Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling
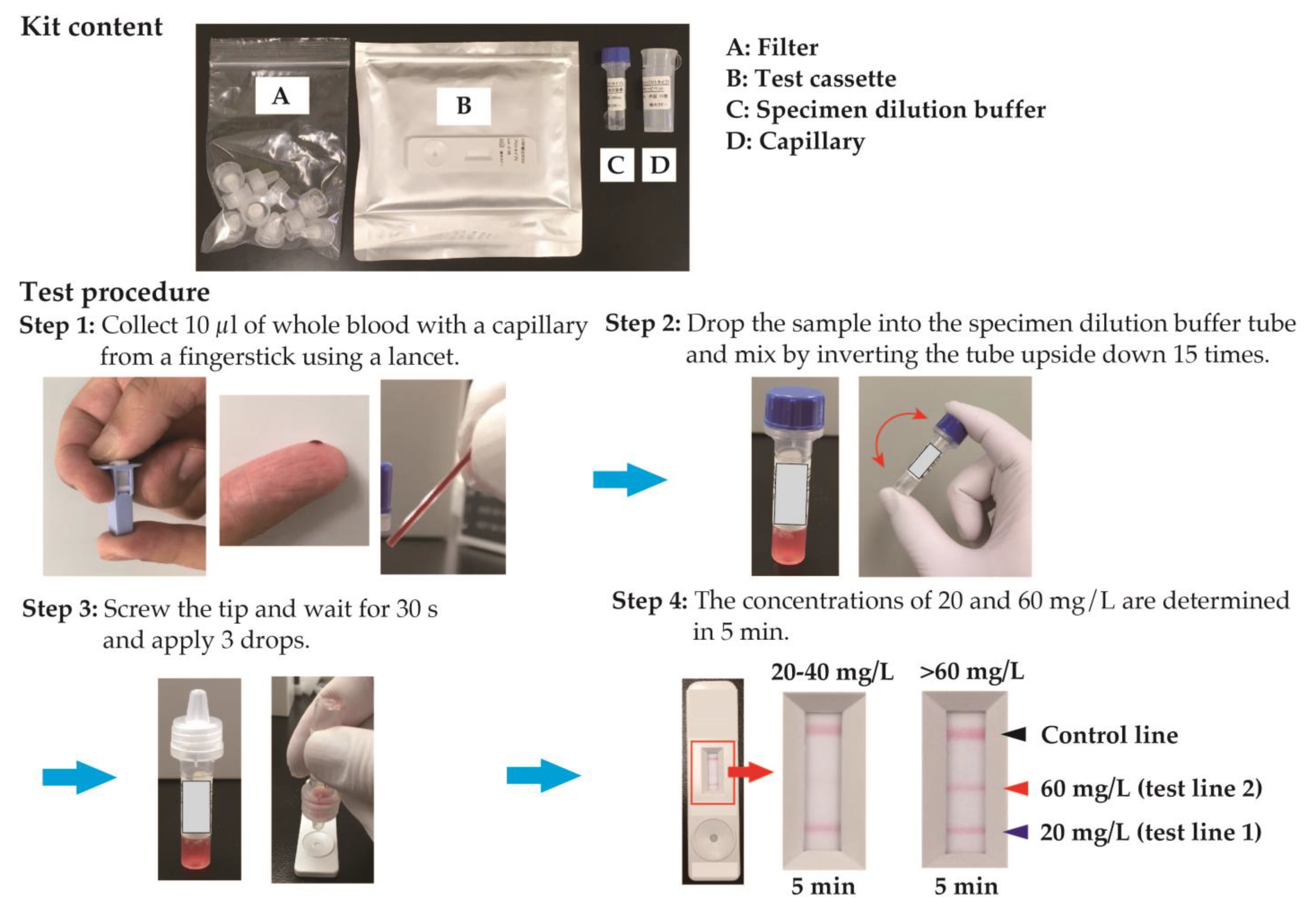
2.2. Measurement of CRP Level
2.3. WBC Differential Counting
2.4. Statistical Analyses
3. Results
3.1. Analytical Accuracy between CRP POCT and Clinical Examination
