Recent Advances of Proteomics in Management of Acute Kidney Injury
Abstract
1. Introduction
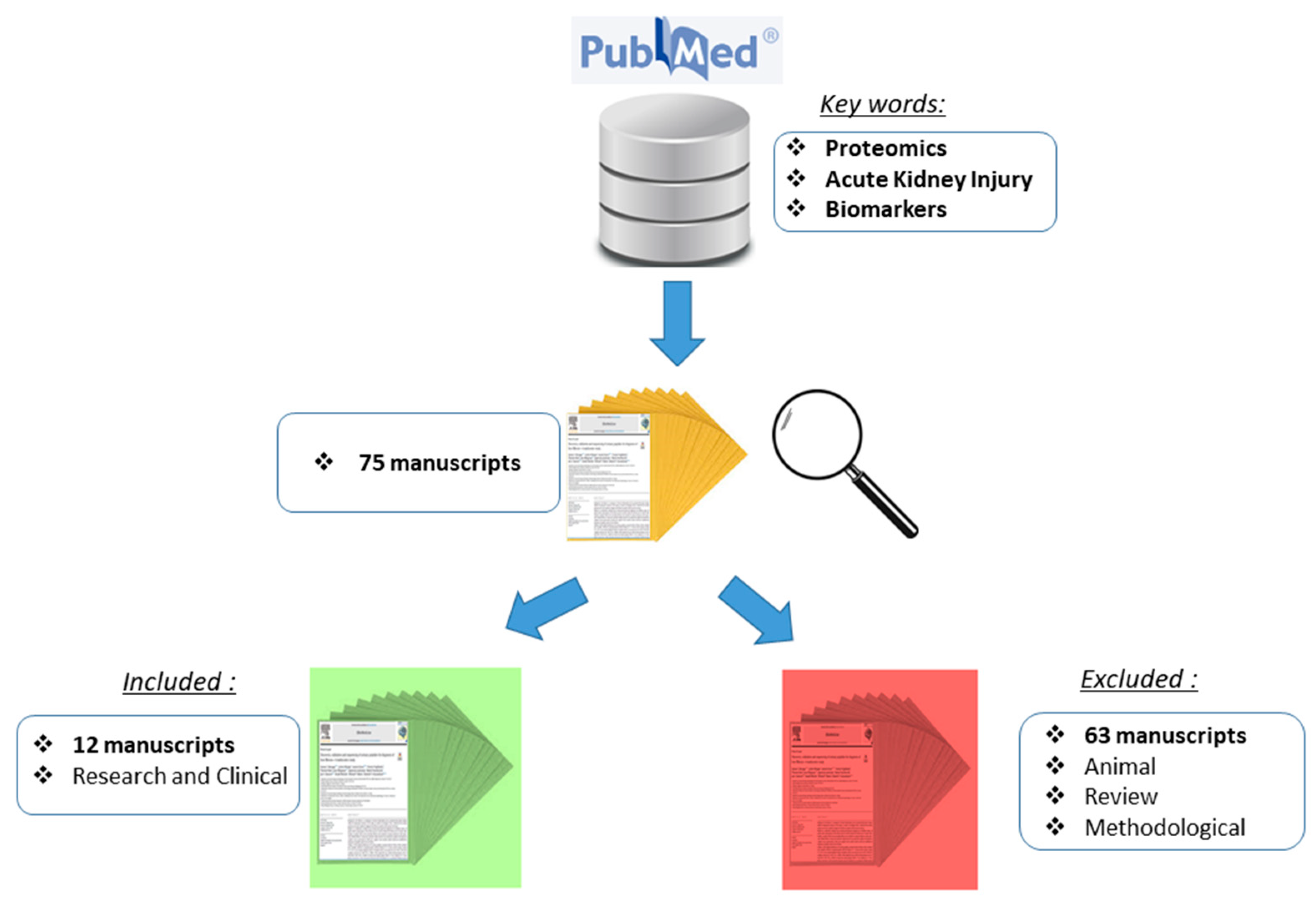
2. Materials and Methods
Study Design and Screening Procedure
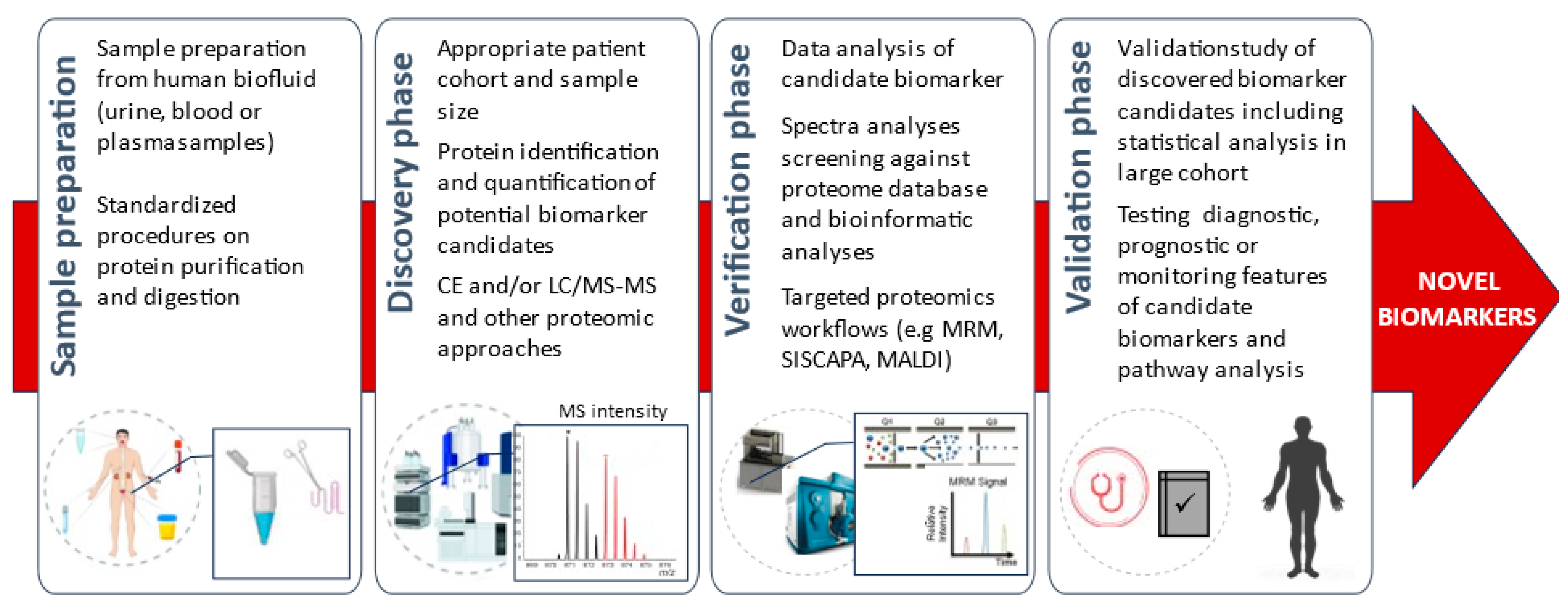
3. Results
3.1. Acute Kidney Injury (AKI)—Related Protein Biomarkers
3.2. AKI—Related Protein Biomarker Types and Their Association
3.3. Diagnostic AKI Biomarkers
3.4. Prognostic AKI Biomarkers
3.5. Monitoring AKI Biomarkers
4. Conclusions
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef] [PubMed]
- Lewington, A.J.; Cerdá, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef]
- Chadwick, N.M.; Perman, M.L.; Leavai, F.; Kaspar, A. Acute Kidney Injury: Incidence, aetiology, management and outcome measures of a Samoan case series. Ann. Med. Surg. 2022, 75, 103362. [Google Scholar] [CrossRef]
- Sawhney, S.; Bell, S.; Black, C.; Christiansen, C.F.; Heide-Jørgensen, U.; Jensen, S.K.; Ronksley, P.E.; Tan, Z.; Tonelli, M.; Walker, H.; et al. Harmonization of epidemiology of acute kidney injury and acute kidney disease produces comparable findings across four geographic populations. Kidney Int. 2022, 101, 1271–1281. [Google Scholar] [CrossRef]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar]
- Brady, H.R.; Singer, G.G. Acute renal failure. Lancet 1995, 346, 1533–1540. [Google Scholar] [CrossRef]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef]
- Pejchinovski, M.; Magalhães, P.; Metzeger, J. Editorial: Clinical application of proteomics in kidney diseases. Front. Med. 2022, 9, 965083. [Google Scholar] [CrossRef]
- Kister, T.S.; Schmidt, M.; Heuft, L.; Federbusch, M.; Haase, M.; Kaiser, T. Laboratory Diagnostic of Acute Kidney Injury and Its Progression: Risk of Underdiagnosis in Female and Elderly Patients. J. Clin. Med. 2023, 12, 1092. [Google Scholar] [CrossRef]
- Siwy, J.; Mischak, H.; Zürbig, P. Proteomics and personalized medicine: A focus on kidney disease. Expert Rev. Proteom. 2019, 16, 773–782. [Google Scholar] [CrossRef]
- Frantzi, M.; Latosinska, A.; Mischak, H. Proteomics in Drug Development: The Dawn of a New Era? Proteom. Clin. Appl. 2019, 13, e1800087. [Google Scholar] [CrossRef]
- O’Connell, S.P.; Frantzi, M.; Latosinska, A.; Webb, M.; Mullen, W.; Pejchinovski, M.; Salji, M.; Mischak, H.; Cooper, C.S.; Clark, J.; et al. On Behalf of the Movember Gap Urine Biomarker Consortium. A Model to Detect Significant Prostate Cancer Integrating Urinary Peptide and Extracellular Vesicle RNA Data. Cancers 2022, 14, 1995. [Google Scholar] [CrossRef]
- Belczacka, I.; Pejchinovski, M.; Krochmal, M.; Magalhães, P.; Frantzi, M.; Mullen, W.; Vlahou, A.; Mischak, H.; Jankowski, V. Urinary Glycopeptide Analysis for the Investigation of Novel Biomarkers. Proteom. Clin Appl. 2019, 13, e1800111. [Google Scholar] [CrossRef]
- Voigtländer, T.; Metzger, J.; Husi, H.; Kirstein, M.M.; Pejchinovski, M.; Latosinska, A.; Frantzi, M.; Mullen, W.; Book, T.; Mischak, H.; et al. Bile and urine peptide marker profiles: Access keys to molecular pathways and biological processes in cholangiocarcinoma. J. Biomed. Sci. 2020, 27, 13. [Google Scholar] [CrossRef]
- Frantzi, M.; van Kessel, K.E.; Zwarthoff, E.C.; Marquez, M.; Rava, M.; Malats, N.; Merseburger, A.S.; Katafigiotis, I.; Stravodimos, K.; Mullen, W.; et al. Development and Validation of Urine-based Peptide Biomarker Panels for Detecting Bladder Cancer in a Multi-center Study. Clin. Cancer Res. 2016, 22, 4077–4086. [Google Scholar] [CrossRef]
- Carrick, E.; Vanmassenhove, J.; Glorieux, G.; Metzger, J.; Dakna, M.; Pejchinovski, M.; Jankowski, V.; Mansoorian, B.; Husi, H.; Mullen, W.; et al. Development of a MALDI MS-based platform for early detection of acute kidney injury. Proteom. Clin. Appl. 2016, 10, 732–742. [Google Scholar] [CrossRef]
- Liu, Y.; Pejchinovski, M.; Wang, X.; Fu, X.; Castelletti, D.; Watnick, T.J.; Arcaro, A.; Siwy, J.; Mullen, W.; Mischak, H.; et al. Dual mTOR/PI3K inhibition limits PI3K-dependent pathways activated upon mTOR inhibition in autosomal dominant polycystic kidney disease. Sci. Rep. 2018, 8, 5584. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Haase, M.; Albert, A.; Zapf, A.; Braun-Dullaeus, R.C.; Haase-Fielitz, A. Biomarker-Guided Risk Assessment for Acute Kidney Injury: Time for Clinical Implementation? Ann. Lab. Med. 2021, 41, 1–15. [Google Scholar] [CrossRef]
- Pejchinovski, M.; Mischak, H. Clinical Proteomics in Kidney Disease: From Discovery to Clinical Application. Prilozi 2017, 38, 39–54. [Google Scholar] [CrossRef][Green Version]
- Cha, S.W.; Shin, I.S.; Kim, D.G.; Kim, S.H.; Lee, J.Y.; Kim, J.S.; Yang, J.W.; Han, B.G.; Choi, S.O. Effectiveness of serum beta-2 microglobulin as a tool for evaluating donor kidney status for transplantation. Sci. Rep. 2020, 10, 8109. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Latoch, E.; Konończuk, K.; Taranta-Janusz, K.; Muszyńska-Rosłan, K.; Sawicka, M.; Wasilewska, A.; Krawczuk-Rybak, M. Urinary Beta-2-Microglobulin and Late Nephrotoxicity in Childhood Cancer Survivors. J. Clin. Med. 2021, 10, 5279. [Google Scholar] [CrossRef]
- Puthiyottil, D.; Priyamvada, P.S.; Kumar, M.N.; Chellappan, A.; Zachariah, B.; Parameswaran, S. Role of Urinary Beta 2 Microglobulin and Kidney Injury Molecule-1 in Predicting Kidney Function at One Year Following Acute Kidney Injury. Int. J. Nephrol. Renov. Dis. 2021, ume 14, 225–234. [Google Scholar] [CrossRef]
- Phanish, M.K.; Chapman, A.N.; Yates, S.; Price, R.; Hendry, B.M.; Roderick, P.J.; Dockrell, M.E.C. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients with Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int. Rep. 2021, 6, 1355–1367. [Google Scholar] [CrossRef]
- Sweetman, D.U.; Onwuneme, C.; Watson, W.R.; O’Neill, A.; Murphy, J.F.; Molloy, E.J. Renal function and novel urinary biomarkers in infants with neonatal encephalopathy. Acta Paediatr. 2016, 105, e513–e519. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; McCarthy, C.P.; Shrestha, S.; Gaggin, H.K.; Mukai, R.; Magaret, C.A.; Rhyne, R.F.; Januzzi, J.L., Jr. A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin. Cardiol. 2019, 42, 292–298. [Google Scholar] [CrossRef]
- Zhu, H.; Chu, W.; Han, S.; Gao, B.; Wang, X. Urinary proteomics investigations into contrast-induced acute kidney injury. PLoS ONE. 2021, 16, e0258736. [Google Scholar] [CrossRef]
- Pirooznia, N.; Hasannia, S.; Arab, S.S.; Lotfi, A.S.; Ghanei, M.; Shali, A. The design of a new truncated and engineered alpha1-antitrypsin based on theoretical studies: An antiprotease therapeutics for pulmonary diseases. Theor. Biol. Med. Model. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Köhnlein, T.; Welte, T. The discovery of α1-antitrypsin and its role in health and disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Karnaukhova, E.; Krupnikova, S.S.; Rajabi, M.; Alayash, A.I. Heme binding to human alpha-1 proteinase inhibitor. Biochim. Biophys. Acta. 2012, 1820, 2020–2029. [Google Scholar] [CrossRef]
