Multiparametric Contrast-Enhanced Ultrasound in Early Prediction of Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival in Breast Cancer
Abstract
1. Introduction
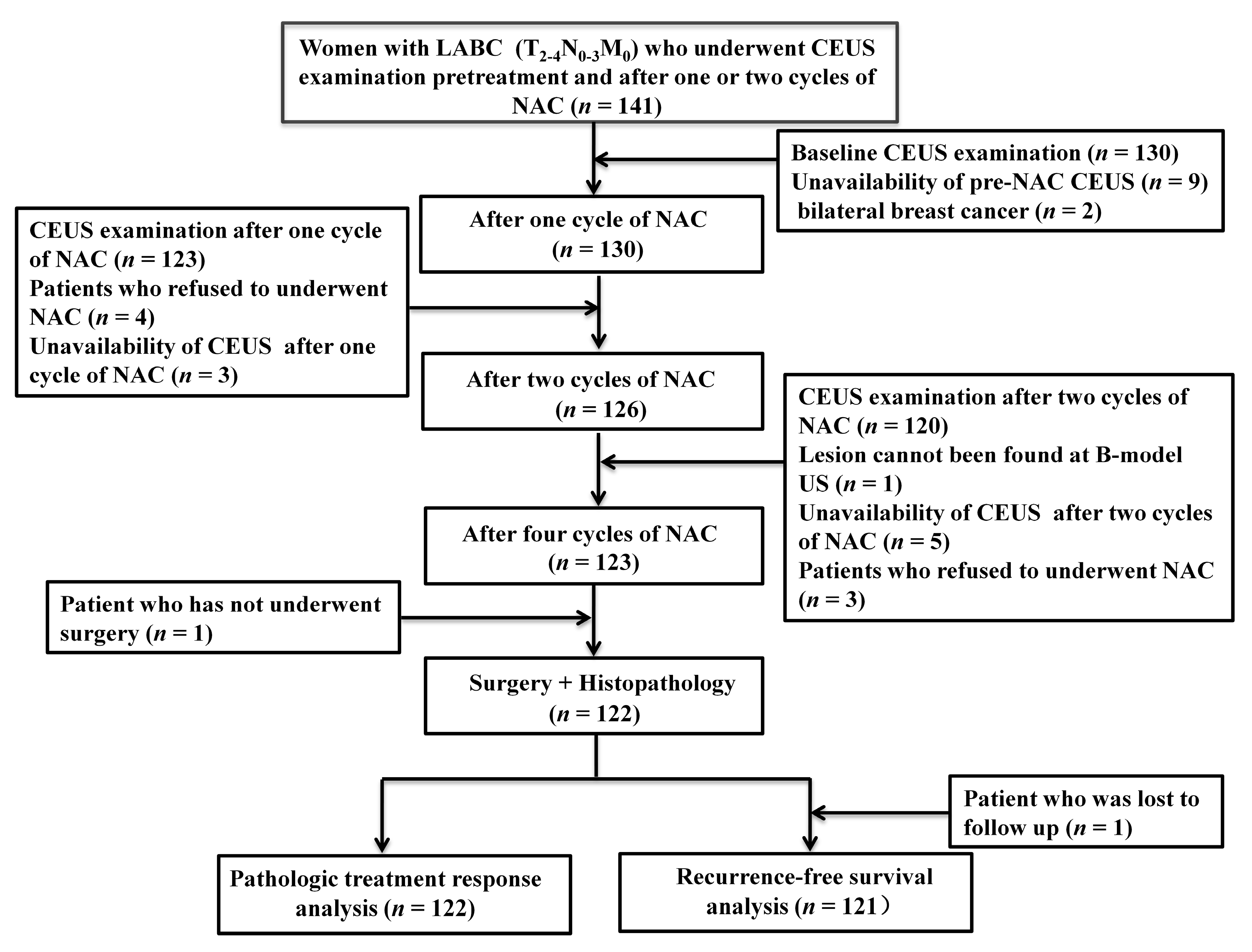
2. Materials and Methods
2.1. Patients
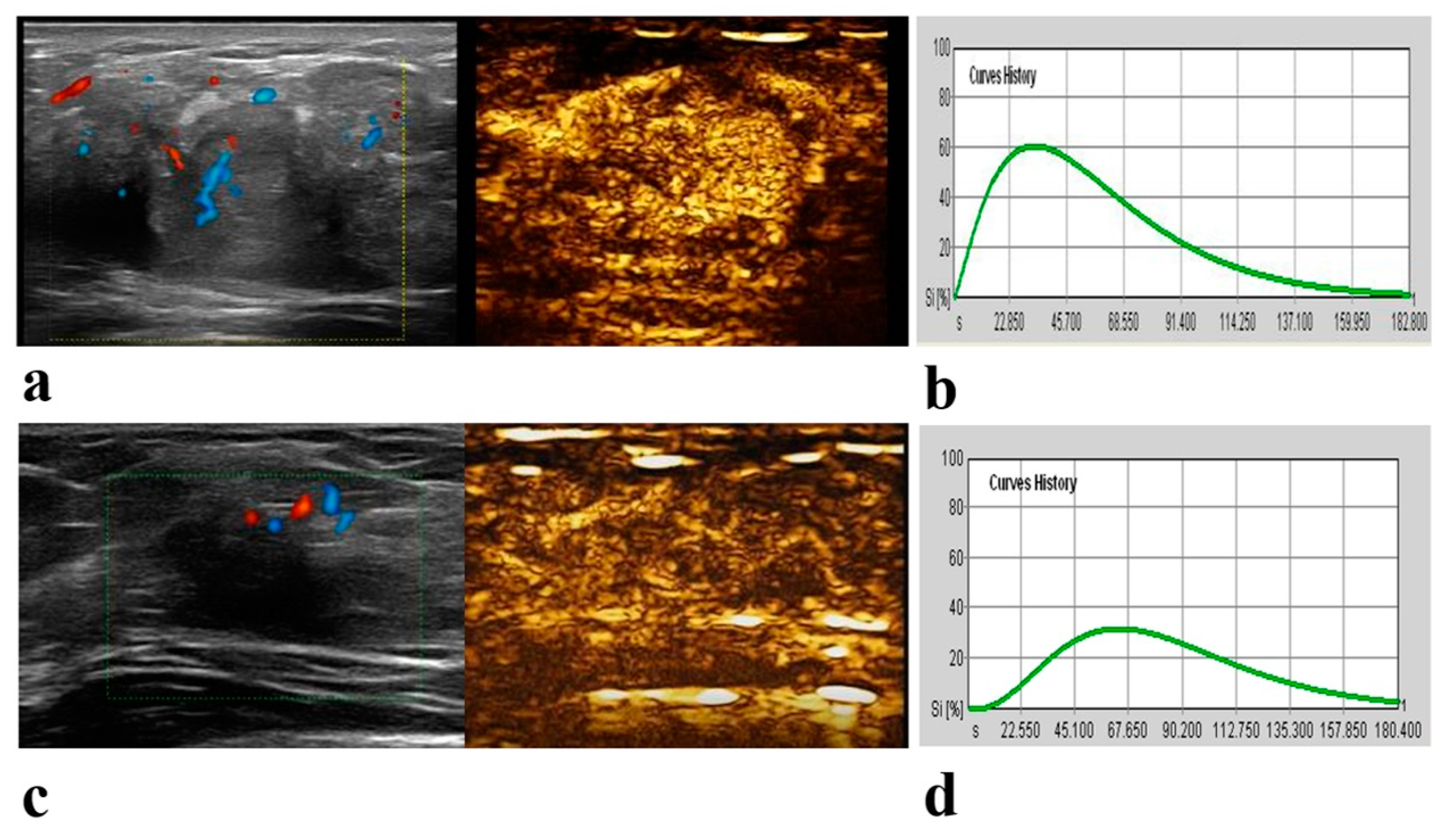
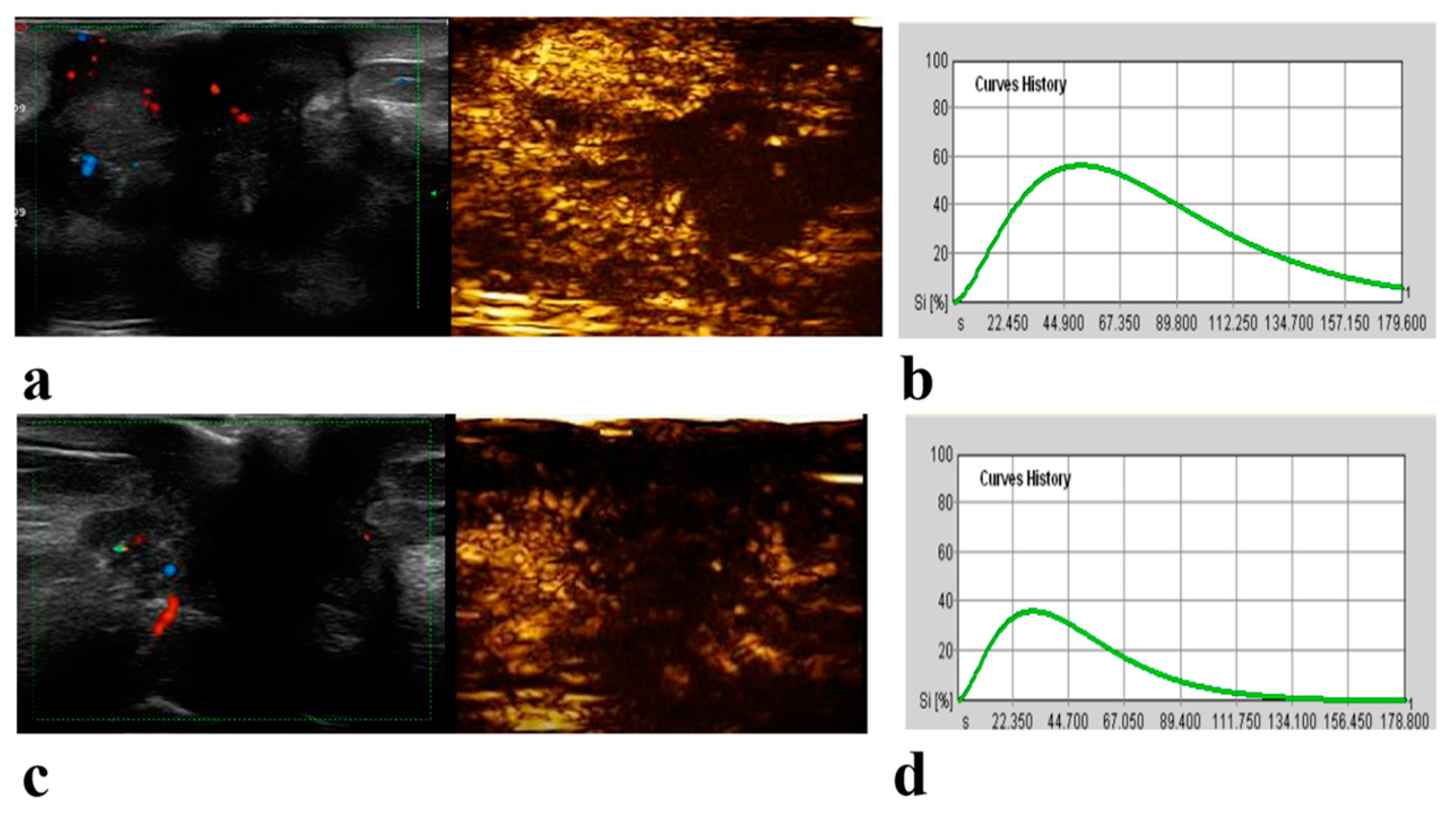
2.2. CEUS Examination and Image Analysis
2.3. Clinical-Pathologic Evaluation and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pathologic Treatment Response
3.3. Factors Associated with PCR
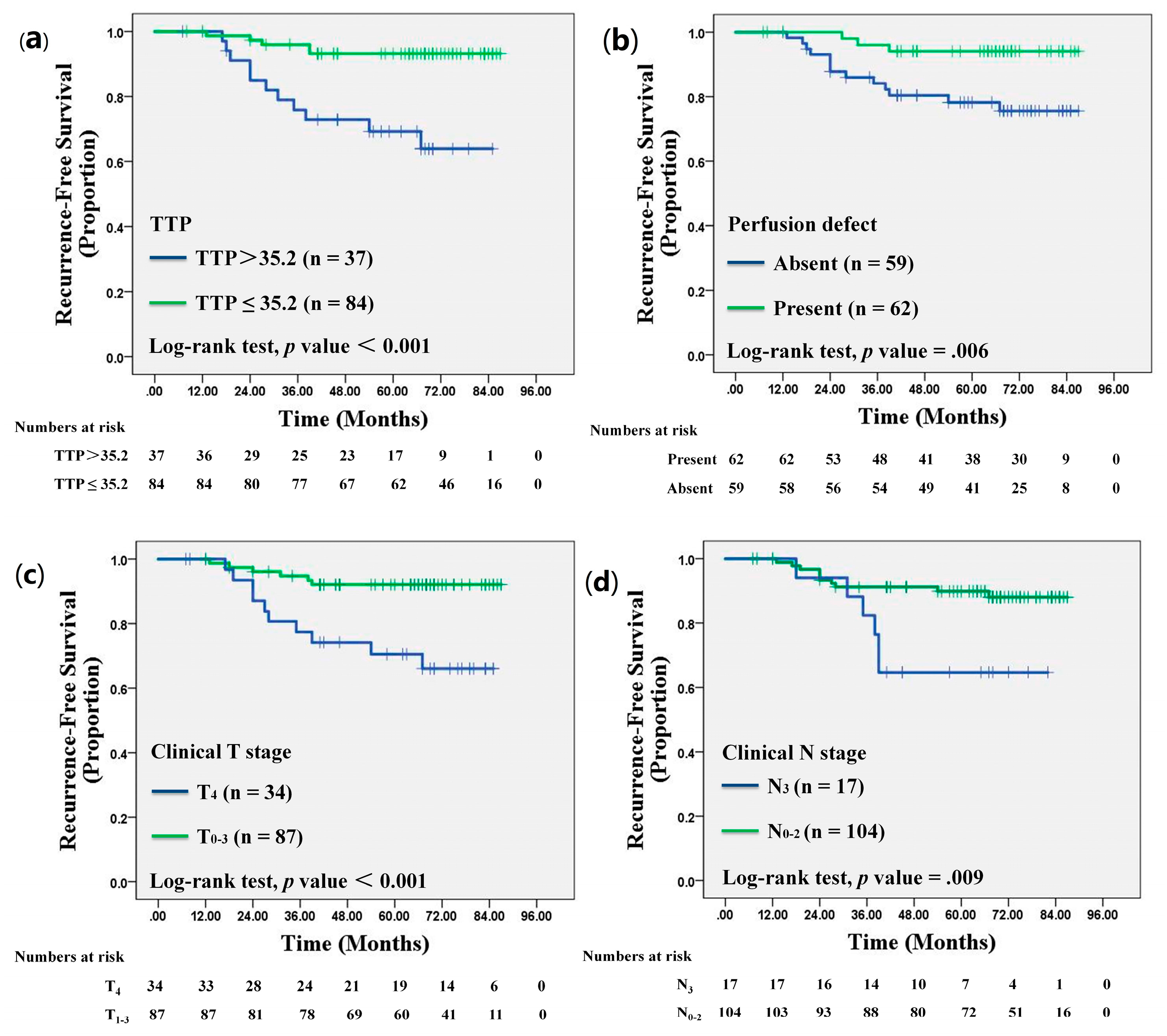
3.4. Recurrence Outcome
3.5. Univariate and Multivariate Cox Proportional Hazards Analyses
3.6. Disease-Free Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEUS | contrast-enhanced ultrasound |
| pCR | pathologic complete response |
| RFS | recurrence-free survival |
| NAC | neoadjuvant chemotherapy |
| PEAK | the maximum intensity |
| RBV | regional blood volume |
| TTP | time to peak |
| RBF | regional blood flow |
| MTT | mean transit time |
| ROC | receiver operating characteristic curve |
References
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Bar, Y.; Isakoff, S.J. The Evolving Role of Neoadjuvant Therapy for Operable Breast Cancer. J. Natl. Compr. Cancer Netw. 2022, 20, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Zhao, R.; Ji, Y.; Li, J.; Zhang, Y.; Lu, H. Development and validation of a nomogram based on pretreatment dynamic contrast-enhanced MRI for the prediction of pathologic response after neoadjuvant chemotherapy for triple-negative breast cancer. Eur. Radiol. 