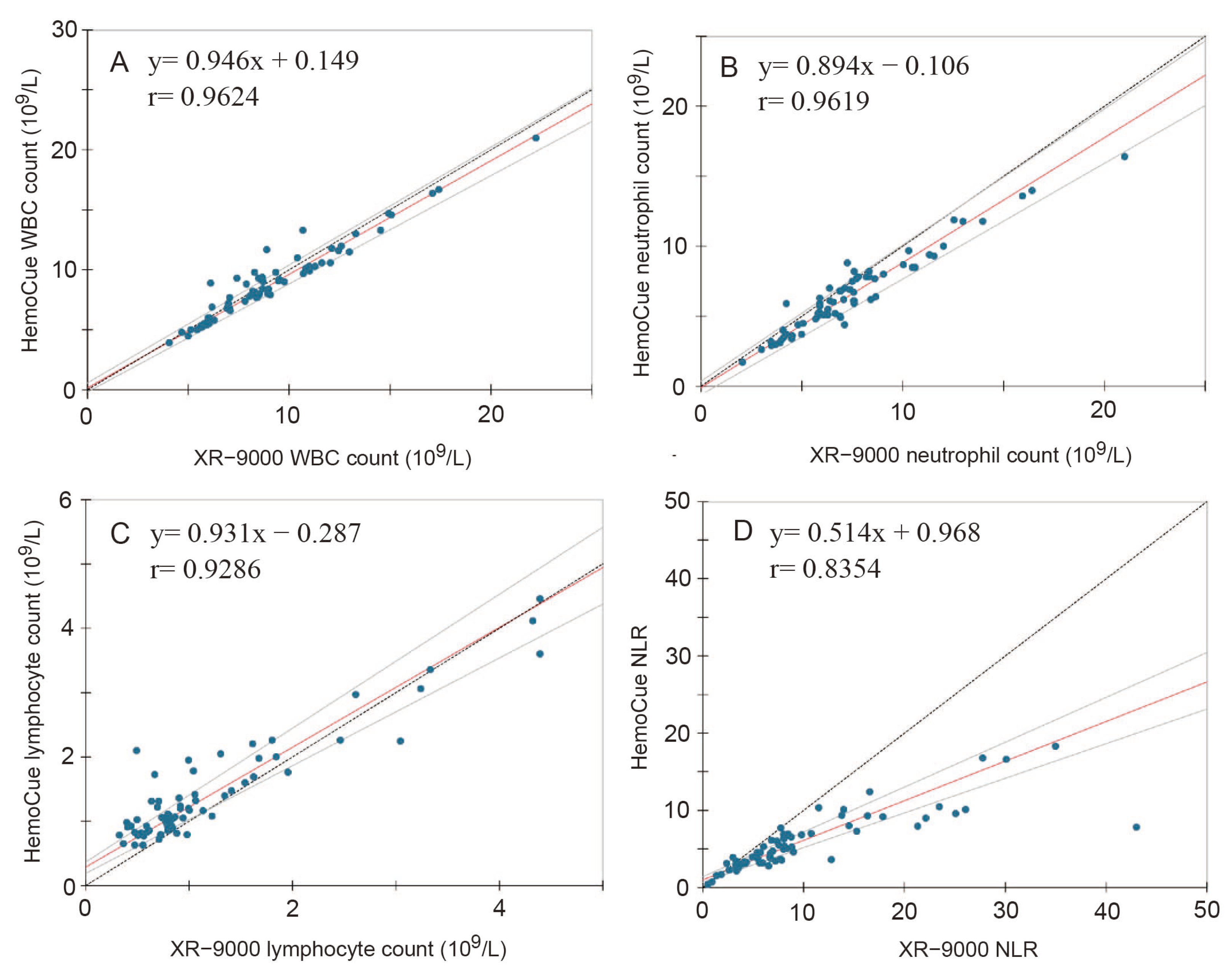
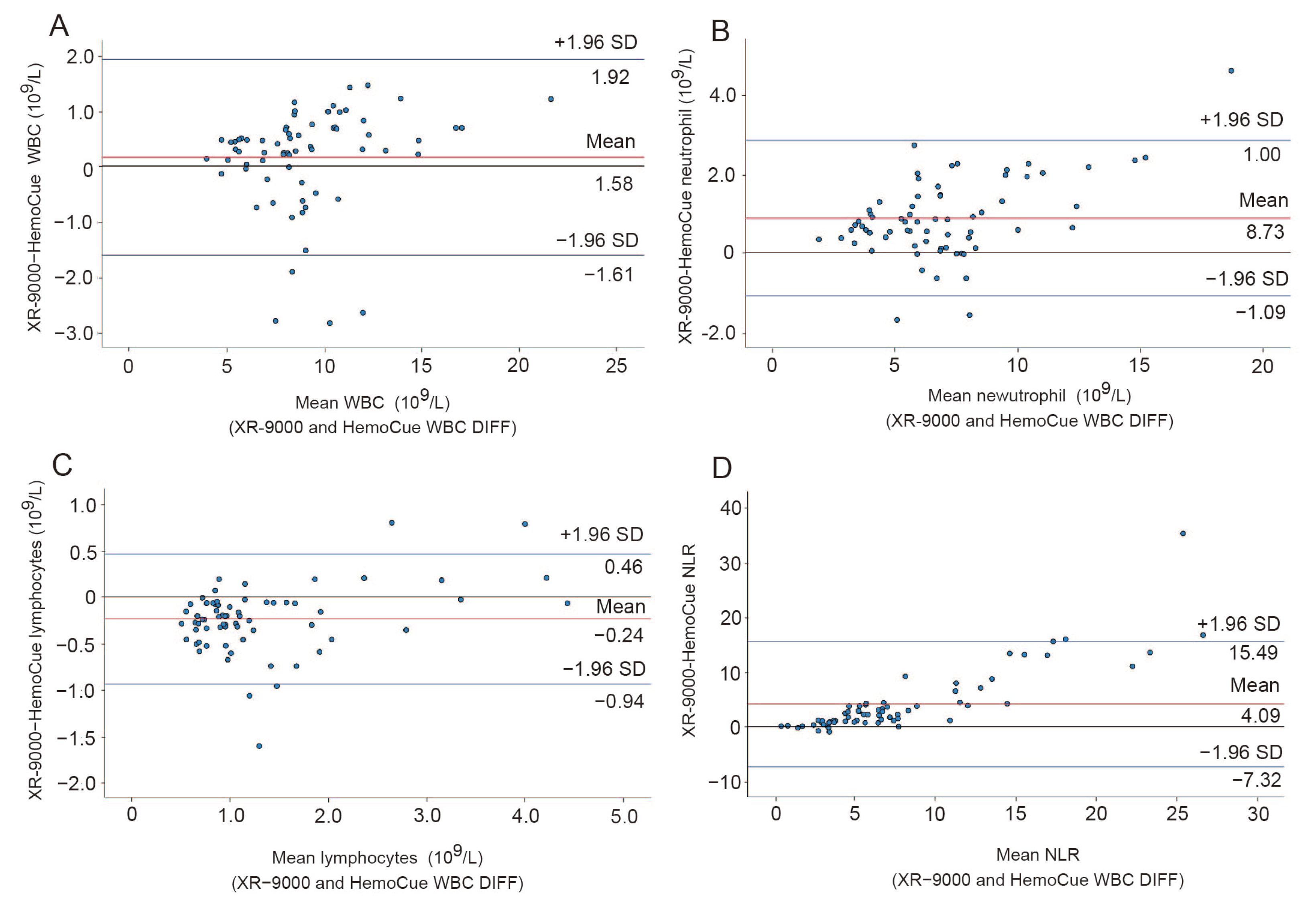
3.2. Analytical Accuracy between POCT and Clinical Examination of WBC Differential Counting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/No.3. 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 11 March 2023).
- Japan Statistics Breau. Statistical Handbook of Japan 2022. Chapter 2: Population. Available online: https://www.stat.go.jp/english/data/handbook/pdf/2022all.pdf#page=23 (accessed on 11 June 2023).