- Maicas, N.; van der Vlag, J.; Bublitz, J.; Florquin, S.; Bakker-van Bebber, M.; Dinarello, C.A.; Verweij, V.; Masereeuw, R.; Joosten, L.A.; Hilbrands, L.B. Human Alpha-1-Antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia-reperfusion injury. PLoS ONE 2017, 12, e0168981. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; Tumpara, S.; Wiese, M.; Wrenger, S.; Vijayan, V.; Gueler, F.; Chen, R.; Madyaningrana, K.; Mahadeva, R.; Welte, T.; et al. Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J. Leukoc. Biol. 2017, 102, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C. Expanding the clinical indications for α(1)-antitrypsin therapy. Mol. Med. 2012, 18, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyam, D.; Virtala, R.; Pawłowski, K.; Clausen, I.G.; Warkentin, S.; Stevens, T.; Janciauskiene, S. TNF-alpha-induced self expression in human lung endothelial cells is inhibited by native and oxidized alpha1-antitrypsin. Int. J. Biochem. Cell Biol. 2008, 40, 258–271. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.; Frostad, K.B. Rapid renal alpha-1 antitrypsin gene induction in experimental and clinical acute kidney injury. PLoS ONE 2014, 9, e98380. [Google Scholar] [CrossRef]
- Jeong, K.H.; Lim, J.H.; Lee, K.H.; Kim, M.J.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, C.D. Protective Effect of Alpha 1-Antitrypsin on Renal Ischemia-Reperfusion Injury. Transplant. Proc. 2019, 51, 2814–2822. [Google Scholar] [CrossRef]
- Tumpara, S.; Ballmaier, M.; Wrenger, S.; König, M.; Lehmann, M.; Lichtinghagen, R.; Martinez-Delgado, B.; Korenbaum, E.; DeLuca, D.; Jedicke, N.; et al. Polymerization of misfolded Z alpha-1 antitrypsin protein lowers CX3CR1 expression in human PBMCs. Elife 2021, 10, e64881. [Google Scholar] [CrossRef]
- Est, C.B.; Murphy, R.M. Retinol binding protein IV purified from Escherichia coli using intein-mediated cleavage as a suitable replacement for serum sources. Protein Expr. Purif. 2020, 167, 105542. [Google Scholar] [CrossRef]
- Raghu, P.; Sivakumar, B. Interactions amongst plasma retinol-binding protein, transthyretin and their ligands: Implications in vitamin A homeostasis and transthyretin amyloidosis. Biochim. Biophys. Acta. 2004, 1703, 1–9. [Google Scholar] [CrossRef]
- Bellovino, D.; Morimoto, T.; Mengheri, E.; Perozzi, G.; Garaguso, I.; Nobili, F.; Gaetani, S. Unique biochemical nature of carp retinol-binding protein. N-linked glycosylation and uncleavable NH2-terminal signal peptide. J. Biol. Chem. 2001, 276, 13949–13956. [Google Scholar] [CrossRef]
- He, T.; Pejchinovski, M.; Mullen, W.; Beige, J.; Mischak, H.; Jankowski, V. Peptides in Plasma, Urine, and Dialysate: Toward Unravelling Renal Peptide Handling. Proteom. Clin. Appl. 2021, 15, e2000029. [Google Scholar] [CrossRef]
- Hall, A.M.; Vilasi, A.; Garcia-Perez, I.; Lapsley, M.; Alston, C.L.; Pitceathly, R.D.; McFarland, R.; Schaefer, A.M.; Turnbull, D.M.; Beaumont, N.J.; et al. The urinary proteome and metabonome differ from normal in adults with mitochondrial disease. Kidney Int. 2015, 87, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.A.; Moreira, S.R.; Gomez, L.; Goulart, A.; Lotufo, P.A.; Benseñor, I.; Titan, S. Urinary Retinol-Binding Protein: Relationship to Renal Function and Cardiovascular Risk Factors in Chronic Kidney Disease. PLoS ONE 2016, 11, e0162782. [Google Scholar] [CrossRef]
- Tiscia, G.L.; Margaglione, M. Human Fibrinogen: Molecular and Genetic Aspects of Congenital Disorders. Int. J. Mol. Sci. 2018, 19, 1597. [Google Scholar] [CrossRef]
- Sörensen-Zender, I.; Rong, S.; Susnik, N.; Lange, J.; Gueler, F.; Degen, J.L.; Melk, A.; Haller, H.; Schmitt, R. Role of fibrinogen in acute ischemic kidney injury. Am. J. Physiol. Physiol. 2013, 305, F777–F785. [Google Scholar] [CrossRef]
- Saenko, E.; Josic, D.; Stadler, M.; Sarafanov, A.; Lim, Y.; Shima, M.; Ananyeva, N.; Schwinn, H. Molecular modifications in factor VIII concentrates produced from different plasma pools. Thromb. Res. 2001, 101, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Gatt, A.; Riddell, A.; Wright, G.; Chowdary, P.; Jalan, R.; Burroughs, A.K.; Davenport, A. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int. 2013, 84, 158–163. [Google Scholar] [CrossRef]
- Hoffmann, D.; Bijol, V.; Krishnamoorthy, A.; Gonzalez, V.R.; Frendl, G.; Zhang, Q.; Goering, P.L.; Brown, R.P.; Waikar, S.S.; Vaidya, V.S. Fibrinogen excretion in the urine and immunoreactivity in the kidney serves as a translational biomarker for acute kidney injury. Am. J. Pathol. 2012, 181, 818–828. [Google Scholar] [CrossRef]
- Park, J.; Joo, M.A.; Choi, H.J.; Hong, S.H.; Park, C.S.; Choi, J.H.; Chae, M.S. Predictive utility of fibrinogen in acute kidney injury in living donor liver transplantation: A propensity score-matching analysis. PLoS ONE 2021, 16, e0252715. [Google Scholar] [CrossRef]
- Celik, I.E.; Kurtul, A.; Duran, M.; Yarlioglues, M.; Elcik, D.; Kilic, A.; Koseoglu, C.; Oksuz, F.; Murat, S.N. Elevated serum fibrinogen levels and risk of contrast-induced acute kidney injury in patients undergoing a percutaneous coronary intervention for the treatment of acute coronary syndrome. Coron. Artery Dis. 2016, 27, 13–18. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L.; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef]
- Hrubá, P.; Brabcová, I.; Gueler, F.; Krejčík, Z.; Stránecký, V.; Svobodová, E.; Malušková, J.; Gwinner, W.; Honsová, E.; Lodererová, A.; et al. Molecular diagnostics identifies risks for graft dysfunction despite borderline histologic changes. Kidney Int. 2015, 88, 785–795. [Google Scholar] [CrossRef]
- Awdishu, L.; Le, A.; Amato, J.; Jani, V.; Bal, S.; Mills, R.H.; Carrillo-Terrazas, M.; Gonzalez, D.J.; Tolwani, A.; Acharya, A.; et al. Urinary Exosomes Identify Inflammatory Pathways in Vancomycin Associated Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 2784. [Google Scholar] [CrossRef]
- Briguori, C.; Visconti, G.; Rivera, N.V.; Focaccio, A.; Golia, B.; Giannone, R.; Castaldo, D.; De Micco, F.; Ricciardelli, B.; Colombo, A.; et al. Cystatin C and Contrast-Induced Acute Kidney Injury. Circulation 2010, 121, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Mitra, P.; Roy, S. Proficient Novel Biomarkers Guide Early Detection of Acute Kidney Injury: A Review. Diseases 2022, 11, 8. [Google Scholar] [CrossRef]
- Duff, S.; Irwin, R.; Cote, J.M.; Redahan, L.; A McMahon, B.; Marsh, B.; Nichol, A.; Holden, S.; Doran, P.; Murray, P.T. Urinary biomarkers predict progression and adverse outcomes of acute kidney injury in critical illness. Nephrol. Dial. Transplant. 2022, 37, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.H.; Bosler, K.; Ortiz-Soriano, V.M.; Gianella, F.; Prado, V.; Lambert, J.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Kidney Biomarkers and Major Adverse Kidney Events in Critically Ill Patients. Kidney360 2020, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.; the RUBY Investigators; Bihorac, A.; Al-Khafaji, A.; Ortega, L.M.; Ostermann, M.; Haase, M.; Zacharowski, K.; Wunderink, R.; Heung, M.; et al. Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensiv. Care Med. 2020, 46, 943–953. [Google Scholar] [CrossRef]
