Eosinophilic Cholecystitis and Eosinophils in Gallbladder Injuries: A Clinicopathological Analysis of 1050 Cholecystectomies
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Patterns of Injury
2.3. Assessment of Eosinophils
2.4. Correlative Parameters
2.5. Statistical Analysis
3. Results
3.1. Incidence
3.2. Clinical Associations
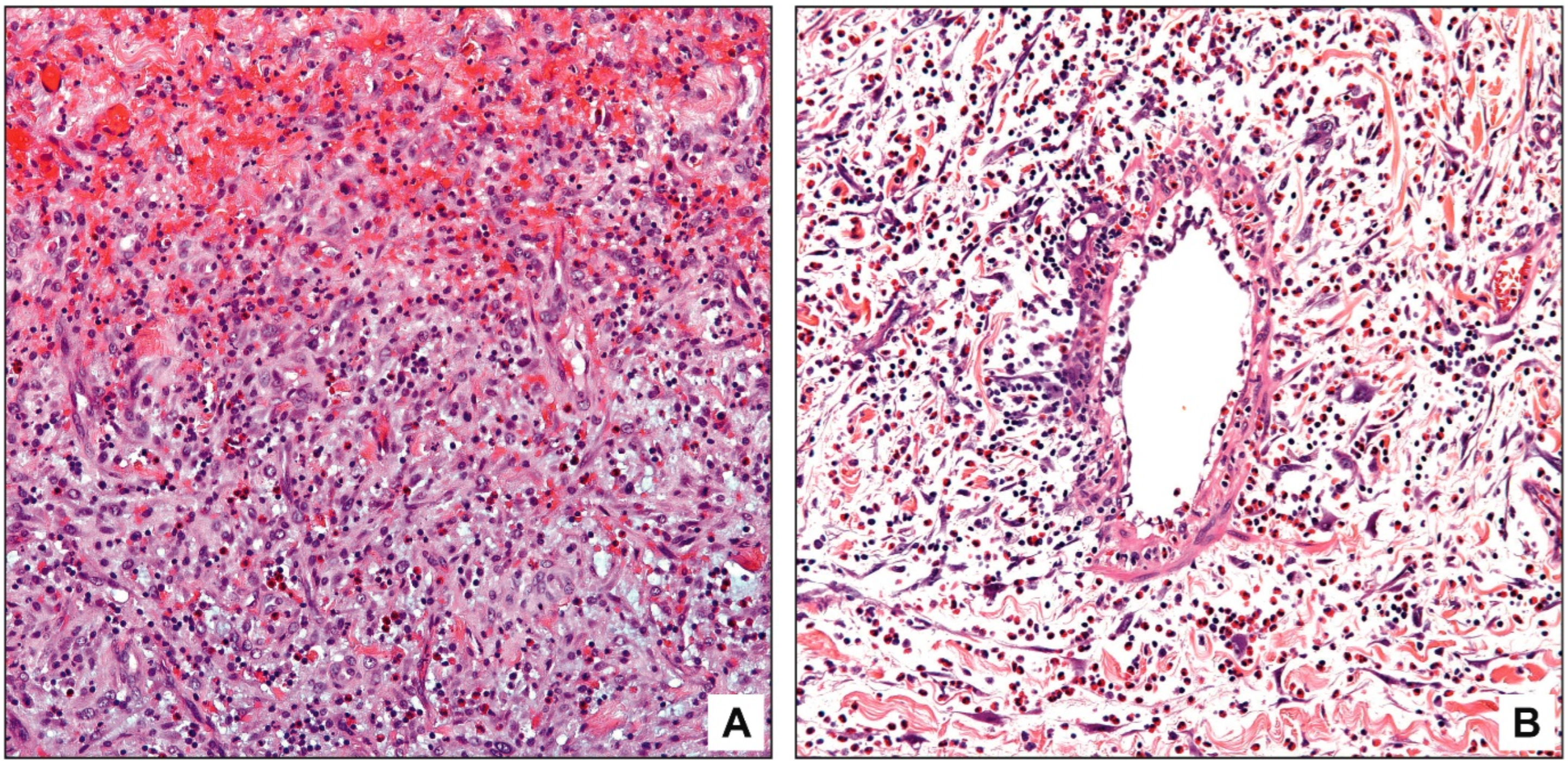
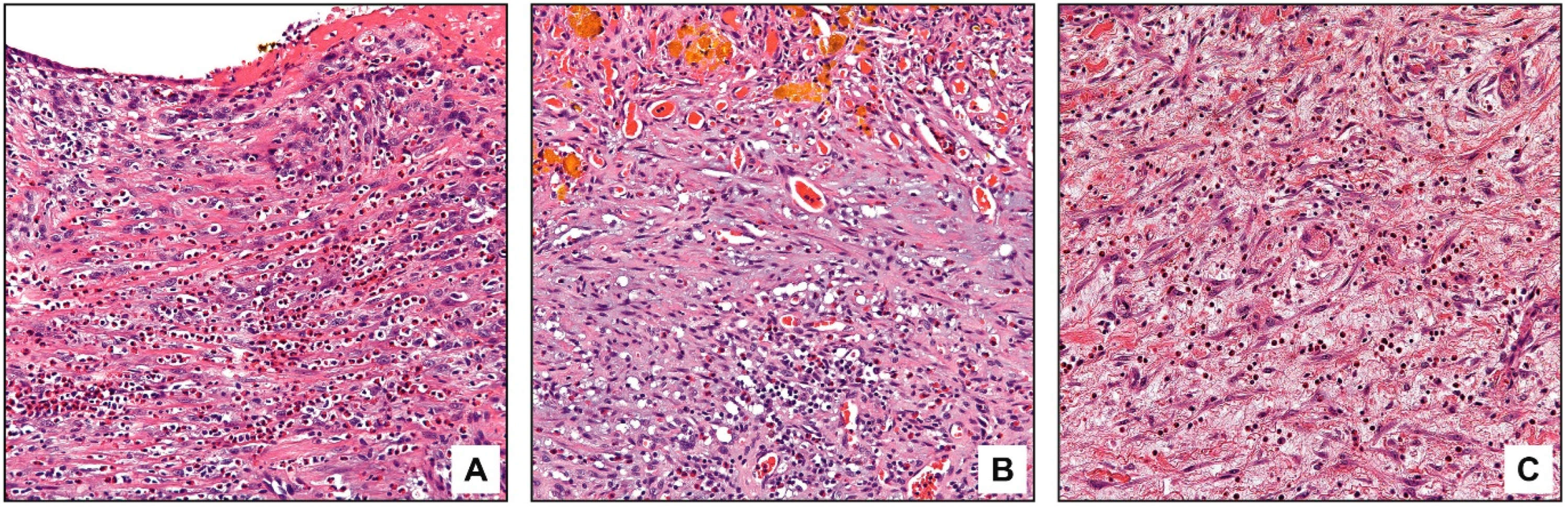
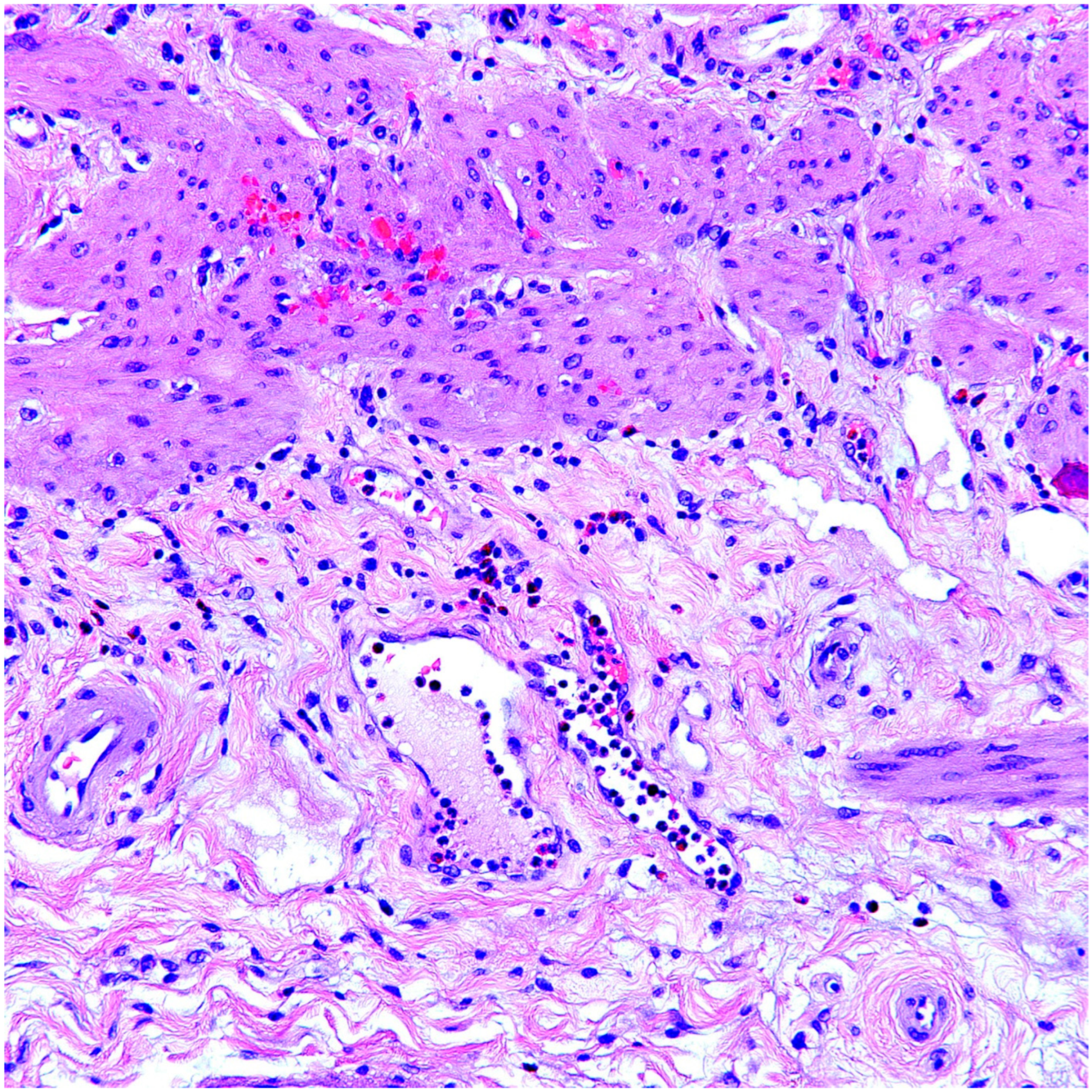
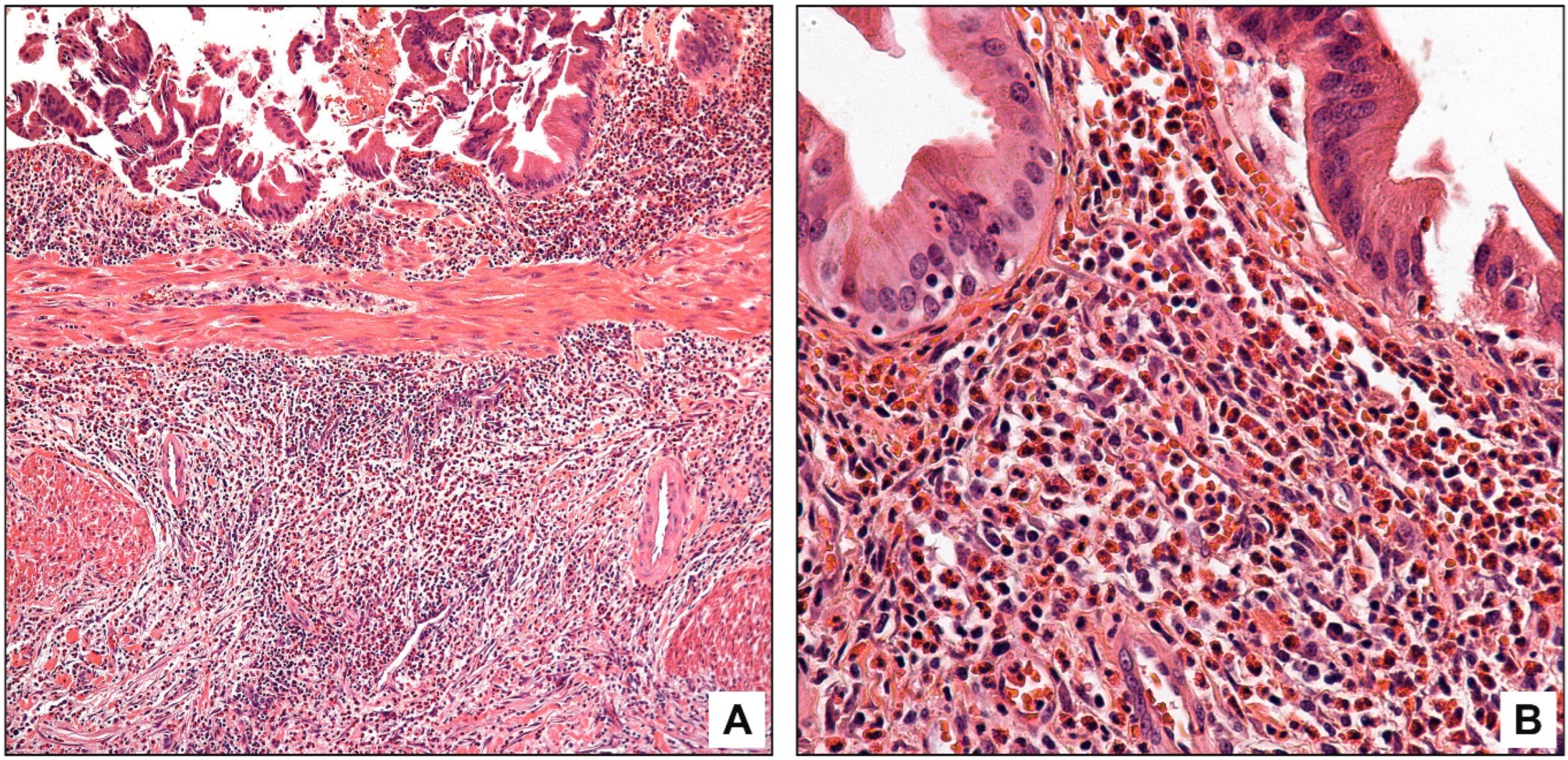
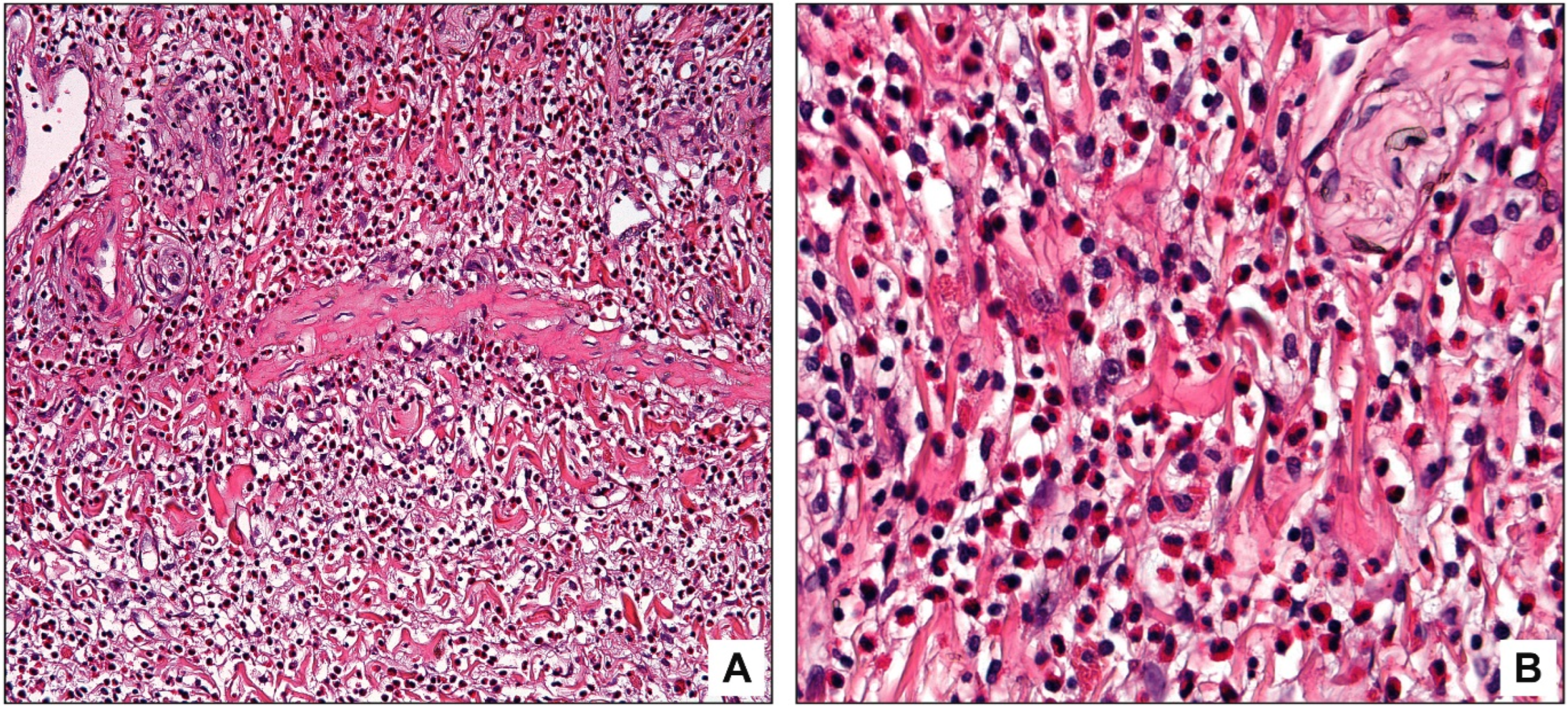
3.3. Pathologic Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adsay, V. Gallbladder, Extrahepatic Bile Ducts and Ampulla. In Sternberg’s Diagnostic Surgical Pathology, 6th ed.; Mills, S.E., Greenson, J.K., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2015. [Google Scholar]
- Basturk, O.; Adsay, V. Diseases of the Gallbladder. In MacSween’s Pathology of the Liver, 8th ed.; Burt, A.D., Ferrell, L.D., Hu, S.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Albot, G.; Poilleux, F. Eosinophilic cholecystitis. Presse Med. 1949, 57, 558. [Google Scholar]
- Sánchez-Pobre, P.; Colina, F.; Yela, C.; Manzano, M.; Rodriguez, S.; Martin, A.; Casís, B.; Garfia, C.; Castellano, G.; Solís-Herruzo, J.A. Eosinophilic cholecystitis: An infrequent cause of cholecystectomy. Gastroenterol. Hepatol. 1997, 20, 21–23. [Google Scholar] [PubMed]