- Song, P.; Tang, W. The community-based integrated care system in Japan: Health care and nursing care challenges posed by super-aged society. BioSci. Trends 2019, 13, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Nugawela, M.D.; Edwards, H.B.; Richards, A.; Roux, H.L.; Pullyblank, A.; Whiting, P. Can early warning scores identify deterioration patients in pre-hospital settings? A systematic review. Resuscitation 2018, 132, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Maciver, M. Pre-hospital use of early warning scores to improve detection and outcomes of sepsis. Br. J. Community Nurs. 2021, 26, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.O.; Stocker, R.; Russell, S.; Roberts, A.; Kingston, A.; Adamson, J.; Hanratty, B. Distribution of the National Early Warning Score (NEWS) in care home residents. Age Ageing 2019, 49, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Stocker, R.; Barker, R.O.; Liddle, J.; Adamson, J.; Hanratty, B. Implementation of the National Early Warning Score in UK care homes: A qualitative evaluation. Br. J. Gen. Pract. 2020, 70, e793–e800. [Google Scholar] [CrossRef]
- Werner, H.; Kuntsche, J. Infection in the elderly- what is different? Z. Gerontol. Geriatr. 2000, 33, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Macovei, D.G.; Irimes, M.B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involve in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2021, 415, 1033–1063. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Cruz, K.R.; Vásques, E.M.M.; de Oliveira, O.N., Jr. Microfluidic point-of-care devices: New trends and future prospects for eHealth diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef]
- Gidske, G.; Sandberg, S.; Fossum, A.L.; Binder, S.; Langsjøen, E.C.; Solsvik, A.E.; Stavelin, A. Point-of-care testing in primary healthcare: A scoring system to determine the frequency of performing internal quality control. Clin. Chem. Lab. Med. 2022, 60, 740–747. [Google Scholar] [CrossRef]
- Larsson, A.; Greig-Pylypczuk, R.; Huisman, A. The state of point-of-care testing: A European perspective. Ups. J. Med. Sci. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited setting. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kokko, E.; Korppim, M.; Helminenm, M.; Hutri-Kähönen, N. Rapid c-reactive protein and white cell tests decrease cost and shorten emergency visits. Pediatr. Int. 2014, 56, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Invest. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to lymphocyte ratio: An emerging marker of the relationships between the immune system and diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Lee, H.; Kim, I.; Kang, B.H.; Um, S.-J. Prognostic value of serial neutrophil-to-lymphocyte ratio measurements in hospitalized community-acquired pneumonia. PLoS ONE 2021, 16, e0250067. [Google Scholar] [CrossRef]
- Cataudella, E.; Giraffa, C.M.; Marca, S.D.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Quattro, R.D.; et al. Nuetrophil-to-lymphocyte ratio: An emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef]
- Latour, K.; Lepeleire, J.D.; Catry, B.; Buntinx, F. Nursing home residents with suspected urinary tract infections: A diagnostic accuracy study. BMC Geriatr. 2022, 22, 187. [Google Scholar] [CrossRef]
- Boere, T.M.; Hopstaken, R.M.; van Tulder, M.W.; Schellevis, F.G.; Verheij, T.J.M.; Hertogh, C.M.P.M.; van Buul, L.W. Implementation and use of point-of-care C-reactive protein testing in nursing homes. J. Am. Med. Dir. Assoc. 2022, 23, 968–975. [Google Scholar] [CrossRef]
- Nouvenne, A.; Ticinesi, A.; Folesani, G.; Cerundolo, N.; Prati, B.; Morelli, I.; Guida, L.; Lauretani, F.; Maggio, M.; Aloe, R.; et al. The association of serum procalcitonin and high-sensitivity C-reactive protein with pneumonia in elderly multimorbid patients with respiratory symptoms: Retrospective cohort study. BMC Geriatr. 2016, 16, 16. [Google Scholar] [CrossRef]