- Daniels, J.R.; Ma, J.Z.; Cao, Z.; Beger, R.D.; Sun, J.; Schnackenberg, L.; Pence, L.; Choudhury, D.; Palevsky, P.M.; Portilla, D.; et al. Discovery of Novel Proteomic Biomarkers for the Prediction of Kidney Recovery from Dialysis-Dependent AKI Patients. Kidney360 2021, 2, 1716–1727. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, L.; Hou, Y.; Ma, J.; Chi, R.; Ye, H.; Zhai, Y.; Zhang, D.; Gao, L.; Hu, L.; et al. The influence of glycemic status on the performance of cystatin C for acute kidney injury detection in the critically ill. Ren. Fail. 2019, 41, 139–149. [Google Scholar] [CrossRef]
- Carneiro, P.; Moreira, A.M.; Figueiredo, J.; Barros, R.; Oliveira, P.; Fernandes, M.S.; Ferro, A.; Almeida, R.; Oliveira, C.; Carneiro, F.; et al. S100P is a molecular determinant of E-cadherin function in gastric cancer. Cell Commun. Signal. 2019, 17, 1–13. [Google Scholar] [CrossRef]
- Emoto, Y.; Kobayashi, R.; Akatsuka, H.; Hidaka, H. Purification and characterization of a new member of the S-100 protein family from human placenta. Biochem. Biophys. Res. Commun. 1992, 182, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Cui, Y.; Wang, S.; Jiang, L.; Cao, J.; Zhu, S.; Birkin, E.; Lane, J.; Ruge, F.; Jiang, W.G.; et al. Calcium-Binding Protein S100P Promotes Tumor Progression but Enhances Chemosensitivity in Breast Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Whiteman, H.J.; Weeks, M.E.; Dowen, S.E.; Barry, S.; Timms, J.F.; Lemoine, N.R.; Crnogorac-Jurcevic, T. The Role of S100P in the Invasion of Pancreatic Cancer Cells Is Mediated through Cytoskeletal Changes and Regulation of Cathepsin D. Cancer Res 2007, 67, 8633–8642. [Google Scholar] [CrossRef] [PubMed]
- Dakhel, S.; Padilla, L.; Adan, J.; Masa, M.; Martinez, J.M.; Roque, L.; Coll, T.; Hervas, R.; Calvis, C.; Messeguer, R.; et al. S100P antibody-mediated therapy as a new promising strategy for the treatment of pancreatic cancer. Oncogenesis 2014, 3, e92. [Google Scholar] [CrossRef]
- Du, M.; Wang, G.; Ismail, T.M.; Gross, S.; Fernig, D.G.; Barraclough, R.; Rudland, P.S. S100P Dissociates Myosin IIA Filaments and Focal Adhesion Sites to Reduce Cell Adhesion and Enhance Cell Migration. J. Biol. Chem. 2012, 287, 15330–15344. [Google Scholar] [CrossRef] [PubMed]
- Heil, A.; Nazmi, A.R.; Koltzscher, M.; Poeter, M.; Austermann, J.; Assard, N.; Baudier, J.; Kaibuchi, K.; Gerke, V. S100P Is a Novel Interaction Partner and Regulator of IQGAP1. J. Biol. Chem. 2011, 286, 7227–7238. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Gou, L.; Wang, X.; Tang, Y.; Wang, X. A novel prognostic model based on urea cycle-related gene signature for colorectal cancer. Front. Surg. 2022, 9, 1027655. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; McNamara, K.M.; Miki, Y.; Iwabuchi, E.; Kanai, A.; Miyashita, M.; Ishida, T.; Sasano, H. S100P and Ezrin promote trans-endothelial migration of triple negative breast cancer cells. Cell. Oncol. 2019, 42, 67–80. [Google Scholar] [CrossRef]
- Ella-Tongwiis, P.; Lamb, R.M.; Makanga, A.; Shergill, I.; Hughes, S.F. The role of antibody expression and their association with bladder cancer recurrence: A single-centre prospective clinical-pilot study in 35 patients. BMC Urol. 2020, 20, 187. [Google Scholar] [CrossRef]
- Jung, Y.H.; Han, D.; Shin, S.H.; Kim, E.-K.; Kim, H.-S. Proteomic identification of early urinary-biomarkers of acute kidney injury in preterm infants. Sci. Rep. 2020, 10, 4057. [Google Scholar] [CrossRef]
- Tinari, N.; Lattanzio, R.; Querzoli, P.; Natoli, C.; Grassadonia, A.; Alberti, S.; Hubalek, M.; Reimer, D.; Nenci, I.; Bruzzi, P.; et al. High expression of 90K (Mac-2 BP) is associated with poor survival in node-negative breast cancer patients not receiving adjuvant systemic therapies. Int. J. Cancer 2009, 124, 333–338. [Google Scholar] [CrossRef]
- Woodman, N.; Pinder, S.E.; Tajadura, V.; LE Bourhis, X.; Gillett, C.; Delannoy, P.; Burchell, J.M.; Julien, S. Two E-selectin ligands, BST-2 and LGALS3BP, predict metastasis and poor survival of ER-negative breast cancer. Int. J. Oncol. 2016, 49, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Noma, N.; Simizu, S.; Kambayashi, Y.; Kabe, Y.; Suematsu, M. Involvement of NF-κB-mediated expression of galectin-3-binding protein in TNF-α-induced breast cancer cell adhesion. Oncol. Rep. 2012, 27, 2080–2084. [Google Scholar] [CrossRef]
- Sun, L.; Chen, L.; Sun, L.; Pan, J.; Yu, L.; Han, L.; Yang, Z.; Luo, Y.; Ran, Y. Functional Screen for Secreted Proteins by Monoclonal Antibody Library and Identification of Mac-2 Binding Protein (Mac-2BP) as a Potential Therapeutic Target and Biomarker for Lung Cancer. Mol. Cell. Proteom. 2013, 12, 395–406. [Google Scholar] [CrossRef]
- Stampolidis, P.; Ullrich, A.; Iacobelli, S. LGALS3BP, lectin galactoside-binding soluble 3 binding protein, promotes oncogenic cellular events impeded by antibody intervention. Oncogene 2015, 34, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Escrevente, C.; Grammel, N.; Kandzia, S.; Zeiser, J.; Tranfield, E.M.; Conradt, H.S.; Costa, J. Sialoglycoproteins and N-Glycans from Secreted Exosomes of Ovarian Carcinoma Cells. PLoS ONE 2013, 8, e78631. [Google Scholar] [CrossRef]
- Samonig, L.; Loipetzberger, A.; Blöchl, C.; Rurik, M.; Kohlbacher, O.; Aberger, F.; Huber, C.G. Proteins and Molecular Pathways Relevant for the Malignant Properties of Tumor-Initiating Pancreatic Cancer Cells. Cells 2020, 9, 1397. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; He, J.; Kuang, Y.; Wang, Z.; Sun, Z.; Zhu, H.; Liu, X. Expression and significance of 90K/Mac-2BP in prostate cancer. Exp. Ther. Med. 2013, 5, 181–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capone, E.; Iacobelli, S.; Sala, G. Role of galectin 3 binding protein in cancer progression: A potential novel therapeutic target. J. Transl. Med. 2021, 19, 405. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.T.; de Matos, A.J.; Gomes, J.; Vilanova, M.; Hespanhol, V.; Manninen, A.; Rutteman, G.; Chammas, R.; Gärtner, F.; Bernardes, E.S. Coordinated expression of galectin-3 and galectin-3-binding sites in malignant mammary tumors: Implications for tumor metastasis. Glycobiology 2010, 20, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, L.; Eliaz, A.; Hu, C.; Qiang, X.; Ke, L.; Chertow, G.; Eliaz, I.; Peng, Z. The potential roles of galectin-3 in AKI and CKD. Front. Physiol. 2023, 14, 1090724. [Google Scholar] [CrossRef]
- Hobson, C.M.; Ozrazgat-Baslanti, T.; Kuxhausen, A.B.; Thottakkara, P.M.E.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Lee, D.D.; Croome, K.P.; Mai, M.L.; Golan, E.; Brotman, R.; Keaveny, A.P.; Taner, C.B. Early Allograft Dysfunction After Liver Transplantation Is Associated With Short- and Long-Term Kidney Function Impairment. Am. J. Transplant. 2016, 16, 850–859. [Google Scholar] [CrossRef]
- Cater, J.H.; Wilson, M.R.; Wyatt, A.R. Alpha-2-Macroglobulin, a Hypochlorite-Regulated Chaperone and Immune System Modulator. Oxidative Med. Cell. Longev. 2019, 2019, 803244. [Google Scholar] [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef]