- Dabbs, D.J. Eosinophilic and lymphoeosinophilic cholecystitis. Am. J. Surg. Pathol. 1993, 17, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hassan, M.J.; JairaJPuri, Z.S.; Jetley, S.; Husain, M. Clinicopathological Study of Eosinophilic Cholecystitis: Five Year Single Institution Experience. J. Clin. Diagn. Res. 2017, 11, EC20–EC23. [Google Scholar] [CrossRef]
- Gutierrez-Moreno, L.I.; Trejo-Avila, M.E.; Díaz-Flores, A.; Davila-Zenteno, M.R.; Montoya-Fuentes, I.M.; Cardenas-Lailson, L.E. Eosinophilic cholecystitis: A retrospective study spanning a fourteen-year period. Rev. Gastroenterol. México (Engl. Ed.) 2018, 83, 405–409. [Google Scholar] [CrossRef]
- Kumar, V.; Verma, R.; Dawan, S.; Choudhary, M.; Verma, S.; Dhaka, J.P. Incidence of eosinophilic cholecystitis in northwestern region of Rajasthan: A study in consecutive 867 cholecystectomies. Int. J. Sci. Study 2016, 4, 147–150. [Google Scholar]
- Yeom, S.S.; Kim, H.H.; Kim, J.C.; Hur, Y.H.; Koh, Y.S.; Cho, C.K.; Kim, H.J.; Shin, S.S.; Kim, H.S. Peripheral eosinophilia—Is it a predictable factor associated with eosinophilic cholecystitis? Korean J. Hepato-Biliary-Pancreat. Surg. 2012, 16, 65–69. [Google Scholar] [CrossRef][Green Version]
- Garzón, G.L.; Jaramillo, B.L.; Valero, H.J.; Quintero, C.E. Eosinophilic cholecystitis in children: Case series. J. Pediatr. Surg. 2021, 56, 550–552. [Google Scholar] [CrossRef]
- Parry, S.W.; Pelias, M.E.; Browder, W. Acalculous hypersensitivity cholecystitis: Hypothesis of a new clinicopathologic entity. Surgery 1988, 104, 911–916. [Google Scholar]