- Dutch Association of Elderly Care Physicians (Verenso). Respiratory Tract Infections in the Vulnerable Elderly Population (Guideline); Verenso: Utrecht, The Netherlands, 2018. [Google Scholar]
- Boere, T.M.; van Buul, L.W.; Hopstaken, R.M.; van Tulder, M.W.; Twisk, J.W.M.R.; Verheij, T.J.M.; Hertogh, C.M.P.M. Effect of C reactive protein point-of-care testing on antibiotic prescribing for lower respiratory tract infections in nursing home residents: Cluster randomized controlled trial. BMJ 2021, 374, n2198. [Google Scholar] [CrossRef] [PubMed]
- Smedemark, S.A.; Aabenhus, R.; Llor, C.; Fournaise, A.; Olsen, O.; Jørgensen, K.J. Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2022, 10, CD010130. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, J.Y.; Lee, J.J.; Goyder, C.; Tan, P.S.; Ananthakumar, T.; Turner, P.J.; Hayward, G.; den Bruel, A.V. Impact of point-of-care C reactive protein in ambulatory care: A systematic review and meta-analysis. BMJ Open 2019, 9, e025036. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, R.; Wu, T.; Wei, X.; Guo, A. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: A systematic review and meta-analysis of primary care studies. Br. J. Gen. Pract. 2013, 63, e787–e794. [Google Scholar] [CrossRef]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef]
- Miravitlles, M.; Moragas, A.; Hernández, S.; Bayona, C.; Llor, C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest 2013, 144, 1571–1577. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujisawa, T.; Ishida, T.; Ito, A.; Oyamada, Y.; Fujimoto, K.; Yoshida, M.; Maeda, H.; Miyashita, N.; Nagai, H.; et al. Therapeutic strategy for all patients with pneumonia: A 3-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin. Infect. Dis. 2019, 68, 1080–1088. [Google Scholar] [CrossRef]
- Jones, B.E.; Jones, J.P.; Vines, C.G.; Dean, N.C. Validating hospital admission criteria for decision support in pneumonia. BMC Pulm. Med. 2014, 14, 149. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. Official Clinical Practice Guidelines of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Brouwer, N.; van Peltm, J. Validation and evaluation of eight commercially available point of care CRP methods. Clin. Chim. Acta 2015, 439, 195–201. [Google Scholar] [CrossRef]
- Karawajczyk, M.; Haile, S.; Grabski, M.; Larsson, A. The HemoCue WBC DIFF system could be used for leucocyte and neutrophil counts but not for full differential counts. Acta Paediatr. 2017, 106, 974–978. [Google Scholar] [CrossRef]
- Nakahori, N.; Sekine, M.; Yamada, M.; Tatsuse, T.; Kido, H.; Suzuki, M. Future projections of the prevalence of dementia in Japan: Results from the Toyama Dementia Survey. BMC Geriatr. 2021, 21, 602. [Google Scholar] [CrossRef]
- Prime Minister of Japan and His Cabinet. Introduction of Nursing Care in Japan. Available online: https://www.kantei.go.jp/jp/singi/kenkouiryou/en/index.html (accessed on 18 June 2023).
- Calarco, S.; Fernandez-Carballo, B.L.; Keller, T.; Weber, S.; Jakobi, M.; Marsall, P.; Schneiderhan-Marra, N.; Dittrich, S. Analytical performance of 17 commercially available point-of-care tests for CRP to support patient management at lower levels of the health system. PLoS ONE 2023, 18, e0267516. [Google Scholar] [CrossRef]
- Gentile, I.; Moriello, N.S.; Hopstaken, R.; Llor, C.; Melbye, H.; Senn, O. The role of CRP testing in the fight against antibiotic overuse in European primary care: Recommendations from a European expert panel. Diagnostics 2023, 13, 320. [Google Scholar] [CrossRef]
- Van den Bruel, A.; Thompson, M.J.; Haj-Hassan, T.; Stevens, R.; Moll, H.; Lakhanpaul, M.; Mant, D. Diagnostic value of laboratory tests in identifying serious infections in febrile children: Systematic review. BMJ 2011, 342, d3082. [Google Scholar] [CrossRef]
- Korppi, M.; Heiskanen-Kosma, T.; Leinonen, M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur. Respir. J. 1997, 10, 1125–1129. [Google Scholar] [CrossRef]
