The Clinical and Microbiological Effects of LANAP Compared to Scaling and Root Planing Alone in the Management of Periodontal Conditions
Abstract
1. Introduction
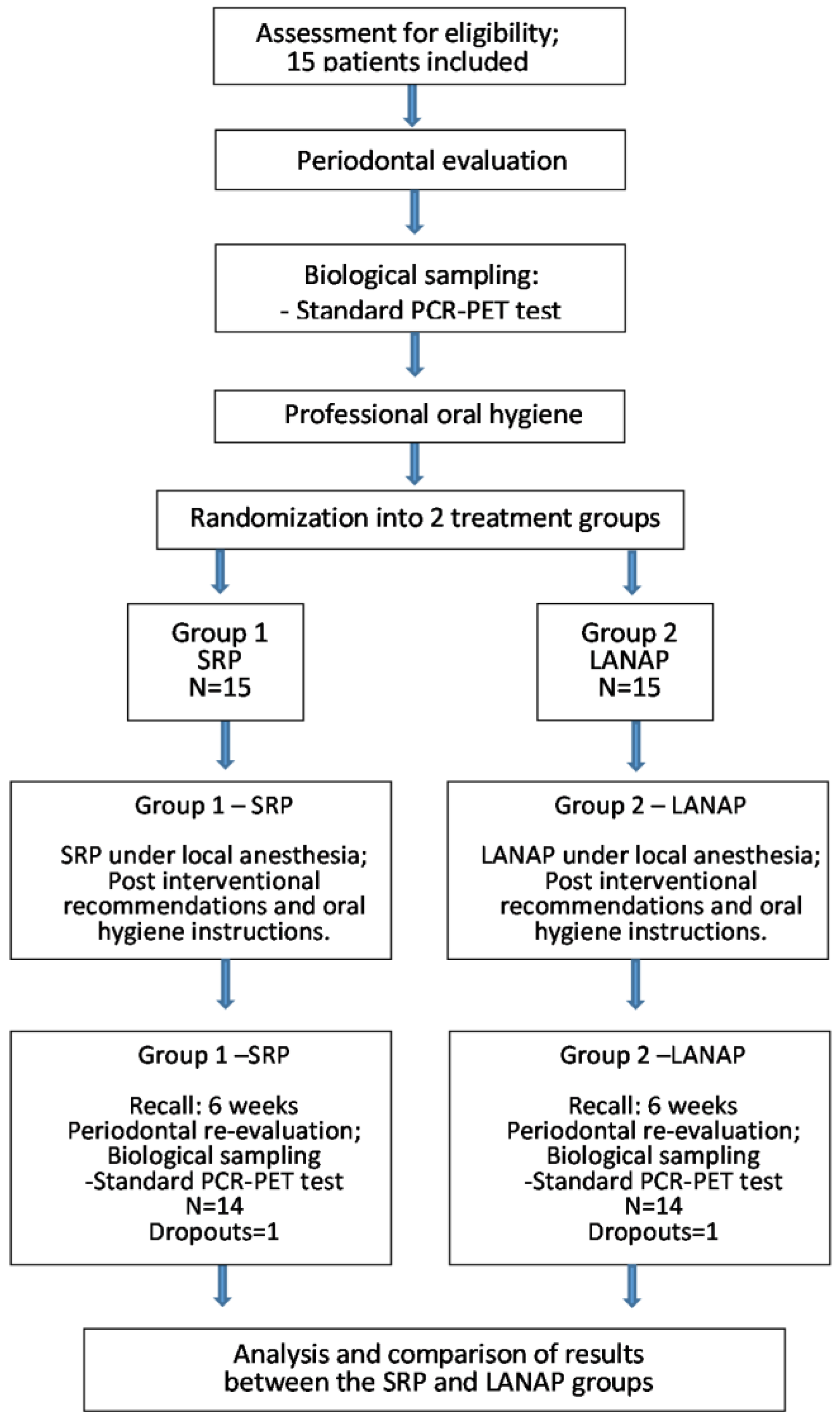
2. Materials and Methods
2.1. Participants
- -
- Specialist consultation, assessment of eligibility, the recording of periodontal parameters in a periodontal chart, and complementary radiographic examinations (OPGs) were carried out;
- -
- Patients were informed of the stage of the condition at the time of presentation;
- -
- Patients with at least stage II, grade B, localised periodontitis, and periodontal pocket depths of at least 4 mm, were selected according to the inclusion and exclusion criteria;
- -
- The selected patients were informed about the requirements of the study and the implementation of the treatment plan, and informed consent was obtained;
- -
- The first biological sampling was carried out using a standard PCR-PET test;