- Lwin, T.; Melton, S.D.; Genta, R.M. Eosinophilic gastritis: Histopathological characterization and quantification of the normal gastric eosinophil content. Mod. Pathol. 2011, 24, 556–563. [Google Scholar] [CrossRef]
- Kuwahara, T.; Kobayashi, Y.; Yun, Y.; Kanda, A.; Asako, M.; Ueki, S.; Iwai, H. Eosinophilic Cholecystitis Occurred in a Patient With Refractory Eosinophilic Airway Inflammation: A Case Report. Allergy Rhinol. 2019, 10, 2152656719869607. [Google Scholar] [CrossRef]
- Shakov, R.; Simoni, G.; Villacin, A.; Baddoura, W. Eosinophilic cholecystitis, with a review of the literature. Ann. Clin. Lab. Sci. 2007, 37, 182–185. [Google Scholar]
- Yantiss, R.K. Eosinophils in the GI tract: How many is too many and what do they mean? Mod. Pathol. 2015, 28 (Suppl. 1), S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Mainwaring, A.R. Eosinophilic infiltration of the gallbladder. Gastroenterology 1972, 63, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Saka, B.; Memis, B.; Seven, I.E.; Pehlivanoglu, B.; Balci, S.; Bagci, P.; Reid, M.; Dursun, N.; Escalano, O.T.; Roa, J.C.; et al. Follicular Cholecystitis: Reappraisal of Incidence, Definition, and Clinicopathologic Associations in an Analysis of 2550 Cholecystectomies. Int. J. Surg. Pathol. 2020, 28, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Lee, B.; Parekh, K.; Farrelly, P.A. Acalculous eosinophilic cholecystitis from herbal medicine: A review of adverse effects of herbal medicine in surgical patients. Surgery 2002, 131, 352–356. [Google Scholar] [CrossRef]