- Eriksson, S.; Granström, L.; Carlström, A. The diagnostic value of repetitive preoperative analyses of C-reactive protein and total leucocyte count in patients with suspected acute appendicitis. Scand. J. Gastroenterol. 1994, 29, 1145–1149. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Bloos, F.; Reinhart, K. Rapid diagnosis of sepsis. Virulence 2014, 5, 154–160. [Google Scholar] [CrossRef]
- Kapasi, A.J.; Dittrich, S.; González, I.J.; Rodwell, T.C. Host biomarkers for distinguishing bacterial from non-bacterial causes of acute febrile illness: A comprehensive review. PLoS ONE 2016, 11, e0160278. [Google Scholar] [CrossRef]
- Mattsson, T.O.; Lindhart, C.L.; Schöley, J.; Friis-Hansen, L.; Herrstedt, J. Patient self-testing of white blood cell count and differentiation: A study of feasibility and measurement performance in a population of Danish cancer patients. Eur. J. Cancer Care 2020, 29, e13189. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.; Ng, J.; Li, S.C.; Giannoutsos, J.; Nayyar, V.; Iredell, J.R.; Dwyer, D.E.; Chen, S.C. Evaluation of point-of-care testing in critically unwell patients: Comparison with clinical laboratory analysers and applicability to patients with Ebolavirus infection. Pathology 2015, 47, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kur, D.K.; Agersnap, N.; Holländer, N.H.; Pedersen, O.B.V.; Friis-Hansen, L. Evaluation of the HemoCue WBC DIFF in leukopenic patient samples. Int. J. Lab. Hematol. 2020, 42, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, L.; Niemelä, J.; Leino, P.; Mertsola, J.; Peltola, V. Accuracy and feasibility of point-of-care white blood cell count and C-reactive protein measurements at the pediatric emergency department. PLoS ONE 2015, 10, e0129920. [Google Scholar] [CrossRef] [PubMed]
- Osei-Bimpong, A.; Jury, C.; McLean, R.; Lewis, S.M. Point-of-care method for total white cell count: An evaluation of the HemoCue WBC device. Int. J. Lab. Hematol. 2009, 31, 657–664. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Mariz, M.; Tiago, I.; Martins, J.; Alarico, S.; Ferreira, P. A review on urinary tract infections diagnostic methods: Laboratory-based and point-of-care approaches. J. Pharm. Biomed. Anal. 2022, 219, 114889. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Q.; Bao, N.; Ding, S.N. A seisitive immunochromatographic test strip based on hydrophobic quantum dots incorporated into Mg/Fe nanoflowers for HCG detection. Chemosensors 2023, 11, 114. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]


| Targets for Comparison | Kappa Index | Degree of Coincidence (%) | ||||
|---|---|---|---|---|---|---|
| Detection range (mg/L) | <20 | 20–60 | >60 | |||
| HC-CRP | TBA-2000FR vs. evaluator 1 | 0.910 | 96.6 | 91.4 | 95.1 | |
| TBA-2000FR vs. evaluator 2 | 0.856 | 93.1 | 84.2 | 97.6 | ||
| Evaluator 1 vs. evaluator 2 | 0.910 | |||||
| Detection range (mg/L) | <10 | 10–40 | 40–80 | >80 | ||
| Actim CRP | TBA-2000FR vs. evaluator 1 | 0.916 | 96.6 | 96.9 | 98.2 | 100 |
| TBA-2000FR vs. evaluator 2 | 0.932 | 89.7 | 96.9 | 94.5 | 100 | |
| Evaluator 1 vs. evaluator 2 | 0.881 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenaka, S.; Moro, H.; Shimizu, U.; Koizumi, T.; Nagano, K.; Edanami, N.; Ohkura, N.; Domon, H.; Terao, Y.; Noiri, Y. Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy. Diagnostics 2023, 13, 2407. https://doi.org/10.3390/diagnostics13142407
Takenaka S, Moro H, Shimizu U, Koizumi T, Nagano K, Edanami N, Ohkura N, Domon H, Terao Y, Noiri Y. Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy. Diagnostics. 2023; 13(14):2407. https://doi.org/10.3390/diagnostics13142407
Chicago/Turabian StyleTakenaka, Shoji, Hiroshi Moro, Utako Shimizu, Takeshi Koizumi, Kei Nagano, Naoki Edanami, Naoto Ohkura, Hisanori Domon, Yutaka Terao, and Yuichiro Noiri. 2023. "Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy" Diagnostics 13, no. 14: 2407. https://doi.org/10.3390/diagnostics13142407
APA StyleTakenaka, S., Moro, H., Shimizu, U., Koizumi, T., Nagano, K., Edanami, N., Ohkura, N., Domon, H., Terao, Y., & Noiri, Y. (2023). Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy. Diagnostics, 13(14), 2407. https://doi.org/10.3390/diagnostics13142407