- -
- Professional dental cleaning was then performed;
- -
- The selected teeth of the patients were randomly divided into two groups;
- -
- SRP was performed on the first group of teeth included in the study and in the remaining quadrants that were not included in the study;
- -
- The LANAP protocol was applied to the second group of teeth;
- -
- Patients underwent training on the implementation of correct dental hygiene procedures at home;
- -
- All patients received postinterventional instructions and the recommendation to use chlorhexidine mouthwash twice a day for two weeks after performing brushing at home. The Bass brushing technique was recommended, with soft bristle manual brushes and fluoride toothpaste;
- -
- A new recording of dental and periodontal status and a second standard PCR-PET test for both groups of teeth included in the study were performed after six weeks.
2.2. Clinical Evaluation and Treatment
2.3. Microbiological Assessment
2.4. Statistical Analysis
3. Results
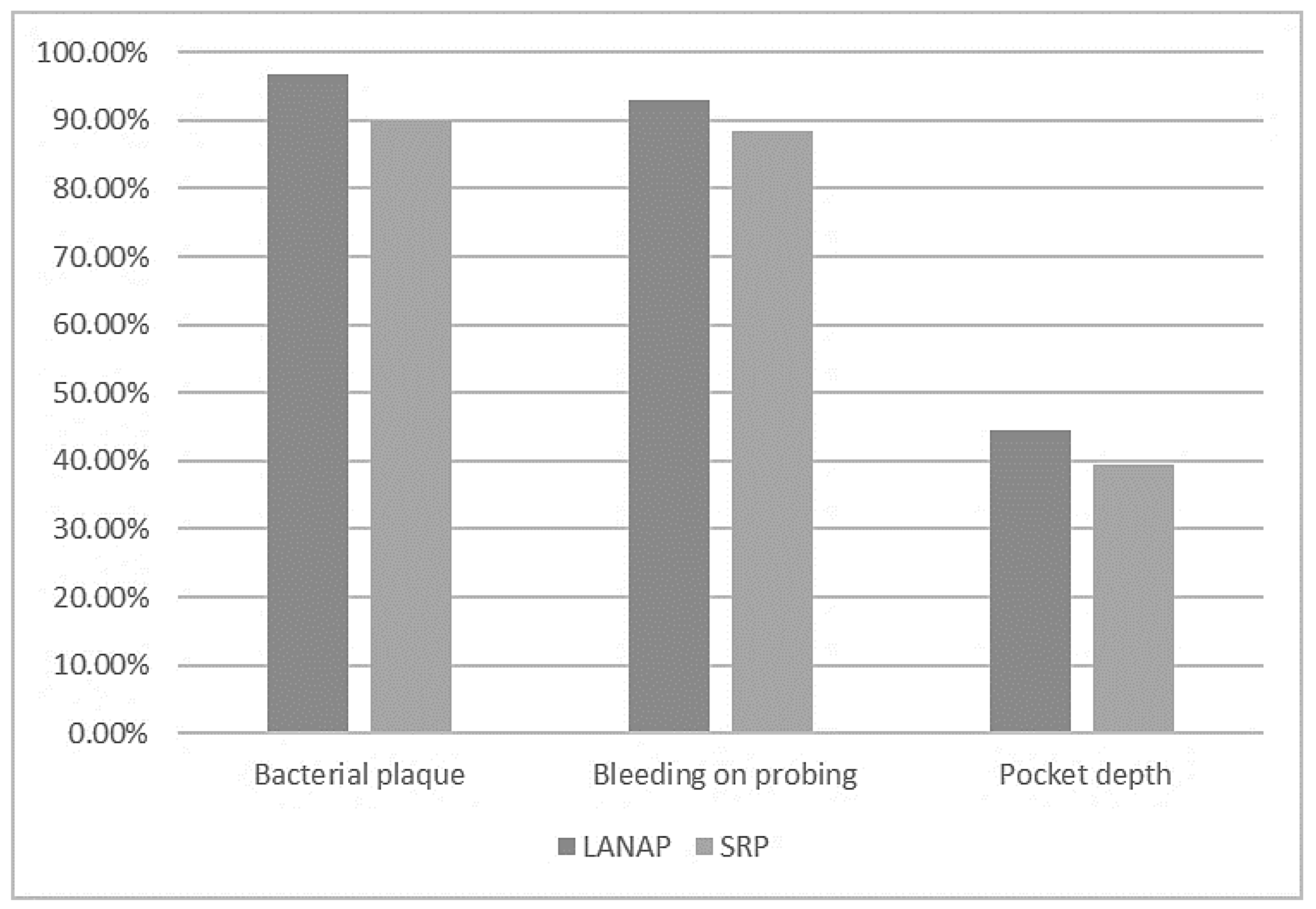
3.1. Clinical Results
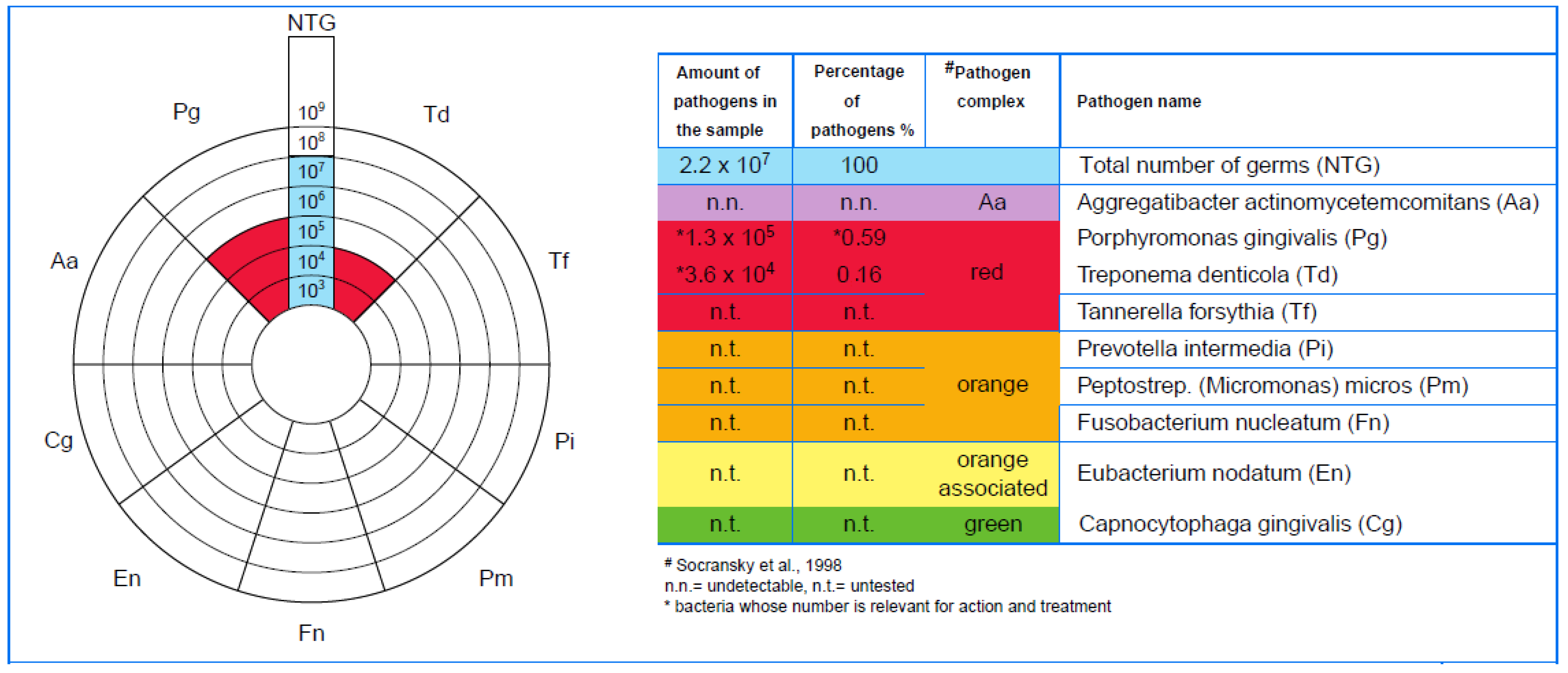
3.2. Microbiological Results
- -
- The amount of Porphyromonas gingivalis (0.05 significance level for LANAP vs. 0.1 for SRP);
- -
- The periodontal pocket depth (44.4% reduction for LANAP vs. 39.5% for SRP);
- -
- The dental plaque index (96.87% reduction for LANAP vs. 90% for SRP);
- -
- The bleeding on the probing index (92.85% reduction for LANAP vs. 88.33% for SRP).
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554590/ (accessed on 30 May 2023).
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontol 2000 2020, 84, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Nirupama, C.; Reddy, P.K.; Koppolu, P.; Alotaibi, D.H. Effect of iatrogenic factors on periodontal health: An epidemiological study. Saudi Dent. J. 2020, 32, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Iarca, R.A.; Mihai, B.M.; Salmen, T.; Dima, V.; Bohiltea, R.E. The management of patients with periodontal disease and anxiety–case report. Rom. J. Stomatol. 2021, 67, 236. [Google Scholar] [CrossRef]
- Muñoz-Carrillo, L.J.; Hernández-Reyes, V.E.; García-Huerta, O.E.; Chávez-Ruvalcaba, F.; Chávez-Ruvalcaba, M.I.; Chávez-Ruvalcaba, K.M.; Díaz-Alfaro, L. Pathogenesis of Periodontal Disease, Periodontal Disease—Diagnostic and Adjunctive Non-Surgical Considerations; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Soderling, F.; Torabi, J. The A, B, Cs, and I, II, and IIIs of Periodontitis Staging and Grading. CDHA J. 2019, 37, 22–28. [Google Scholar]
- Tsai, C.Y.; Tang, C.Y.; Tan, T.S.; Chen, K.H.; Liao, K.H.; Liou, M.L. Subgingival microbiota in individuals with severe chronic periodontitis. J. Microbiol. Immunol. Infect. 2018, 51, 226–234. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.homd.org (accessed on 18 June 2023).