- Shah, N.; Shah, B.; Khalighi, K.; Debnath, R. Acne Treatment Gone Erratic: A Rare Case of Minocycline-induced Eosinophilic Cholecystitis, Myopericarditis, and CNS Vasculitis: 2469. Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, S1371–S1372. [Google Scholar] [CrossRef]
- Hepburn, A.; Coady, A.; Livingstone, J.; Pandit, N. Eosinophilic cholecystitis as a possible late manifestation of the eosinophilia-myalgia syndrome. Clin. Rheumatol. 2000, 19, 470–472. [Google Scholar] [CrossRef]
- Gutierrez-Alvarez, M.; Vallarta, S.; Cruz, R.; Visag-Castillo, V. Eosinophilic Cholecystitis as an Atypical Etiology of Biliary Colic: A Case Report and Review of the Literature. Cureus 2023, 15, e35831. [Google Scholar] [CrossRef]
- Kasprzak, A.; Malkowski, W.; Biczysko, W.; Seraszek, A.; Sterzyńska, K.; Zabel, M. Histological alterations of gallbladder mucosa and selected clinical data in young patients with symptomatic gallstones. Pol. J. Pathol. 2011, 62, 41–49. [Google Scholar]
- Hui, C.K. Eosinophilic gastroenteritis complicating acute calculus eosinophilic cholecystitis 6 months after cholecystectomy. J. Dig. Dis. 2018, 19, 693–695. [Google Scholar] [CrossRef]
