Depression and Malnutrition for Prediction of Mortality after Transcatheter Aortic Valve Replacement: A Registry Study of a Tertiary Referral Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics
2.3. Clinical Characteristics
2.4. Procedural and Hospitalization-Related Characteristics
2.5. Geriatric Frailty Markers
2.6. Study Endpoint
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population and Clinical Endpoint Groups
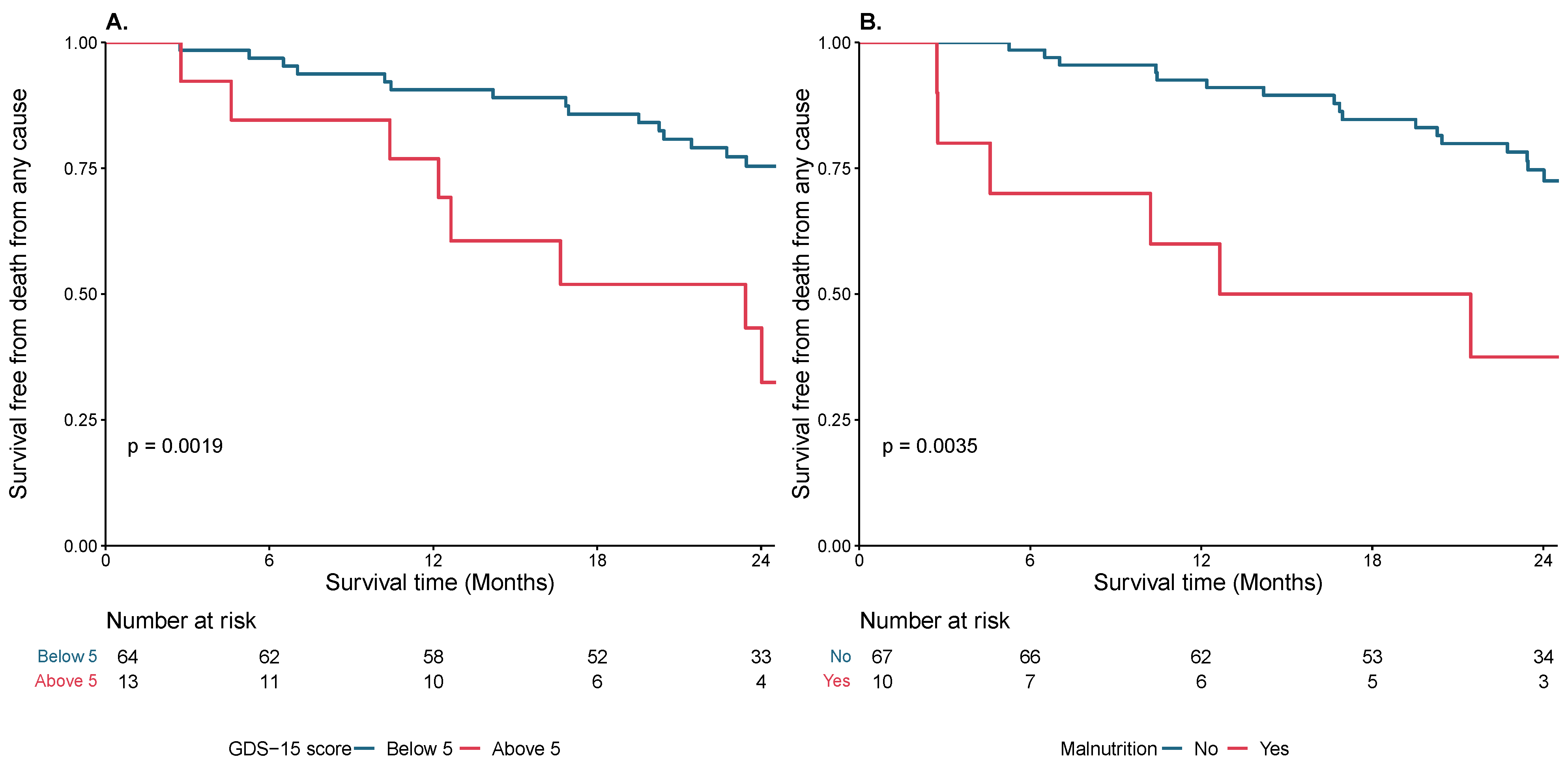
3.2. Geriatric Frailty Characteristics in Relation to Clinical Endpoint
3.3. Frailty Marker Risk Models to Predict All-Cause Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic Stenosis in the Elderly: Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement: A Meta-Analysis and Modeling Study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Brennan, J.M.; Rumsfeld, J.S.; Dai, D.; O’brien, S.M.; Vemulapalli, S.; Edwards, F.H.; Carroll, J.; Shahian, D.; Grover, F.; et al. Clinical Outcomes at 1 Year Following Transcatheter Aortic Valve Replacement. JAMA 2015, 313, 1019–1028. [Google Scholar] [CrossRef]
- Barili, F.; Pacini, D.; Capo, A.; Rasovic, O.; Grossi, C.; Alamanni, F.; Di Bartolomeo, R.; Parolari, A. Does EuroSCORE II perform better than its original versions? A multicentre validation study. Eur. Heart J. 2013, 34, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Speir, A.M.; Head, S.J.; Fonner, C.E.; Fonner, E.; Kappetein, A.P.; Rich, J.B. Performance of EuroSCORE II in a large US database: Implications for transcatheter aortic valve implantation. Eur. J. Cardio-Thorac. Surg. 2014, 46, 400–408. [Google Scholar] [CrossRef]
- Kumar, A.; Sato, K.; Narayanswami, J.; Banerjee, K.; Andress, K.; Lokhande, C.; Mohananey, D.; Anumandla, A.K.; Khan, A.R.; Sawant, A.C.; et al. Current Society of Thoracic Surgeons Model Reclassifies Mortality Risk in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e006664. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Shi, S.; Afilalo, J.; Lipsitz, L.A.; Popma, J.J.; Khabbaz, K.R.; Laham, R.J.; Guibone, K.; Grodstein, F.; Lux, E.; Kim, D.H. Frailty Phenotype and Deficit Accumulation Frailty Index in Predicting Recovery after Transcatheter and Surgical Aortic Valve Replacement. J. Gerontol. Ser. A 2018, 74, 1249–1256. [Google Scholar] [CrossRef]