- Marrero, A.; Duquerroy, S.; Trapani, S.; Goulas, T.; Guevara, T.; Andersen, G.R.; Navaza, J.; Sottrup-Jensen, L.; Gomis-Rüth, F.X. The Crystal Structure of Human α2-Macroglobulin Reveals a Unique Molecular Cage. Angew. Chem. Int. Ed. 2012, 51, 3340–3344. [Google Scholar] [CrossRef]
- Arimura, Y.; Funabiki, H. Structural Mechanics of the Alpha-2-Macroglobulin Transformation. J. Mol. Biol. 2022, 434, 167413. [Google Scholar] [CrossRef]
- Rehman, A.A.; Ahsan, H.; Khan, F.H. Alpha-2-macroglobulin: A physiological guardian. J. Cell. Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef]
- Zia, M.K.; Siddiqui, T.; Ali, S.S.; Ahsan, H.; Khan, F.H. Interaction of anti-cancer drug-cisplatin with major proteinase inhibitor-alpha-2-macroglobulin: Biophysical and thermodynamic analysis. Int. J. Biol. Macromol. 2018, 116, 721–727. [Google Scholar] [CrossRef]
- Ling, X.; Delorme, M.; Berry, L.; Ofosu, F.; Mitchell, L.; Paes, B.; Andrew, M. alpha 2-Macroglobulin remains as important as antithrombin III for thrombin regulation in cord plasma in the presence of endothelial cell surfaces. Pediatr. Res. 1995, 37, 373–378. [Google Scholar] [CrossRef]
- Schaefer, U.; Brücker, B.; Elbers, A.; Neugebauer, E. The capacity of alpha2-macroglobulin to inhibit an exogenous protease is significantly increased in critically ill and septic patients. Shock 2004, 22, 16–22. [Google Scholar] [CrossRef]
- Liu, S.; Gui, Y.; Wang, M.S.; Zhang, L.; Xu, T.; Pan, Y.; Zhang, K.; Yu, Y.; Xiao, L.; Qiao, Y.; et al. Serum integrative omics reveals the landscape of human diabetic kidney disease. Mol. Metab. 2021, 54, 101367. [Google Scholar] [CrossRef]
- Trink, J.; Li, R.; Palarasah, Y.; Troyanov, S.; Andersen, T.E.; Sidelmann, J.J.; Inman, M.D.; Pizzo, S.V.; Gao, B.; Krepinsky, J.C. Activated Alpha 2-Macroglobulin Is a Novel Mediator of Mesangial Cell Profibrotic Signaling in Diabetic Kidney Disease. Biomedicines 2021, 9, 1112. [Google Scholar] [CrossRef]
- Anania, V.G.; Yu, K.; Pingitore, F.; Li, Q.; Rose, C.M.; Liu, P.; Sandoval, W.; Herman, A.E.; Lill, J.R.; Mathews, W.R. Discovery and Qualification of Candidate Urinary Biomarkers of Disease Activity in Lupus Nephritis. J. Proteome Res. 2019, 18, 1264–1277. [Google Scholar] [CrossRef]
- Zhao, K.-W.; Murray, E.J.B.; Murray, S.S. HK2 Proximal Tubule Epithelial Cells Synthesize and Secrete Plasma Proteins Predominantly Through the Apical Surface. J. Cell. Biochem. 2017, 118, 924–933. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Knox, K.B.; Kelly, M.E.; Hunter, G.; Popescu, B.; Nichol, H.; Kopciuk, K.; Ntanda, H.; Gailer, J. Quantification of human plasma metalloproteins in multiple sclerosis, ischemic stroke and healthy controls reveals an association of haptoglobin-hemoglobin complexes with age. PLoS ONE 2022, 17, e0262160. [Google Scholar] [CrossRef]
- Mitaki, S.; Wada, Y.; Sheikh, A.M.; Yamaguchi, S.; Nagai, A. Proteomic analysis of extracellular vesicles enriched serum associated with future ischemic stroke. Sci. Rep. 2021, 11, 24024. [Google Scholar] [CrossRef]
- Gunner, C.B.; Azmoon, P.; Mantuano, E.; Das, L.; Zampieri, C.; Pizzo, S.V.; Gonias, S.L. An antibody that targets cell-surface glucose-regulated protein-78 inhibits expression of inflammatory cytokines and plasminogen activator inhibitors by macrophages. J. Cell. Biochem. 2023, 124, 743–752. [Google Scholar] [CrossRef]
- Montavon, B.; Winter, L.E.; Gan, Q.; Arasteh, A.; Montaño, A.M. Mucopolysaccharidosis Type IVA: Extracellular Matrix Biomarkers in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 829111. [Google Scholar] [CrossRef]
- Barnum-Huckins, K.M.; Martinez, A.O.; Rivera, E.V.; Adrian, E.K., Jr.; Herbert, D.C.; Weaker, F.J.; Walter, C.A.; Adrian, G.S. A comparison of the suppression of human transferrin synthesis by lead and lipopolysaccharide. Toxicology 1997, 118, 11–22. [Google Scholar] [CrossRef]
- Morimoto, C.; Schlossman, S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998, 161, 55–70. [Google Scholar] [CrossRef]
- Xin, Y.; Min, P.; Xu, H.; Zhang, Z.; Zhang, Y.; Zhang, Y. CD26 upregulates proliferation and invasion in keloid fibroblasts through an IGF-1-induced PI3K/AKT/mTOR pathway. Burn. Trauma 2020, 8, tkaa025. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Matheeussen, V.; Jungraithmayr, W.; De Meester, I. Dipeptidyl peptidase 4 as a therapeutic target in ischemia/reperfusion injury. Pharmacol. Ther. 2012, 136, 267–282. [Google Scholar] [CrossRef]
- Du, J.; Sun, Q.; Wang, Z.; Wang, F.; Chen, F.; Wang, H.; Shang, G.; Chen, X.; Ding, S.; Li, C.; et al. Tubular epithelial cells derived-exosomes containing CD26 protects mice against renal ischemia/reperfusion injury by maintaining proliferation and dissipating inflammation. Biochem. Biophys. Res. Commun. 2021, 553, 134–140. [Google Scholar] [CrossRef]
- Sun, A.-L.; Deng, J.-T.; Guan, G.-J.; Chen, S.-H.; Liu, Y.-T.; Cheng, J.; Li, Z.-W.; Zhuang, X.-H.; Sun, F.-D.; Deng, H.-P. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vasc. Dis. Res. 2012, 9, 301–308. [Google Scholar] [CrossRef]
- Kanasaki, K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin. Sci. 2018, 132, 489–507. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Sun, Q.; Wang, Z.; Wang, F.; Chen, F.; Wang, H.; Liu, Y.; Zhou, H.; Shang, G.; et al. Urinary exosomal CD26 is associated with recovery from acute kidney injury in intensive care units: A prospective cohort study. Clin. Chem. Lab. Med. 2021, 59, 1535–1546. [Google Scholar] [CrossRef]
- Rodriguez, E.; Nan, R.; Li, K.; Gor, J.; Perkins, S.J. A Revised Mechanism for the Activation of Complement C3 to C3b: A molecular explanation of a disease-associated polymorphism. J. Biol. Chem. 2015, 290, 2334–2350. [Google Scholar] [CrossRef]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef]
- Hawksworth, O.A.; Coulthard, L.G.; Woodruff, T.M. Complement in the fundamental processes of the cell. Mol. Immunol. 2017, 84, 17–25. [Google Scholar] [CrossRef]
- Gorelik, A.; Sapir, T.; Ben-Reuven, L.; Reiner, O. Complement C3 Affects Rac1 Activity in the Developing Brain. Front. Mol. Neurosci. 2018, 11, 150. [Google Scholar] [CrossRef]
- Caravaca-Fontán, F.; Lucientes, L.; Cavero, T.; Praga, M. Update on C3 Glomerulopathy: A Complement-Mediated Disease. Nephron 2020, 144, 272–280. [Google Scholar] [CrossRef]
- Fischer, L.-M.; Fichte, L.A.; Büttner-Herold, M.; Ferrazzi, F.; Amann, K.; Benz, K.; Daniel, C. Complement in Renal Disease as a Potential Contributor to Arterial Hypertension. Kidney Blood Press. Res. 2021, 46, 362–376. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, D.; Korsten, P.; Ströbel, P.; Tampe, B. Complement Components C3 and C4 Indicate Vasculitis Manifestations to Distinct Renal Compartments in ANCA-Associated Glomerulonephritis. Int. J. Mol. Sci. 2021, 22, 6588. [Google Scholar] [CrossRef]