- Jastrząb, B.; Paśnik-Chwalik, B.; Dębska-Łasut, K.; Konopka, T.; Krajewski, P.K.; Szepietowski, J.C.; Matusiak, Ł. The Composition of Subgingival Microbiome in Hidradenitis Suppurativa and Periodontitis Patients. Pathogens 2023, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol 2000 2023. Early View. [Google Scholar] [CrossRef]
- Badanian, A.B.L.; Papone, V. Comparative bacterial analysis of chronic and aggressive periodontitis in a sample population from Uruguay. Odontoestomatología 2019, 21, 5–13. [Google Scholar] [CrossRef]
- Howard, K.C.; Gonzalez, O.A.; Garneau-Tsodikova, S. Porphyromonas gingivalis: Where do we stand in our battle against this oral pathogen? RSC Med. Chem. 2021, 12, 666–704. [Google Scholar] [CrossRef]
- Schulz, S.; Stein, J.M.; Schumacher, A.; Kupietz, D.; Yekta-Michael, S.S.; Schittenhelm, F.; Conrads, G.; Schaller, H.-G.; Reichert, S. Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota. J. Clin. Med. 2022, 11, 1187. [Google Scholar] [CrossRef]
- Roccuzzo, A.; De Ry, S.P.; Sculean, A.; Roccuzzo, M.; Salvi, G.E. Current Approaches for the Non-surgical Management of Peri-implant Diseases. Curr. Oral Health Rep. 2020, 7, 274–282. [Google Scholar] [CrossRef]
- Salehuddin, N.Q.; Sabri, B.A.M.; Ariffin, F. Patients’ view on non-surgical and surgical periodontal therapy in relation to oral health: A narrative review. Dent. Rev. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Pardo, A.; Butera, A.; Giordano, A.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Abu-Ta’a, M.; Karameh, R. Laser and Its Application in Periodontology: A Review of Literature. Open J. Stomatol. 2022, 12, 305–320. [Google Scholar] [CrossRef]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A Review With Their Applications in Oral Medicine. J. Lasers Med. Sci. 2019, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, S.; Qayyum, H.; Rafaqat, Z.; Qadir, A.; Fayyaz, S.; Khan, A.; Jabeen, H.; Muhammad, N.; Khan, A.M. Laser as an innovative tool, its implications and advances in dentistry: A systematic review. J. Photochem. Photobiol. 2022, 12, 100148. [Google Scholar] [CrossRef]
- Silva, A.S.; Nunes, A.M.M.; Neves, P.A.M.; Lago, A.D.N. Use of high-power lasers in pediatric dental surgeries: Case reports. Gen. Dent. 2022, 70, 56–59. [Google Scholar] [PubMed]
- Varma, S.R.; AlShayeb, M.; Narayanan, J.; Abuhijleh, E.; Hadi, A.; Jaber, M.; Abu Fanas, S. Applications of Lasers in Refractory Periodontitis: A Narrative Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Dortaj, D.; Bassir, S.H.; Hakimiha, N.; Hong, H.; Aslroosta, H.; Fekrazad, R.; Moslemi, N. Efficacy of Nd:YAG laser-assisted periodontal therapy for the management of periodontitis: A double-blind split-mouth randomized controlled clinical trial. J. Periodontol. 2022, 93, 662–672. [Google Scholar] [CrossRef]
- Markou, N.; Papadelli, A.; Nikolidakis, D.; Pepelassi, E.; Madianos, P.; Karoussis, I.K. Adjunctive Nd:YAG laser irradiation in the treatment of stage III/IV periodontitis: A 12-month, randomized, controlled trial. Clin. Oral Investig. 2023, 27, 3045–3056. [Google Scholar] [CrossRef]
- Laky, M.; Müller, M.; Laky, B.; Arslan, M.; Wehner, C.; Husejnagic, S.; Lettner, S.; Moritz, A.; Rausch-Fan, X. Short-term results of the combined application of neodymium-doped yttrium aluminum garnet (Nd:YAG) laser and erbium-doped yttrium aluminum garnet (Er:YAG) laser in the treatment of periodontal disease: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 6119–6126. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Gupta, V.; Adinarayan, R. LANAP, Periodontics and Beyond: A Review. J. Lasers Med. Sci. 2018, 9, 76–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajesh, K.S.; Siva Pillai, A.S.; Shashikanth, H.; Kumar, A.M.S.; Boloor, V. The LANAP Procedure—An Update. IOSR J. Dent. Med. Sci. IOSR-JDMS 2021, 20, 1–6. [Google Scholar]