- Adachi, W.; Kishimoto, K.; Shiozawa, H.; Komatsu, O.; Matsushita, T.; Fukushima, M. Eosinophilic gastroenteritis with eosinophilic infiltration of the gall bladder. Clin. J. Gastroenterol. 2008, 1, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.J. Eosinophilic cholecystitis caused by Ascaris lumbricoides. World J. Gastroenterol. 2008, 14, 2783. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chin, C.; Chung, H.C.; Liu, H.; Hwang, J.C.; Lin, H.H. Clonorchiasis-associated perforated eosinophilic cholecystitis. Am. J. Trop. Med. Hyg. 2007, 76, 396–398. [Google Scholar] [CrossRef]
- Shah, O.J.; Shah, P. Ascariasis-induced eosinophilic cholecystitis—A unique case. HPB 2006, 8, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Nagata, H. Idiopathic hypereosinophilic syndrome manifesting with eosinophilic cholecystitis and recurrent gastroenteritis. Ann. Hematol. 2017, 96, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, X.; Liu, Y. Eosinophilic Granulomatosis with Polyangiitis Presenting with Skin Rashes, Eosinophilic Cholecystitis, and Retinal Vasculitis. Am. J. Case Rep. 2016, 17, 864–868. [Google Scholar] [CrossRef][Green Version]
- Ito, H.; Mishima, Y.; Cho, T.; Ogiwara, N.; Shinma, Y.; Yokota, M.; Anzai, K.; Tsuda, S.; Nagata, J.; Kojima, S.; et al. Eosinophilic Cholecystitis Associated with Eosinophilic Granulomatosis with Polyangiitis. Case Rep. Gastroenterol. 2020, 14, 668–674. [Google Scholar] [CrossRef]