- Stepniewska, J.; Dolegowska, B.; Golembiewska, E.; Marchelek-Mysliwiec, M.; Domanski, M.; Ciechanowski, K.; Zair, L. The activation of complement system in different types of renal replacement therapy. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Pajenda, S.; Zawedde, F.; Kapps, S.; Wagner, L.; Schmidt, A.; Winnicki, W.; O’connell, D.; Gerges, D. Urinary C3 levels associated with sepsis and acute kidney injury—A pilot study. PLoS ONE 2021, 16, e0259777. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, W.; Zhang, L.; Yang, X.; Wang, L.; Chen, Y.; Wang, J.; Zhang, C.; Wu, G. The role of complement activation in rhabdomyolysis-induced acute kidney injury. PLoS ONE 2018, 13, e0192361. [Google Scholar] [CrossRef]
- Leaf, D.E.; Srivastava, A.; Zeng, X.; McMahon, G.M.; Croy, H.E.; Mendu, M.L.; Kachalia, A.; Waikar, S.S. Excessive diagnostic testing in acute kidney injury. BMC Nephrol. 2016, 17, 9. [Google Scholar] [CrossRef]
- McCullough, J.W.; Renner, B.; Thurman, J.M. The Role of the Complement System in Acute Kidney Injury. Semin. Nephrol. 2013, 33, 543–556. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Magalhães, P.; Pontillo, C.; Pejchinovski, M.; Siwy, J.; Krochmal, M.; Makridakis, M.; Carrick, E.; Klein, J.; Mullen, W.; Jankowski, J.; et al. Comparison of Urine and Plasma Peptidome Indicates Selectivity in Renal Peptide Handling. Proteom. -Clin. Appl. 2018, 12, e1700163. [Google Scholar] [CrossRef]
- Ferrando, E.S.; Hanslin, K.; Hultström, M.; Larsson, A.; Frithiof, R.; Lipcsey, M. Soluble TNF receptors predict acute kidney injury and mortality in critically ill COVID-19 patients: A prospective observational study. Cytokine 2022, 149, 155727. [Google Scholar] [CrossRef]
- Ramseyer, V.D.; Garvin, J.L.; Pham, G.S.; Wang, L.A.; Mathis, K.W.; Graham, L.A.; Dominiczak, A.F.; Ferreri, N.R.; Crowley, S.D.; Jeffs, A.D.; et al. Tumor necrosis factor-α: Regulation of renal function and blood pressure. Am. J. Physiol. Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef]
- Wilson, M.; Packington, R.; Sewell, H.; Bartle, R.; McCole, E.; Kurth, M.J.; Richardson, C.; Shaw, S.; Akani, A.; Banks, R.E.; et al. Biomarkers During Recovery From AKI and Prediction of Long-term Reductions in Estimated GFR. Am. J. Kidney Dis. 2022, 79, 646–656.e1. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef]
- Enrich, C.; Lu, A.; Tebar, F.; Rentero, C.; Grewal, T. Annexins Bridging the Gap: Novel Roles in Membrane Contact Site Formation. Front. Cell Dev. Biol. 2022, 9, 797949. [Google Scholar] [CrossRef]
- Mui, L.; Martin, C.M.; Tschirhart, B.J.; Feng, Q. Therapeutic Potential of Annexins in Sepsis and COVID-19. Front. Pharmacol. 2021, 12, 735472. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89. [Google Scholar] [CrossRef]
- Kundranda, M.N.; Ray, S.; Saria, M.; Friedman, D.; Matrisian, L.M.; Lukyanov, P.; Ochieng, J. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochim. et Biophys. Acta (BBA) -Mol. Cell Res. 2004, 1693, 111–123. [Google Scholar] [CrossRef]
- Mozaffari, M.S. Therapeutic Potential of Annexin A1 Modulation in Kidney and Cardiovascular Disorders. Cells 2021, 10, 3420. [Google Scholar] [CrossRef]
- Gavins, F.N.E.; Hickey, M.J. Annexin A1 and the regulation of innate and adaptive immunity. Front. Immunol. 2012, 3, 354. [Google Scholar] [CrossRef]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Grindheim, A.K.; Saraste, J.; Vedeler, A. Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim. et Biophys. Acta (BBA) -Gen. Subj. 2017, 1861 (Pt A), 2515. [Google Scholar] [CrossRef]
- Fujii, A.; Sunatani, Y.; Furuichi, K.; Fujimoto, K.; Adachi, H.; Iwabuchi, K.; Yokoyama, H. DNA damage in human glomerular endothelial cells induces nodular glomerulosclerosis via an ATR and ANXA2 pathway. Sci. Rep. 2020, 10, 22206. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Biomarkers of vascular calcification in serum. Adv. Clin. Chem. 2020, 98, 91–147. [Google Scholar] [CrossRef]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and Molecular Implications of Osteopontin in Heart Failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 1996, 33, 141–164. [Google Scholar] [CrossRef]
- Marx, D.; Metzger, J.; Pejchinovski, M.; Gil, R.B.; Frantzi, M.; Latosinska, A.; Belczacka, I.; Heinzmann, S.S.; Husi, H.; Zoidakis, J.; et al. Proteomics and Metabolomics for AKI Diagnosis. Semin. Nephrol. 2018, 38, 63–87. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Hafer, C.; Faulhaber-Walter, R.; Kümpers, P.; Kielstein, J.T.; Haller, H.; Fliser, D. Osteopontin predicts survival in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 2011, 26, 531–537. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Montesanti, A.; Hunley, H.; Koralkar, R.; Pawar, P.; Shuaib, F.; Liwo, A.; Devarajan, P.; Ambalavanan, N. Urine Biomarkers Predict Acute Kidney Injury and Mortality in Very Low Birth Weight Infants. J. Pediatr. 2011, 159, 907–912.e1. [Google Scholar] [CrossRef]
- Du Clos, T.W. Pentraxins: Structure, Function, and Role in Inflammation. ISRN Inflamm. 2013, 2013, 379040. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, J.; Hashmi, S.; Ali, A.; Siddiqui, A.J.; Sami, S.A.; Bokhari, S.S.; Sharif, H.; Uddin, J.; El-Seedi, H.R.; et al. Dysregulation of metalloproteins in ischemic heart disease patients with systolic dysfunction. Int. J. Biol. Macromol. 2023, 232, 123435. [Google Scholar] [CrossRef]
- Liu, B.; Lv, D. Prognostic value of C-reactive protein to albumin ratio for mortality in acute kidney injury. BMC Nephrol. 2023, 24, 44. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Lu, J.; Marjon, K.D.; Marnell, L.L.; Wang, R.; Mold, C.; Du Clos, T.W.; Sun, P. Recognition and functional activation of the human IgA receptor (FcαRI) by C-reactive protein. Proc. Natl. Acad. Sci. USA 2011, 108, 4974–4979. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, X.R.; Lv, J.; Chung, A.C.-K.; Zhang, Y.; Chen, J.-Z.; Szalai, A.J.; Xu, A.; Lan, H.Y. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin. Sci. 2014, 126, 645–659. [Google Scholar] [CrossRef]
- Novo-Veleiro, I.; Pose-Reino, A.; Gullón, A.; Díez-Manglano, J.; Cepeda, J.-M.; Formiga, F.; Camafort, M.; Mostaza, J.-M.; Suárez, C. Acute kidney injury is linked to higher mortality in elderly hospitalized patients with non-valvular atrial fibrillation. Aging Clin. Exp. Res. 2019, 31, 455–461. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, D.K.; Kim, S.; Chin, H.J.; Chae, D.-W.; Na, K.Y. C-Reactive Protein Predicts Acute Kidney Injury and Death After Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2017, 104, 804–810. [Google Scholar] [CrossRef]
- Law, S.K.A.; Dodds, A.W. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 1997, 6, 263–274. [Google Scholar] [CrossRef]
- Sim, R.B.; Sim, E. AUTOLYTIC FRAGMENTATION OF COMPLEMENT COMPONENTS C3 AND C4 AND ITS RELATIONSHIP TO COVALENT BINDING ACTIVITY. Ann. N. Y. Acad. Sci. 1983, 421, 259–276. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Ohtani, K. Complement-Related Proteins and Their Measurements: The Current Status of Clinical Investigation. Nephron 2020, 144 (Suppl. 1), 7–12. [Google Scholar] [CrossRef]
- Kohzuma, T.; Tao, X.; Koga, M. Glycated albumin as biomarker: Evidence and its outcomes. J. Diabetes Complicat. 2021, 35, 108040. [Google Scholar] [CrossRef]