- Shimura, T.; Yamamoto, M.; Kano, S.; Kagase, A.; Kodama, A.; Koyama, Y.; Tsuchikane, E.; Suzuki, T.; Otsuka, T.; Kohsaka, S.; et al. Impact of the Clinical Frailty Scale on Outcomes after Transcatheter Aortic Valve Replacement. Circulation 2017, 135, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Stapor, M.; Dudek, D. Impact of frailty on mortality after transcatheter aortic valve implantation. Am. Hearth J. 2017, 185, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Puls, M.; Sobisiak, B.; Bleckmann, A.; Jacobshagen, C.; Danner, B.C.; Hünlich, M.; Beißbarth, T.; Schöndube, F.; Hasenfuß, G.; Seipelt, R.; et al. Impact of frailty on short- and long-term morbidity and mortality after transcatheter aortic valve implantation: Risk assessment by Katz Index of activities of daily living. Eurointervention 2014, 10, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.B.; Xu, J.; Orkaby, A.R.; Shen, C.; Song, Y.; Charest, B.R.; Kim, D.H.; Cohen, D.J.; Kramer, D.B.; Spertus, J.A.; et al. Role of Frailty in Identifying Benefit from Transcatheter Versus Surgical Aortic Valve Replacement. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e008566. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.; Erten, O.; Ramlawi, B. Transcatheter Aortic Valve Implantation in Elderly Patients with Aortic Valve Stenosis: The Role of Frailty, Malnutrition, and Sarcopenia. J. Am. Heart Assoc. 2022, 11, 27705. [Google Scholar] [CrossRef] [PubMed]
- Bs, Q.P.H.; Maltagliati, A.J.; Shi, S.M.; Afilalo, J.; Popma, J.J.; Khabbaz, K.R.; Laham, R.J.; Np, K.G.; Kim, D.H. A Practical Two-Stage Frailty Assessment for Older Adults Undergoing Aortic Valve Replacement. J. Am. Geriatr. Soc. 2019, 67, 2031–2037. [Google Scholar] [CrossRef]
- Green, P.; Woglom, A.E.; Genereux, P.; Daneault, B.; Paradis, J.-M.; Schnell, S.; Hawkey, M.; Maurer, M.S.; Kirtane, A.J.; Kodali, S.; et al. The Impact of Frailty Status on Survival after Transcatheter Aortic Valve Replacement in Older Adults with Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2012, 5, 974–981. [Google Scholar] [CrossRef]
- Anand, A.; Harley, C.; Visvanathan, A.; Shah, A.S.V.; Cowell, J.; MacLullich, A.; Shenkin, S.; Mills, N.L. The relationship between preoperative frailty and outcomes following transcatheter aortic valve implantation: A systematic review and meta-analysis. Eur. Heart. J. Qual. Care Clin. Outcomes 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Senior Health Calculator: Online Tool for Providers|BIDMC of Boston. Available online: https://www.bidmc.org/research/research-by-department/medicine/gerontology/calculator (accessed on 7 April 2023).