- Kaji, K.; Yoshiji, H.; Yoshikawa, M.; Yamazaki, M.; Ikenaka, Y.; Noguchi, R.; Sawai, M.; Ishikawa, M.; Mashitani, T.; Kitade, M.; et al. Eosinophilic cholecystitis along with pericarditis caused by Ascaris lumbricoides: A case report. World J. Gastroenterol. 2007, 13, 3760–3762. [Google Scholar] [CrossRef]
- Tajima, K.; Katagiri, T. Deposits of eosinophil granule proteins in eosinophilic cholecystitis and eosinophilic colitis associated with hypereosinophilic syndrome. Dig. Dis. Sci. 1996, 41, 282–288. [Google Scholar] [CrossRef]
- Tenner, S.; Roston, A.; Lichtenstein, D.; Brooks, D.; Herlihy, E.; Carr-Locke, D. Eosinophilic cholangiopathy. Gastrointest. Endosc. 1997, 45, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Miura, F.; Asano, T.; Amano, H.; Yoshida, M.; Toyota, N.; Wada, K.; Kato, K.; Takada, T.; Fukushima, J.; Kondo, F.; et al. Resected case of eosinophilic cholangiopathy presenting with secondary sclerosing cholangitis. World J. Gastroenterol. 2009, 15, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
| Acute Cholecystitis (n = 55) | Subacute Cholecystitis (n = 100) | Chronic Cholecystitis (n = 895) | p Value | ||
|---|---|---|---|---|---|
| Ordinary Chronic Cholecystitis (n = 885) | Eosinophilic Cholecystitis (n = 10) | ||||
| Mean age (year) | 52.5 | 51 | 49 | 43 | 0.23 |
| Female/Male | 1.5 | 2.5 | 3 | 9 | 0.05 |
| Cholelithiasis (%) | 72 | 79 | 82 | Patchy pattern: 100 Diffuse pattern: 50 | 0.85 |