- Genest, D.S.; Bonnefoy, A.; Khalili, M.; Merlen, C.; Genest, G.; Lapeyraque, A.-L.; Patey, N.; Smail, N.; Royal, V.; Troyanov, S. Comparison of Complement Pathway Activation in Autoimmune Glomerulonephritis. Kidney Int. Rep. 2022, 7, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Stenson, E.K.; You, Z.; Reeder, R.; Norris, J.; Scott, H.F.; Dixon, B.P.; Thurman, J.M.; Frazer-Abel, A.; Mourani, P.; Kendrick, J. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Complement Activation Fragments Are Increased in Critically Ill Pediatric Patients with Severe AKI. Kidney360 2021, 2, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Bi, T.-D.; Zheng, J.-N.; Zhang, J.-X.; Yang, L.-S.; Liu, N.; Yao, L.; Liu, L.-L. Serum complement C4 is an important prognostic factor for IgA nephropathy: A retrospective study. BMC Nephrol. 2019, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Sise, M.E.; Strohbehn, I.; Chute, D.; Corey, K.E.; Fusco, D.N.; Sabbisetti, V.S.; Waikar, S.S.; Chung, R.T. Low Complement C4 Predicts Improvement of Kidney Function After Direct-Acting Antiviral Therapy for Hepatitis C Virus. Hepatol. Commun. 2020, 4, 1206–1217. [Google Scholar] [CrossRef]
- Ayano, M.; Horiuchi, T. Complement as a Biomarker for Systemic Lupus Erythematosus. Biomolecules 2023, 13, 367. [Google Scholar] [CrossRef]
- Wally, J.; Buchanan, S.K. A structural comparison of human serum transferrin and human lactoferrin. Biometals 2007, 20, 249–262. [Google Scholar] [CrossRef]
- Luck, A.N.; Mason, A.B. Transferrin-mediated cellular iron delivery. Curr. Top. Membr. 2012, 69, 3–35. [Google Scholar] [CrossRef]
- Jamnongkan, W.; Lebrilla, C.B.; Barboza, M.; Techasen, A.; Loilome, W.; Sithithaworn, P.; Khuntikeo, N.; Pairojkul, C.; Chamadol, N.; Thanan, R.; et al. Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma. Biomolecules 2019, 9, 538. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Wang, M.; Hao, Q.; Sun, H. Iron and bismuth bound human serum transferrin reveals a partially-opened conformation in the N-lobe. Sci. Rep. 2012, 2, 999. [Google Scholar] [CrossRef]
- Satomi, Y.; Shimonishi, Y.; Takao, T. N-glycosylation at Asn491in the Asn-Xaa-Cys motif of human transferrin. FEBS Lett. 2004, 576, 51–56. [Google Scholar] [CrossRef]
- Ma, J.; Sanda, M.; Wei, R.; Zhang, L.; Goldman, R. Quantitative analysis of core fucosylation of serum proteins in liver diseases by LC-MS-MRM. J. Proteom. 2018, 189, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pourcelot, E.; Lénon, M.; Mobilia, N.; Cahn, J.-Y.; Arnaud, J.; Fanchon, E.; Moulis, J.-M.; Mossuz, P. Iron for proliferation of cell lines and hematopoietic progenitors: Nailing down the intracellular functional iron concentration. Biochim. Biophys. Acta 2015, 1853, 1596–1605. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leaf, D.E.; Rajapurkar, M.; Lele, S.S.; Mukhopadhyay, B.; Boerger, E.A.; Mc Causland, F.R.; Eisenga, M.F.; Singh, K.; Babitt, J.L.; Kellum, J.A.; et al. Iron, Hepcidin, and Death in Human AKI. J. Am. Soc. Nephrol. 2019, 30, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.G.; Vicente-Vicente, L.; Hernández-Sánchez, M.T.; Prieto, M.; Rihuete, M.I.; Ramis, L.M.; del Barco, E.; Cruz, J.J.; Ortiz, A.; Cruz-González, I.; et al. Urinary transferrin pre-emptively identifies the risk of renal damage posed by subclinical tubular alterations. Biomed. Pharmacother. 2020, 121, 109684. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Wijeysundera, D.N.; Yau, T.M.; McCluskey, S.A.; Chan, C.T.; Wong, P.-Y.; Crowther, M.A.; Hozhabri, S.; Beattie, W.S. Advance Targeted Transfusion in Anemic Cardiac Surgical Patients for Kidney Protection: An unblinded randomized pilot clinical trial. Anesthesiology 2012, 116, 613–621. [Google Scholar] [CrossRef]
- Schnabolk, G.; Coughlin, B.; Joseph, K.; Kunchithapautham, K.; Bandyopadhyay, M.; O’Quinn, E.C.; Nowling, T.; Rohrer, B. Local Production of the Alternative Pathway Component Factor B Is Sufficient to Promote Laser-Induced Choroidal Neovascularization. Investig. Opthalmology Vis. Sci. 2015, 56, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, J.; Philbrook, H.T.; Parikh, C.R.; Thurman, J.M. Urine complement activation fragments are increased in patients with kidney injury after cardiac surgery. Am. J. Physiol. Renal Physiol. 2019, 317, F650–F657. [Google Scholar] [CrossRef]
- Burne-Taney, M.J.; Rabb, H. The role of adhesion molecules and T cells in ischemic renal injury. Curr. Opin. Nephrol. Hypertens. 2003, 12, 85–90. [Google Scholar] [CrossRef]
- Fuquay, R.; Renner, B.; Kulik, L.; McCullough, J.W.; Amura, C.; Strassheim, D.; Pelanda, R.; Torres, R.; Thurman, J.M. Renal Ischemia-Reperfusion Injury Amplifies the Humoral Immune Response. J. Am. Soc. Nephrol. 2013, 24, 1063–1072. [Google Scholar] [CrossRef]
- Thurman, J.M. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 2007, 123, 7–13. [Google Scholar] [CrossRef]
- Koyner, J.L.; Parikh, C.R. Clinical Utility of Biomarkers of AKI in Cardiac Surgery and Critical Illness. Clin. J. Am. Soc. Nephrol. 2013, 8, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Brier, M.E.; Slaughter, M.S.; Klein, J.B.; McLeish, K.R. Biomarker enhanced risk prediction for development of AKI after cardiac surgery. BMC Nephrol. 2018, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell Biol. 2005, 83, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich Glycoprotein Binds to Cell-surface Heparan Sulfate via Its N-terminal Domain following Zn2+ Chelation. J. Biol. Chem. 2004, 279, 30114–30122. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef]
- Li, H.-X.; Hwang, B.-Y.; Laxmikanthan, G.; Blaber, S.I.; Blaber, M.; Golubkov, P.A.; Ren, P.; Iverson, B.L.; Georgiou, G. Substrate specificity of human kallikreins 1 and 6 determined by phage display. Protein Sci. 2008, 17, 664–672. [Google Scholar] [CrossRef]
- Kang, S.W.; Shih, P.-A.B.; O Mathew, R.; Mahata, M.; Biswas, N.; Rao, F.; Yan, L.; Bouchard, J.; Malhotra, R.; Tolwani, A.; et al. Renal kallikrein excretion and epigenetics in human acute kidney injury: Expression, mechanisms and consequences. BMC Nephrol. 2011, 12, 27. [Google Scholar] [CrossRef]
- Kalinska, M.; Meyer-Hoffert, U.; Kantyka, T.; Potempa, J. Kallikreins—The melting pot of activity and function. Biochimie 2016, 122, 270–282. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Perianayagam, M.C.; Kang, S.W.; Zhang, W.; Rao, F.; O’Connor, D.T.; Jaber, B.L. Association of Functional Kallikrein-1 Promoter Polymorphisms and Acute Kidney Injury: A Case-Control and Longitudinal Cohort Study. Nephron Clin. Pr. 2013, 122, 107–113. [Google Scholar] [CrossRef]