- Kim, D.H.; Glynn, R.J.; Avorn, J.; Lipsitz, L.A.; Rockwood, K.; Pawar, A.; Schneeweiss, S. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J. Gerontol. Ser. A 2018, 74, 1271–1276. [Google Scholar] [CrossRef]
- Risk Calculator|STS. Available online: https://www.sts.org/resources/risk-calculator (accessed on 7 April 2023).
- EuroScore Website—Calculator. Available online: https://www.euroscore.org/index.php?id=17 (accessed on 7 April 2023).
- Puls, M.; Sobisiak, B.; Jacobshagen, C.; Danner, B.; Schoendube, F.; Hasenfuss, G.; Seipelt, R.; Schillinger, W. Katz-Index effectively predicts long-term mortality after Transcatheter Aortic Valve Implantation (TAVI). Eur. Heart J. 2013, 34, 2580. [Google Scholar] [CrossRef][Green Version]
- Kukull, W.; Larson, E.; Teri, L.; Bowen, J.; McCormick, W.; Pfanschmidt, M. The mini-mental state examination score and the clinical diagnosis of dementia. J. Clin. Epidemiol. 1994, 47, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document†. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.A.; Ma, J.; Anderson, L.N.; Mayhew, A.J.; So, H.Y.; Griffith, L.E.; Gilsing, A.; Raina, P. Age-appropriate BMI cut-points for cardiometabolic health risk: A cross-sectional analysis of the Canadian Longitudinal Study on Aging. Int. J. Obes. 2022, 46, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.L.D.C.; Teixeira, A.L.; Guimarães, H.C.; Barbosa, M.T.; Resende, E.D.P.F.; Beato, R.G.; Carmona, K.C.; Caramelli, P. Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community-dwelling oldest-old sample: The Pietà Study. Trends Psychiatry Psychother. 2017, 39, 276–279. [Google Scholar] [CrossRef][Green Version]
- Bautmans, I.; Onyema, O.; Van Puyvelde, K.; Pleck, S.; Mets, T. Grip work estimation during sustained maximal contraction: Validity and relationship with dependency and inflammation in elderly persons. J. Nutr. Health Aging 2011, 15, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Buatois, S.; Perret-Guillaume, C.; Gueguen, R.; Miget, P.; Vançon, G.; Perrin, P.; Benetos, A. A Simple Clinical Scale to Stratify Risk of Recurrent Falls in Community-Dwelling Adults Aged 65 Years and Older. Phys. Ther. 2010, 90, 550–560. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk Factors for Falls among Elderly Persons Living in the Community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chang, S.S.; Weiss, C.O.; Xue, Q.-L.; Fried, L.P. Association between inflammatory-related disease burden and frailty: Results from the Women’s Health and Aging Studies (WHAS) I and II. Arch. Gerontol. Geriatr. 