2022, 32, 1676–1687. [Google Scholar] [CrossRef]
- Baysal, H.; Serdaroglu, A.Y.; Ozemir, I.A.; Baysal, B.; Gungor, S.; Erol, C.I.; Ozsoy, M.S.; Ekinci, O.; Alimoglu, O. Comparison of Magnetic Resonance Imaging With Positron Emission Tomography/Computed Tomography in the Evaluation of Response to Neoadjuvant Therapy of Breast Cancer. J. Surg. Res. 2022, 278, 223–232. [Google Scholar] [CrossRef]
- Hottat, N.A.; Badr, D.A.; Lecomte, S.; Besse-Hammer, T.; Jani, J.C.; Cannie, M.M. Value of diffusion-weighted MRI in predicting early response to neoadjuvant chemotherapy of breast cancer: Comparison between ROI-ADC and whole-lesion-ADC measurements. Eur. Radiol. 2022, 32, 4067–4078. [Google Scholar] [CrossRef]
- Han, X.; Yang, H.; Jin, S.; Sun, Y.; Zhang, H.; Shan, M.; Cheng, W. Prediction of pathological complete response to neoadjuvant chemotherapy in patients with breast cancer using a combination of contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging. Cancer Med. 2022, 12, 1389–1398. [Google Scholar] [CrossRef]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging Neoadjuvant Therapy Response in Breast Cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.