- Wald, R.; Quinn, R.R.; Luo, J.; Li, P.; Scales, D.C.; Mamdani, M.M.; Ray, J.G.; for the University of Toronto Acute Kidney Injury Research Group. Chronic Dialysis and Death Among Survivors of Acute Kidney Injury Requiring Dialysis. JAMA 2009, 302, 1179–1185. [Google Scholar] [CrossRef]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term Risk of Mortality and Other Adverse Outcomes After Acute Kidney Injury: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ma, T.; Zhang, C.; Tang, X.; Xu, Q.; Meng, X.; Ma, T. Identification of urinary candidate biomarkers of cisplatin-induced nephrotoxicity in patients with carcinoma. J. Proteom. 2020, 210, 103533. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, L.; Nalesso, F.; Garzotto, F.; Xie, Y.; Yang, B.; Virzì, G.M.; Passannante, A.; Bonato, R.; Carta, M.; Giavarina, D.; et al. Predicting Acute Kidney Injury in Intensive Care Unit Patients: The Role of Tissue Inhibitor of Metalloproteinases-2 and Insulin-Like Growth Factor-Binding Protein-7 Biomarkers. Blood Purif. 2018, 45, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Wilkins, J.A.; Chan, F.V.; Ye, B.; Nickerson, P.; Ho, J. Phospholipase A2 group XV activity during cardiopulmonary bypass surgery. Clin. Biochem. 2021, 88, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Ho, J.; Dwivedi, R.C.; Choi, N.; Ezzati, P.; Spicer, V.; Arora, R.C.; Rigatto, C.; Wilkins, J.A. Activity-Based Protein Profiling of Intraoperative Serine Hydrolase Activities during Cardiac Surgery. J. Proteome Res. 2018, 17, 3547–3556. [Google Scholar] [CrossRef]
- Pejchinovski, M.; Klein, J.; Ramírez-Torres, A.; Bitsika, V.; Mermelekas, G.; Vlahou, A.; Mullen, W.; Mischak, H.; Jankowski, V. Comparison of higher energy collisional dissociation and collision-induced dissociation MS/MS sequencing methods for identification of naturally occurring peptides in human urine. Proteom. -Clin. Appl. 2015, 9, 531–542. [Google Scholar] [CrossRef]
- Heil, L.R.; Damoc, E.; Arrey, T.N.; Pashkova, A.; Denisov, E.; Petzoldt, J.; Peterson, A.; Hsu, C.; Searle, B.C.; Shulman, N.; et al. Evaluating the performance of the Astral mass analyzer for quantitative proteomics using data independent acquisition. bi-oRxiv 2023, 2023, 543570. [Google Scholar] [CrossRef]
- Lian, G.; Li, L.; Ye, F.; Wang, D.; Liu, J.; Shi, Y.; Jing, C.; Suo, J.; Zhang, D.Y.; Chen, M. The screening and analysis of protein signatures and signaling associated with chemoresistance based on Protein Pathway Array technology in gastric cancer. Oncol. Rep. 2018, 39, 307–315. [Google Scholar] [CrossRef]
- Zlobec, I.; Suter, G.; Perren, A.; Lugli, A. A Next-generation Tissue Microarray (ngTMA) Protocol for Biomarker Studies. J. Vis. Exp. 2014, e51893. [Google Scholar] [CrossRef]
- Zlobec, I.; Koelzer, V.H.; Dawson, H.; Perren, A.; Lugli, A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: An example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J. Transl. Med. 2013, 11, 104. [Google Scholar] [CrossRef]
- Jiang, Z.; Kamerud, J.; You, Z.; Basak, S.; Seletskaia, E.; Steeno, G.S.; Gorovits, B. Feasibility of singlicate-based analysis in bridging ADA assay on Meso-Scale Discovery platform: Comparison with duplicate analysis. Bioanalysis 2021, 13, 1123–1134. [Google Scholar] [CrossRef]
- Lasseter, H.C.; Provost, A.C.; Chaby, L.E.; Daskalakis, N.P.; Haas, M.; Jeromin, A. Cross-platform comparison of highly sensitive immunoassay technologies for cytokine markers: Platform performance in post-traumatic stress disorder and Parkinson’s disease. Cytokine X 2020, 2, 100027. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. New trends in single-molecule bioanalytical detection. Anal. Bioanal. Chem. 2020, 412, 5005–5014. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Markó, L.; Paragas, N.; Barasch, J.; Dragun, D.; Müller, D.N.; Budde, K.; Schmidt-Ott, K.M. Neutrophil gelatinase-associated lipocalin: Pathophysiology and clinical applications. Acta Physiol. 2013, 207, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Li, Y.; Wen, D.; Liu, M.; Ma, Y.; Cong, B. NGAL protects against endotoxin-induced renal tubular cell damage by suppressing apoptosis. BMC Nephrol. 2018, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.S.; Cruz, D.; Bobek, I.; Chionh, C.Y.; Nalesso, F.; Lentini, P.; de Cal, M.; Corradi, V.; Virzi, G.; Ronco, C. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int. Urol. Nephrol. 2010, 42, 141–150. [Google Scholar] [CrossRef]
- Neely, B.A.; Palmblad, M. Machine Learning in Proteomics and Metabolomics. J. Proteome Res. 2022, 21, 2553–2554. [Google Scholar] [CrossRef]
- Palmblad, M.; Böcker, S.; Degroeve, S.; Kohlbacher, O.; Käll, L.; Noble, W.S.; Wilhelm, M. Interpretation of the DOME Recommendations for Machine Learning in Proteomics and Metabolomics. J. Proteome Res. 2022, 21, 1204–1207. [Google Scholar] [CrossRef]
- Diaz-Uriarte, R.; de Lope, E.G.; Giugno, R.; Fröhlich, H.; Nazarov, P.V.; Nepomuceno-Chamorro, I.A.; Rauschenberger, A.; Glaab, E. Ten quick tips for biomarker discovery and validation analyses using machine learning. PLOS Comput. Biol. 2022, 18, e1010357. [Google Scholar] [CrossRef]

| Biomarkers | Biomarker Type | Study Type | Affected Area of Kidney | Affected Kidney Cell Types | AKI Category |
|---|---|---|---|---|---|
| NGAL | Diagnostic | Urine analysis | Renal pelvis | Collecting duct epithelial cells | Prerenal |
| B2M | Diagnostic | Urine analysis | Proximal tubule | Tubular epithelial cells | Intrinsic renal |
| SERPINA1 (AAT) | Diagnostic | Urine analysis | Proximal tubule | Tubular epithelial cells | Intrinsic renal |
| RBP4 | Diagnostic | Plasma analysis | Proximal tubule | Tubular epithelial cells | Postrenal |
| FBG | Diagnostic | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| GDF15 | Diagnostic | Urine analysis | Nephron | Renal endothelial cells | Intrinsic renal (after MI) ** |
| LRG1 | Diagnostic | Urine analysis | Nephron | Renal endothelial cells | Intrinsic renal (after MI) ** |
| SPP1 | Diagnostic | Urine analysis | Nephron | Renal endothelial cells | Intrinsic renal (after MI) ** |
| ANXA5 | Diagnostic | Urine analysis | Nephron | Renal endothelial cells | Prerenal |
| 6-PGLS | Diagnostic | Urine analysis | Nephron | Renal endothelial cells | Prerenal |
| TIMP-2 IGFBP7 * | Diagnostic | Urine/serum | Proximal tubule | Proximal tubular epithelial cells | Intrinsic renal (after MI) ** |
| C3 | Diagnostic or prognostic | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| C4 | Diagnostic or prognostic | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| GAL-3BP | Prognostic | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| Cys C | Prognostic | Plasma analysis | Proximal tubule | Tubular epithelial cells | Prerenal |
| S100P | Prognostic | Urine analysis | Glomerulus | Urothelium cells | Prerenal |
| α2M | Prognostic | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| CD26 * | Prognostic | Urine analysis | Glomerulus/Proximal tubule | Renal brush border epithelium | Intrinsic renal (after MI) ** |
| sTNFR1, sTNFR2 | Monitoring | Plasma analysis | Glomerulus | Tubular epithelial & mesangial cells | Intrinsic renal |
| ANXA-2 | Monitoring | Urine analysis | Glomerulus | Renal glomerular endothelial cells | Intrinsic renal |
| CRP | Monitoring | Blood analysis | Renal cortex | Renal Cortical Epithelial Cells | Intrinsic renal (after MI) ** |
| OPN | Monitoring | Blood analysis | Nephron-loop of Henle | Renal epithelial cells | Intrinsic renal (after MI) ** |
| CD5 & Factor VII * | Monitoring | Blood analysis | Nephron | Filtrating cells | Intrinsic renal (after MI) ** |