2012, 54, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Drudi, L.M.; Ades, M.; Turkdogan, S.; Huynh, C.; Lauck, S.; Webb, J.G.; Piazza, N.; Martucci, G.; Langlois, Y.; Perrault, L.P.; et al. Association of Depression with Mortality in Older Adults Undergoing Transcatheter or Surgical Aortic Valve Replacement. JAMA Cardiol. 2018, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kagansky, N.; Berner, Y.; Koren-Morag, N.; Perelman, L.; Knobler, H.; Levy, S. Poor nutritional habits are predictors of poor outcome in very old hospitalized patients. Am. J. Clin. Nutr. 2005, 82, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Gupta, S.; Aggarwal, M.; Jain, V.; Gad, M.M.; Verma, B.R.; Kapadia, S.R. Impact of Malnutrition on Outcomes among Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 141, 157–160. [Google Scholar] [CrossRef]
- Schoenenberger, A.W.; Stortecky, S.; Neumann, S.; Moser, A.; Jüni, P.; Carrel, T.; Huber, C.; Gandon, M.; Bischoff, S.; Schoenenberger, C.-M.; et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur. Heart J. 2012, 34, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Alexander, K.P.; O’Gara, P.T.; Afilalo, J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2014, 7, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Yamamoto, M.; Kano, S.; Sago, M.; Tsunaki, T.; Kagase, A.; Koyama, Y.; Tsujimoto, S.; Otsuka, T.; Yashima, F.; et al. Predictors and Prognostic Impact of Nutritional Changes After Transcatheter Aortic Valve Replacement. Cardiovasc. Revascularization Med. 2020, 23, 68–76. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Kim, D.H.; Afilalo, J.; Shi, S.M.; Popma, J.J.; Khabbaz, K.R.; Laham, R.J.; Grodstein, F.; Guibone, K.; Lux, E.; Lipsitz, L.A. Evaluation of Changes in Functional Status in the Year after Aortic Valve Replacement. JAMA Intern. Med. 2019, 179, 383–391. [Google Scholar] [CrossRef]
- Ribeiro, G.S.; Melo, R.D.; Deresz, L.F.; Lago, P.D.; Pontes, M.R.; Karsten, M. Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: Systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
| Overall Population (n = 100) | Primary Endpoint (n = 28) | No Primary Endpoint (n = 72) | p-Value | |
|---|---|---|---|---|
| Clinical | ||||
| Male, n (%) | 39 (39) | 14 (50) | 25 (35) | 0.160 |
| Age (years) | 84 ± 4 | 84 ± 4 | 84 ± 4 | 0.979 |
| Body mass index (kg/m2) | 27.5 ± 4.9 | 27.3 ± 4.8 | 27.6 ± 5.0 | 0.793 |
| Hypertension, n (%) | 78 (78) | 22 (79) | 56 (78) | 0.931 |
| Diabetes mellitus, n (%) | 24 (24) | 8 (29) | 16 (22) | 0.504 |
| Atrial fibrillation, n (%) | 33 (33) | 8 (29) | 25 (35) | 0.557 |
| Prior MI, n (%) | 15 (15) | 4 (14) | 11 (15) | 0.901 |
| CAD, n (%) | 42 (42) | 12 (43) | 30 (42) | 0.914 |
| Prior stroke or TIA, n (%) | 9 (9) | 4 (14) | 5 (7) | 0.249 |
| Sternotomy, n (%) | 24 (24) | 7 (25) | 17 (24) | 0.884 |
| COPD, n (%) | 19 (19) | 5 (18) | 14 (19) | 0.856 |