-S.; Liu, S.-Y.; Shen, K.-W. Accuracy of MRI in Prediction of Pathologic Complete Remission in Breast Cancer After Preoperative Therapy: A Meta-Analysis. Am. J. Roentgenol. 2010, 195, 260–268. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Houssami, N.; Macaskill, P.; Sardanelli, F.; Irwig, L.; Mamounas, E.P.; von Minckwitz, G.; Brennan, M.E.; Ciatto, S. Meta-Analysis of Magnetic Resonance Imaging in Detecting Residual Breast Cancer After Neoadjuvant Therapy. Gynecol. Oncol. 2013, 105, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Trahan, T.J.; Xiao, J.; Taghipour, M.; Mena, E.; Connolly, R.M.; Subramaniam, R.M. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist 2016, 21, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-L.; Pan, S.-M.; Ren, J.; Yang, Z.-X.; Jiang, G.-Q. Role of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: A Meta-analysis. Clin. Breast Cancer 2017, 17, 245–255. [Google Scholar] [CrossRef]
- Chen, J.H.; Feig, B.; Agrawal, G.; Yu, H.; Carpenter, P.M.; Mehta, R.S.; Nalcioglu, O.; Su, M.Y. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer 2007, 112, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; Von Minckwitz, G.; Mamounas, E.P.; Brennan, M.; Ciatto, S.; Houssami, N. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. Br. J. Cancer 2013, 109, 1528–1536. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.-F.; Liu, X.-S.; Wang, L.; Zhang, J.; Lu, J.-S.; Li, F.-H. Quantitative contrast-enhanced ultrasound evaluation of pathological complete response in patients with locally advanced breast cancer receiving neoadjuvant chemotherapy. Eur. J. Radiol. 2018, 103, 118–123. [Google Scholar] [CrossRef]
- Saracco, A.; Szabó, B.K.; Tánczos, E.; Bergh, J.; Hatschek, T. Contrast-enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol. 2016, 58, 394–402. [Google Scholar] [CrossRef]
- Huang, Y.; Le, J.; Miao, A.; Zhi, W.; Wang, F.; Chen, Y.; Zhou, S.; Chang, C. Prediction of treatment responses to neoadjuvant chemotherapy in breast cancer using contrast-enhanced ultrasound. Gland. Surg. 2021, 10, 1280–1290. [Google Scholar] [CrossRef]
- Peng, J.; Pu, H.; Jia, Y.; Chen, C.; Ke, X.-K.; Zhou, Q. Early prediction of response to neoadjuvant chemotherapy using contrast-enhanced ultrasound in breast cancer. Medicine 2021, 100, e25908. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, T.; Huang, M.; Wang, J.; Chu, Y.; Zhong, L.; Zheng, S. Evaluation of the response of breast cancer patients to neoadjuvant chemotherapy by combined contrast-enhanced ultrasonography and ultrasound elastography. Exp. Ther. Med. 2019, 17, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Y.; Wang, Y.; Zhou, L.; Xu, S.; Wu, Y.; Peng, J.; Zhang, J.; Yin, W.; Lu, J. Association