| IgHM | Monitoring | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| Serotransferrin | Monitoring | Urine analysis | Glomerulus | Tubular epithelial cells | Intrinsic renal (after MI) ** |
| HRG | Monitoring | Urine analysis | Glomerulus | Proximal tubule epithelial cells | Intrinsic renal (after MI) ** |
| CFB | Monitoring | Urine analysis | Glomerulus | Proximal tubule epithelial cells | Intrinsic renal (after MI) ** |
| CD59 | Monitoring | Urine analysis | Glomerulus/Proximal tubule | Renal brush border epithelium | Intrinsic renal (after MI) ** |
| AGT | Monitoring | Urine analysis | Glomerulus | Proximal tubule epithelial cells | Intrinsic renal (after MI) ** |
| KRK1 * | Monitoring | Urine analysis | Glomerulus | Proximal tubule epithelial cells | Intrinsic renal (after MI) ** |
| Authors | Biofluid | Method | Patient Cohort | Investigated Biomarkers | Most Significant Biomarkers | Conclusion |
|---|---|---|---|---|---|---|
| Ibrahim et al. [20,21] | Blood | Luminex xMAP immunoassay | 44 AKI 745 non- AKI | 109 | CRP; OPN; CD5; FACTOR VII | The biomarker panel using machine learning was developed and showed a performance with an AUC of 0.79 for predicting procedural AKI. The optimal score cutoff had 77% sensitivity, 75% specificity, and a negative predictive value of 98% for procedural AKI. An elevated score was predictive of procedural AKI in all subjects (odds ratio = 9.87; p < 0.001). |
| Zhu et al. [21,22] | Urine | LC-MS/MS | 4 CI-AKI 20 CI-non AKI | 99 | NGAL; S100- P; ANXA2; B2M; SERPINA1; RBP4 | In relatively small patient cohort, urine proteome of CI-AKI vs. non-CI-AKI were compared. Upregulation was observed in CI-AKI with ratio of 7.40 (B2M), 6.63(S100-P), 4.25 (NGAL) and 4.27 (SERPINA1). |
| Awdishu et al. [23] | Urine/blood | LC-MS/MS | 10 V-AKI 12 HC | 251 | C3; C4; GAL-3BP, FBG, α2M; IgHM; SEROTRANSFERRIN | Urinary exosome proteins in response to V-AKI might provide vulnerable molecular information that helps elucidate mechanisms of injury and identify novel biomarkers among patients with confirmed drug-induced kidney injury. |
| Jung et al. [24] | Urine | LC-MS/MS | 14 AKI 14 non-AKI | 174 | NGAL; ANXA5;GAL3; 6-PGLS; S100-P | Proteomic urinary-based biomarkers that can predict early AKI occurrences in infants were identified. Three biomarkers performed well, showing AUC values of 0.75, 0.88 and 0.74 for NGAL, ANXA5 and S100-P, respectively. There was higher beneficial effect of the classifier performance when NGAL + AXA5 (AUC of 0.92) and NGAL + AXA5 + S100-P (AUC of 0.93) were applied. |
| Du et al. [25] | Urine | Flow cytometry | 133 AKI 68 non-AKI | 1 | CD26 | Urinary exosomal CD26 was negatively correlated with AKI compared with non-AKI patients (β = −15.95, p < 0.001). Similar results were obtained for the AKI cohort with major adverse events. On the other hand, AKI survivors exhibited high-CD26 levels compared AKI patients with low-CD26 levels for early reversal, recovery and reversal, respectively, after adjustment for clinical factors (ORs (95% CI) were 4.73 (1.77–11.48), 5.23 (1.72–13.95) and 6.73 (2.00–19.67), respectively). Prediction performance was moderate for AKI survivors (AUC 0.65; 95% CI, 0.53–0.77; p = 0.021) but improved for non-septic AKI survivors (AUC, 0.83; 95% CI, 0.70–0.97; p = 0.003) |
| Wilson et al. [22] | Plasma | Randox’s multiplexed Biochip Arrays | 500 AKI | 11 | sTNFR1; sTNFR2; CYSTATIN C; NGAL | A multivariable panel containing sTNFR1, sTNFR2, cystatin C, and eGFR discriminated between those with and without kidney disease progression (AUC 0.79 [95% CI, 0.70–0.83]). Optimization of the panel showed 95% sensitivity and a negative predictive value of 92% used to stratify patients at low risk for disease severity. |
| Merchant et al. [26] | Urine | ELISA | 15 AKI 32 non-AKI | 29 | HRG; CFB; CD59; C3; AGT | Two proteins, HRG and CFB were upregulated in AKI patients, showing moderate predictive performance (AUC 0.79; 95% CI, 0.65–0.94; p = 0.001 and AUC 0.75; 95% CI, 0.57–0.93; p = 0.007). Significant improvement in the risk prediction for primary outcome was observed, specifically for NRI, IDI in addition to CFB and HRG. Only HRG was a significant predictor in the 21 patients with AKI defined by KDIGO criteria. |
| Coca et al. [27] | Serum | Randox’s multiplexed Biochip Arrays | 769 AKI 769 non-AKI | 2 | sTNFR1; sTNFR2 | Plasma sTNFR1 and sTNFR2 measured 3 months after discharge were associated with renal deterioration independent of AKI (HR 4.7, 95% CI, 2.6–8.6) and significant association with renal failure. In this regards, clinical classifier performance was with AUC of 0.83. There was also association of the both biomarkers with Heart failure ((sTNFR1-1.9 (95% CI, 1.4–2.5) and sTNFR2-1.5 (95% CI, 1.2–2.0)) and death ((sTNFR1- 3.3 (95% CI, 2.5–4.2) and sTNFR2-1.5 (95% CI, 1.9–3.1)). |
| Jiang et al. [28] | Urine | LC-MS/MS | 90 CP-AKI | 12 | GDF15; LRG1; SPP1 | Urinary proteomic profiles of GDF15 (1.77-fold) and LRG1 (4.25-fold) were significantly elevated by CP treatment compared to the baseline. |
| Di Leo et al. [29] | Urine/serum | NephroCheck® (NC) Immunoassay | 719 patients at ICU | 2 | TIMP-2; IGFBP7 | TIMP-2 and IGFBP7 levels yielded good performance in prediction AKI development at first 4 days at ICU and in all critically ill patients (AUC of 0.65). The Kaplan-Meier analysis predicted lower risk for AKI development only for those patients who NC test was negative. |
| Navarrete et al. [30] | Urine/serum | ELISA assay | 21 AKI 21 non-AKI | 1 | PLA2G15/LPLA2 | Urinary PLA2G15/LPLA2 activity was associated with subsequent AKI development during/ongoing CPB. There was similar association with PLA2G15/LPLA2 activity from serum. No association was observed between PLA2G15/LPLA2 activity from both biofluids, suggesting that this biomarker might be an early sign of renal response to CPB events. |
| Navarrete et al. [31] | Urine | Nano RPLC-MS/MS | 8 AKI 8 non-AKI | 28 | KRK1 | Investigation on KLK1, confirmed the activity of this enzyme in AKI and non- AKI patients. In fact, increased action of KLK1 was confirmed only in AKI patients who arrived at ICU and had highest activity in comparison to other enzymes, hence providing novel finding related to intraoperative events in human ischemia reperfusion injury during CPB. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejchinovski, I.; Turkkan, S.; Pejchinovski, M. Recent Advances of Proteomics in Management of Acute Kidney Injury. Diagnostics 2023, 13, 2648. https://doi.org/10.3390/diagnostics13162648
Pejchinovski I, Turkkan S, Pejchinovski M. Recent Advances of Proteomics in Management of Acute Kidney Injury. Diagnostics. 2023; 13(16):2648. https://doi.org/10.3390/diagnostics13162648
Chicago/Turabian StylePejchinovski, Ilinka, Sibel Turkkan, and Martin Pejchinovski. 2023. "Recent Advances of Proteomics in Management of Acute Kidney Injury" Diagnostics 13, no. 16: 2648. https://doi.org/10.3390/diagnostics13162648
APA StylePejchinovski, I., Turkkan, S., & Pejchinovski, M. (2023). Recent Advances of Proteomics in Management of Acute Kidney Injury. Diagnostics, 13(16), 2648. https://doi.org/10.3390/diagnostics13162648