- Srimaneepong, V.; Heboyan, A.; Zafar, M.S.; Khurshid, Z.; Marya, A.; Fernandes, G.V.O.; Rokaya, D. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J. Funct. Biomater. 2022, 13, 15. [Google Scholar] [CrossRef]
- Rakhewar, P.S.; Birla, A.; Thorat, M.; Chacko, L.; Patil, S.; Muley, A.; Bhalla, N. Application of Lasers in Periodontics: True Innovation or Myth? IOSR J. Dent. Med. Sci. IOSR-JDMS 2019, 18, 58–66. [Google Scholar]
- Bakar, O.; Lektemür Alpan, A.; Akar, T. Management of Gingival Recession around the Prosthesis. Arch. Basic Clin. Res. 2019, 1, 34–36. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mappangara, S.; Oktawati, S.; Chandha, M.H.; Hatta, R. Antimicrobial properties of laser treatment in periodontal therapy. J. Phys. Conf. Ser. 2018, 1073, 052017. [Google Scholar] [CrossRef]
- El Mobadder, M.; Namour, A.; Nammour, S. Laser-Assisted Non-Surgical Treatments of Periodontitis. Encyclopedia 2023, 3, 458–467. [Google Scholar] [CrossRef]
- McCawley, T.K.; McCawley, M.N.; Rams, T.E. Immediate effect of Nd:YAG laser monotherapy on subgingival periodontal pathogens: A pilot clinical study. J. Periodontal Implant. Sci. 2022, 52, 77–87. [Google Scholar] [CrossRef]
- Agop-Forna, D.; Sălceanu, M.; Topoliceanu, C.; Creţu, C.; Vasincu, D.; Forna, N. Dental lasers in restorative dentistry: A review. Rom. J. Oral Rehabil. RJOR 2021, 13, 7–17. [Google Scholar]
- Giannelli, M.; Lasagni, M.; Bani, D. Photonic Therapy in Periodontal Diseases an Overview with Appraisal of the Literature and Reasoned Treatment Recommendations. Int. J. Mol. Sci. 2019, 20, 4741. [Google Scholar] [CrossRef]
- Cios, A.; Cieplak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Özberk, S.S.; Gündoğar, H.; Özkaya, M.; Taner, I.L.; Erciyas, K. The effect of photobiomodulation therapy on nonsurgical periodontal treatment in patients with type 2 diabetes mellitus: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2019, 35, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Bani, D.; Viti, C.; Tani, A.; Lorenzini, L.; Zecchi-Orlandini, S.; Formigli, L. Comparative evaluation of the effects of different photoablative laser irradiation protocols on the gingiva of periodontopathic patients. Photomed. Laser Surg. 2012, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A.; Carr, R.L.; Evans, G.H. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int. J. Periodontics Restor. Dent. 2007, 27, 577–587. [Google Scholar]
- Yukna, R.A. Clinical evaluation of Laser-Assisted New Attachment Procedure® (LANAP®) surgical treatment of chronic periodontitis: A retrospective case series of 1-year results in 22 consecutive patients. J. Periodontal Implant. Sci. 2022, 53, 173. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Du, J.; Fu, J.; Liu, Y.; Guo, L.; Liu, Y. Clinical and biochemical effect of laser as an adjunct to non-surgical treatment of chronic periodontitis. Oral Dis. 2022, 28, 1042–1057. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Sculean, A.; Gašpirc, B. Laser reduction of specific microorganisms in the periodontal pocket using Er:YAG and Nd:YAG lasers: A randomized controlled clinical study. Lasers Med. Sci. 2018, 33, 1461–1470. [Google Scholar] [CrossRef]
- Available online: https://www.pet-mip.de/en/ (accessed on 24 May 2023).