| Mean wall thickness of cases with noticeable eosinophils (mm) | 6.6 | 5.9 | 4.2 | Patchy pattern: 3.5 Diffuse pattern: 3.2 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memis, B.; Saka, B.; Roa, J.C.; Bandyopadhyay, S.; Reid, M.; Bagci, P.; Aktas, B.K.; Armutlu, A.; Basturk, O.; Adsay, N.V. Eosinophilic Cholecystitis and Eosinophils in Gallbladder Injuries: A Clinicopathological Analysis of 1050 Cholecystectomies. Diagnostics 2023, 13, 2559. https://doi.org/10.3390/diagnostics13152559
Memis B, Saka B, Roa JC, Bandyopadhyay S, Reid M, Bagci P, Aktas BK, Armutlu A, Basturk O, Adsay NV. Eosinophilic Cholecystitis and Eosinophils in Gallbladder Injuries: A Clinicopathological Analysis of 1050 Cholecystectomies. Diagnostics. 2023; 13(15):2559. https://doi.org/10.3390/diagnostics13152559
Chicago/Turabian StyleMemis, Bahar, Burcu Saka, Juan Carlos Roa, Sudeshna Bandyopadhyay, Michelle Reid, Pelin Bagci, Berk Kaan Aktas, Ayse Armutlu, Olca Basturk, and N. Volkan Adsay. 2023. "Eosinophilic Cholecystitis and Eosinophils in Gallbladder Injuries: A Clinicopathological Analysis of 1050 Cholecystectomies" Diagnostics 13, no. 15: 2559. https://doi.org/10.3390/diagnostics13152559
APA StyleMemis, B., Saka, B., Roa, J. C., Bandyopadhyay, S., Reid, M., Bagci, P., Aktas, B. K., Armutlu, A., Basturk, O., & Adsay, N. V. (2023). Eosinophilic Cholecystitis and Eosinophils in Gallbladder Injuries: A Clinicopathological Analysis of 1050 Cholecystectomies. Diagnostics, 13(15), 2559. https://doi.org/10.3390/diagnostics13152559









