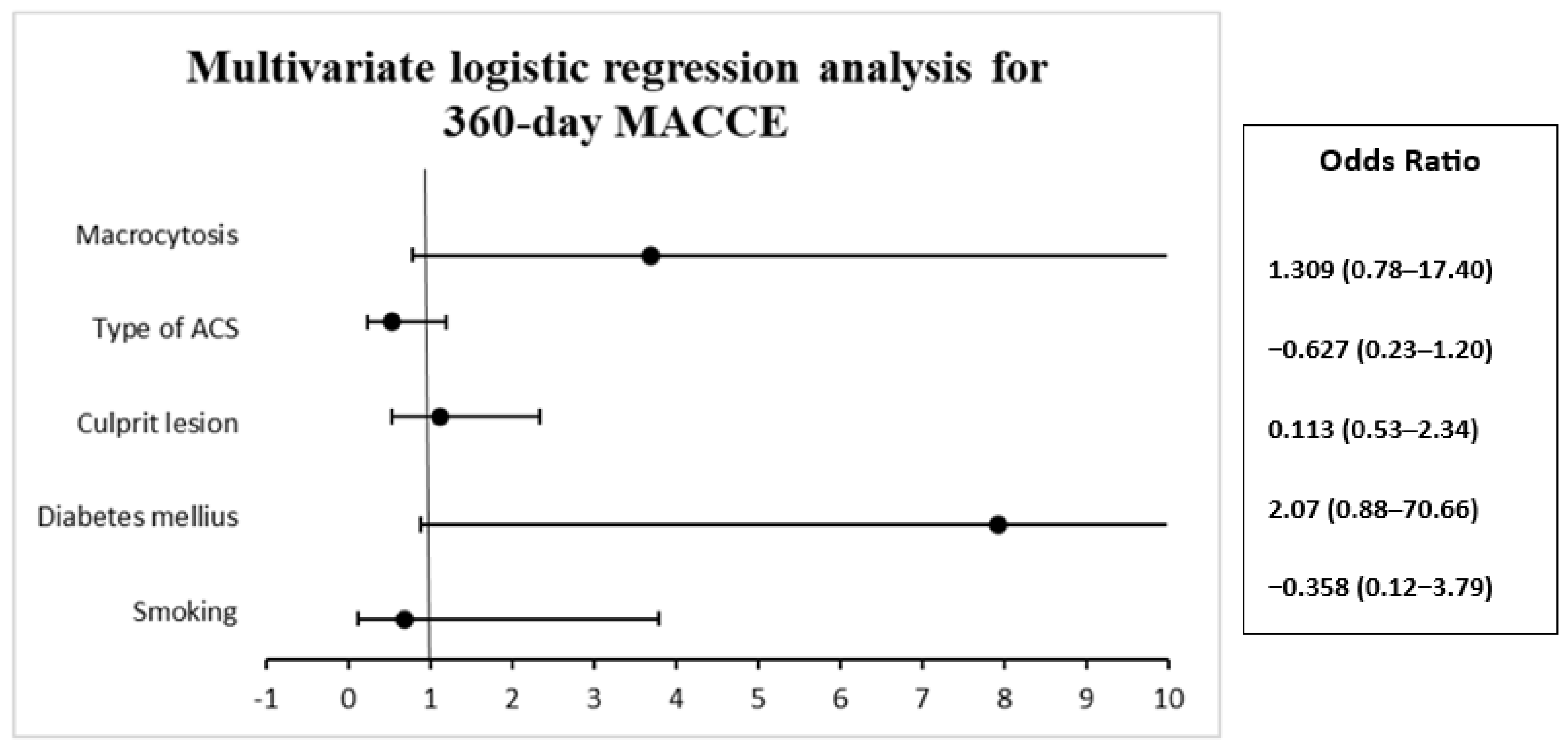
| Parameter | B | S.E. | Wald | Df | Val. P | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Smoking | −0.358 | 0.864 | 0.173 | 1 | 0.678 | 0.699 | 0.129 | 3.790 |
| Diabetes mellitus | 2.070 | 1.117 | 3.435 | 1 | 0.064 | 7.921 | 0.888 | 70.661 |
| Culprit lesion | 0.113 | 0.378 | 0.089 | 1 | 0.765 | 1.119 | 0.534 | 2.347 |
| Type of ACS | −0.627 | 0.414 | 2.296 | 1 | 0.130 | 0.534 | 0.238 | 1.202 |
| Macrocytosis | 1.309 | 0.790 | 2.744 | 1 | 0.098 | 3.701 | 0.787 | 17.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajdechi, M.; Gurghean, A.; Bataila, V.; Scafa-Udriște, A.; Bajdechi, G.-E.; Radoi, R.; Oprea, A.C.; Chioncel, V.; Mateescu, I.; Zekra, L.; et al. Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome. Diagnostics 2023, 13, 2682. https://doi.org/10.3390/diagnostics13162682
Bajdechi M, Gurghean A, Bataila V, Scafa-Udriște A, Bajdechi G-E, Radoi R, Oprea AC, Chioncel V, Mateescu I, Zekra L, et al. Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome. Diagnostics. 2023; 13(16):2682. https://doi.org/10.3390/diagnostics13162682
Chicago/Turabian StyleBajdechi, Mircea, Adriana Gurghean, Vlad Bataila, Alexandru Scafa-Udriște, Georgiana-Elena Bajdechi, Roxana Radoi, Anca Cristiana Oprea, Valentin Chioncel, Iuliana Mateescu, Lucia Zekra, and et al. 2023. "Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome" Diagnostics 13, no. 16: 2682. https://doi.org/10.3390/diagnostics13162682
APA StyleBajdechi, M., Gurghean, A., Bataila, V., Scafa-Udriște, A., Bajdechi, G.-E., Radoi, R., Oprea, A. C., Chioncel, V., Mateescu, I., Zekra, L., Cernat, R., Dumitru, I. M., & Rugina, S. (2023). Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome. Diagnostics, 13(16), 2682. https://doi.org/10.3390/diagnostics13162682