- Ito, T.; Mori, G.; Oda, Y.; Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Yajima, Y. Clinical evaluation of periodontal pathogen levels by real-time polymerase chain reaction in peri-implantitis patients. Int. J. Implant. Dent. 2021, 7, 105. [Google Scholar] [CrossRef]
- Shirmohammadi, A.; Babaloo, A.; Maleki Dizaj, S.; Lotfipour, F.; Sharifi, S.; Ghavimi, M.A.; Khezri, K. A View on Polymerase Chain Reaction as an Outstanding Molecular Diagnostic Technique in Periodontology. Biomed. Res. Int. 2021, 19, 9979948. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Kumar Mujawdiya, P.; Noor, S.; Gasmi, A. Porphyromonas Gingivalis in the Development of Periodontitis: Impact on Dysbiosis and Inflammation. Arch. Razi Inst. 2022, 77, 1539–1551. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]

| Procedure | Variables | Before Treatment | After Treatment | p-Value | ||
|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | |||
| LANAP | Periodontal pocket depth (mm) | 6.4286 | 1.98898 | 3.5714 | 1.01635 | 0 |
| Visible plaque index | 2.2857 | 1.81568 | 0.0714 | 0.26726 | 0 | |
| Bleeding on probing | 4 | 1.83973 | 0.2857 | 0.82542 | 0 | |
| SRP | Periodontal pocket depth (mm) | 5.7857 | 0.97496 | 3.5 | 0.75955 | 0 |
| Visible plaque index | 2.8571 | 2.34872 | 0.2857 | 0.46881 | 0.001 | |
| Bleeding on probing | 4.2857 | 1.5898 | 0.5 | 0.75955 | 0 | |
| Variables | LANAP | SRP | p-Value | ||
|---|---|---|---|---|---|
| Mean | Std | Mean | Std | ||
| Periodontal pocket depth (mm) | 2.8571 | 1.70326 | 2.3571 | 1.27745 | 0.302 |
| Visible plaque index | 2.2143 | 1.71772 | 2.5714 | 2.1018 | 0.413 |
| Bleeding on probing | 3.7143 | 1.72888 | 3.7857 | 1.84718 | 0.808 |
| Treatment | Variable | Before | After | p-Value | ||
|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | |||
| LANAP | Aggregatibacter actinomycetemcomitans | 58.5714 | 219.15422 | 0 | 0 | 0.336 |
| Porphyromonas gingivalis | 699,385.7143 | 788,725.1972 | 50,660 | 97,481.48353 | 0.007 | |
| Treponema denticola | 158,746.1538 | 142,394.5377 | 127,672.31 | 386,171.8167 | 0.79 | |
| Total number of pathogens | 463,607,142.9 | 261,684,884 | 302,575,000 | 787,427,411.5 | 0.144 | |
| SRP | Aggregatibacter actinomycetemcomitans | 0 | 0 | 3538.4615 | 12,758.10451 | 0.337 |
| Porphyromonas gingivalis | 436,246.1538 | 568,000.8988 | 129,896.92 | 336,900.2959 | 0.052 | |
| Treponema denticola | 113,942.8571 | 30,612.73475 | 43,185.714 | 105,652.3898 | 0.11 | |
| Total number of pathogens | 363,428,571.4 | 651,730,561.9 | 357,499,286 | 763,468,672.3 | 0.984 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechir, E.S. The Clinical and Microbiological Effects of LANAP Compared to Scaling and Root Planing Alone in the Management of Periodontal Conditions. Diagnostics 2023, 13, 2450. https://doi.org/10.3390/diagnostics13142450
Bechir ES. The Clinical and Microbiological Effects of LANAP Compared to Scaling and Root Planing Alone in the Management of Periodontal Conditions. Diagnostics. 2023; 13(14):2450. https://doi.org/10.3390/diagnostics13142450
Chicago/Turabian StyleBechir, Edwin Sever. 2023. "The Clinical and Microbiological Effects of LANAP Compared to Scaling and Root Planing Alone in the Management of Periodontal Conditions" Diagnostics 13, no. 14: 2450. https://doi.org/10.3390/diagnostics13142450
APA StyleBechir, E. S. (2023). The Clinical and Microbiological Effects of LANAP Compared to Scaling and Root Planing Alone in the Management of Periodontal Conditions. Diagnostics, 13(14), 2450. https://doi.org/10.3390/diagnostics13142450