| EuroSCORE II | 4.6 [2.8–7.7] | 6.1 [3.7–10.4] | 4.0 [2.7–6.0] | 0.025 |
| STS-PROM | 3.9 [2.6–5.0] | 3.6 [2.5–5.0] | 4.1 [2.7–5.0] | 0.539 |
| Medication, n (%) | ||||
| ADP inhibitor | 7 (7) | 2 (7) | 5 (7) | 0.972 |
| ACE/ARB inhibitor | 57 (57) | 18 (64) | 39 (54) | 0.359 |
| Antidepressants | 8 (8) | 2 (7) | 6 (8) | 0.844 |
| Hemodynamic and laboratory | ||||
| AVA (cm2) | 0.76 ± 0.22 | 0.74 ± 0.16 | 0.76 ± 0.24 | 0.654 |
| PG (mmHg) | 71.3 ± 27.8 | 68.9 ± 19.4 | 72.2 ± 30.5 | 0.593 |
| LVEF (%) | 55 [50–60] | 50 [50–59] | 55 [50–60] | 0.326 |
| TAPSE (mm) | 19.7 ± 5.3 | 19.1 ± 5.9 | 19.9 ± 5.1 | 0.604 |
| SPAP (mmHg) | 42.0 ± 15.3 | 45.4 ± 21.1 | 40.5 ± 11.8 | 0.279 |
| LAVI (mL/m2) | 50.9 ± 15.6 | 57.5 ± 17.2 | 48.7 ± 14.5 | 0.042 |
| eGFR (mL/min/1.73 m2) | 53.2 ± 14.3 | 49.4 ± 14.6 | 54.6 ± 13.9 | 0.100 |
| Hematocrit (%) | 34.5 ± 11.5 | 33.3 ± 11.8 | 35.0 ± 11.5 | 0.509 |
| WBC (×103/mm3) | 7.0 [5.6–8.1] | 7.1 [5.2–8.0] | 7.0 [5.7–8.2] | 0.514 |
| Albumin (g/L) | 39.6 ± 5.5 | 37.8 ± 4.4 | 40.4 ± 5.8 | 0.046 |
| Procedural and hospitalization | ||||
| Full anesthesia, n (%) | 75 (75) | 22 (79) | 53 (74) | 0.607 |
| TF approach, n (%) | 97 (97) | 27 (96) | 70 (97) | 0.835 |
| Length of stay (days) | 8 [6–12] | 10 [6–23] | 8 [6–12] | 0.263 |
| Pacemaker, n (%) | 18 (18) | 6 (21) | 12 (17) | 0.578 |
| MR ≥ 2/4, n (%) | 29 (29) | 10 (36) | 19 (26) | 0.356 |
| AR ≥ 2/4, n (%) | 10 (10) | 2 (8) | 8 (12) | 0.581 |
| Geriatric follow-up, n (%) | 34 (34) | 6 (21) | 28 (39) | 0.098 |
| Overall Population | Primary Endpoint | No Primary Endpoint | p-Value | |
|---|---|---|---|---|
| Katz (n = 97) | 7 [6–8] | 7 [6–8] | 7 [6–8] | 0.818 |
| MMSE (n = 97) | 27 [25–29] | 27 [25–29] | 27 [24–29] | 0.420 |
| Polypharmacy (n = 98) | ||||
| ≥5 medicines | 90 (92) | 24 (89) | 66 (93) | 0.511 |
| Timed up and go (n = 93) | 15.4 ± 9.8 | 13.7 ± 6.9 | 16.1 ± 10.6 | 0.305 |
| ≥20 s | 18 (19) | 5 (20) | 13 (19) | 0.924 |
| Chair stand test (n = 90) | 15.9 ± 9.6 | 14.2 ± 8.7 | 16.4 ± 9.9 | 0.347 |
| ≥14 s | 66 (73) | 16 (70) | 50 (75) | 0.636 |
| Gait speed (n = 75) | 0.83 ± 0.26 | 0.80 ± 0.33 | 0.84 ± 0.24 | 0.393 |
| ≤0.8 m/s | 30 (40) | 7 (39) | 23 (40) | 0.912 |
| Tinetti (n = 81) | 25 [21–27] | 24 [20–27] | 26 [21–27] | 0.711 |
| <20 | 16 (20) | 5 (23) | 11 (19) | 0.681 |
| SPPB (n = 80) | 7.7 ± 2.8 | 8.2 ± 2.4 | 7.6 ± 3.0 | 0.393 |
| <10 | 54 (68) | 14 (70) | 40 (67) | 0.784 |
| Grip strength (n = 68) | 42.5 ± 16.4 | 46.8 ± 14.8 | 41.2 ± 16.8 | 0.235 |
| GDS-15 (n = 91) | 1 [1–4] | 3.5 [1–5] | 1 [0–3] | 0.045 |
| ≥5 | 18 (20) | 9 (38) | 9 (13) | 0.011 |
| Malnutrition (n = 80) | ||||
| BMI < 22 or albumin < 3.5 g/L | 11 (14) | 7 (28) | 4 (7) | 0.013 |
| BMI < 22 | 3 (3) | 2 (7) | 1 (1) | 0.063 |
| Albumin < 3.5 g/L | 11 (14) | 7 (28) | 4 (6) | 0.002 |
| CCI (n = 100) | 5.0 ± 1.1 | 5.1 ± 1.2 | 4.9 ± 1.1 | 0.399 |
| CGA-FI (n = 100) | 0.14 ± 0.05 | 0.15 ± 0.06 | 0.14 ± 0.05 | 0.200 |
| >0.2 | 14 (14) | 5 (18) | 9 (12) | 0.488 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Unadjusted | |||
| GDS-15 ≥ 5 | 3.60 | 1.52–8.52 | 0.004 |
| Malnutrition | 3.67 | 1.44–9.34 | 0.006 |
| Adjusted for age and sex | |||
| GDS-15 ≥ 5 | 5.07 | 2.00–12.87 | <0.001 |
| Malnutrition | 3.83 | 1.45–10.12 | 0.007 |
| Adjusted for EuroSCORE II | |||
| GDS-15 ≥ 5 | 4.15 | 1.72–10.01 | 0.002 |
| Malnutrition | 2.89 | 1.09–7.67 | 0.033 |
| Combined model with EuroSCORE II | |||
| GDS-15 ≥ 5 | 4.38 | 1.79–10.74 | 0.001 |
| Malnutrition | 3.08 | 1.15–8.22 | 0.025 |
| C-Index | ΔC-Index | |
|---|---|---|
| Unadjusted | ||
| GDS-15 | 0.628 | |
| Malnutrition | 0.593 | |
| EuroSCORE II | 0.702 | |
| Adjusted model | ||
| EuroSCORE II + GDS-15 | 0.727 | 0.025 |
| EuroSCORE II + Malnutrition | 0.741 | 0.039 |
| EuroSCORE II + GDS-15 + Malnutrition | 0.746 | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geers, J.; Van den Bussche, K.; Vandeloo, B.; Kimenai, D.M.; Van Loo, I.; Michiels, V.; Plein, D.; Beckers, S.; Muylle, T.; Lieten, S.; et al. Depression and Malnutrition for Prediction of Mortality after Transcatheter Aortic Valve Replacement: A Registry Study of a Tertiary Referral Hospital. Diagnostics 2023, 13, 2561. https://doi.org/10.3390/diagnostics13152561
Geers J, Van den Bussche K, Vandeloo B, Kimenai DM, Van Loo I, Michiels V, Plein D, Beckers S, Muylle T, Lieten S, et al. Depression and Malnutrition for Prediction of Mortality after Transcatheter Aortic Valve Replacement: A Registry Study of a Tertiary Referral Hospital. Diagnostics. 2023; 13(15):2561. https://doi.org/10.3390/diagnostics13152561
Chicago/Turabian StyleGeers, Jolien, Karen Van den Bussche, Bert Vandeloo, Dorien M. Kimenai, Ines Van Loo, Vincent Michiels, Daniele Plein, Stefan Beckers, Teun Muylle, Siddhartha Lieten, and et al. 2023. "Depression and Malnutrition for Prediction of Mortality after Transcatheter Aortic Valve Replacement: A Registry Study of a Tertiary Referral Hospital" Diagnostics 13, no. 15: 2561. https://doi.org/10.3390/diagnostics13152561
APA StyleGeers, J., Van den Bussche, K., Vandeloo, B., Kimenai, D. M., Van Loo, I., Michiels, V., Plein, D., Beckers, S., Muylle, T., Lieten, S., Cosyns, B., Compté, N., & Argacha, J.-F. (2023). Depression and Malnutrition for Prediction of Mortality after Transcatheter Aortic Valve Replacement: A Registry Study of a Tertiary Referral Hospital. Diagnostics, 13(15), 2561. https://doi.org/10.3390/diagnostics13152561