of Neo-Family History Score with pathological complete response, safety, and survival outcomes in patients with breast cancer receiving neoadjuvant platinum-based chemotherapy: An exploratory analysis of two prospective trials. Eclinicalmedicine 2021, 38, 101031. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef]
- Jia, W.-R.; Tang, L.; Wang, D.-B.; Chai, W.-M.; Fei, X.-C.; He, J.-R.; Chen, M.; Wang, W.-P. Three-dimensional Contrast-enhanced Ultrasound in Response Assessment for Breast Cancer: A Comparison with Dynamic Contrast-enhanced Magnetic Resonance Imaging and Pathology. Sci. Rep. 2016, 6, srep33832. [Google Scholar] [CrossRef]
- Wang, J.-W.; Zheng, W.; Liu, J.-B.; Chen, Y.; Cao, L.-H.; Luo, R.-Z.; Li, A.-H.; Zhou, J.-H. Assessment of Early Tumor Response to Cytotoxic Chemotherapy with Dynamic Contrast-Enhanced Ultrasound in Human Breast Cancer Xenografts. PLoS ONE 2013, 8, e58274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Grant, E.; Sheth, P.; Garcia, A.A.; Desai, B.; Ji, L.; Groshen, S.; Hwang, D.; Yamashita, M.; Hovanessian-Larsen, L. Accuracy of Contrast-Enhanced Ultrasound Compared With Magnetic Resonance Imaging in Assessing the Tumor Response After Neoadjuvant Chemotherapy for Breast Cancer. J. Ultrasound Med. 2017, 36, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Grover, S.B.; Mani, C.; Ahluwalia, C. Contrast enhanced ultrasound quantitative parameters for assessing neoadjuvant chemotherapy response in patients with locally advanced breast cancer. Br. J. Radiol. 2021, 94, 20201160. [Google Scholar] [CrossRef]
- Dietzel, M.; Zoubi, R.; Vag, T.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A.; Baltzer, P.A. Association between survival in patients with primary invasive breast cancer and computer aided MRI. J. Magn. Reson. Imaging 2012, 37, 146–155. [Google Scholar] [CrossRef]
- Wan, C.; Du, J.; Fang, H.; Li, F.; Wang, L. Evaluation of breast lesions by contrast enhanced ultrasound: Qualitative and quantitative analysis. Eur. J. Radiol. 2012, 81, e444–e450. [Google Scholar] [CrossRef]
- Dzik-Jurasz, A.; Domenig, C.; George, M.; Wolber, J.; Padhani, A.; Brown, G.; Doran, S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002, 360, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Moon, W.K.; Cho, N.; Song, I.C.; Chang, J.M.; Park, I.-A.; Han, W.; Noh, D.-Y.; Iima, M.; Kataoka, M.; et al. Diffusion-weighted MR Imaging: Pretreatment Prediction of Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Radiology 2010, 257, 56–63. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | pCR Status | Recurrence Status | ||||
|---|---|---|---|---|---|---|
| pCR | NonpCR | p Value | Recurrence | Nonrecurrence | p Value | |
| Number of lesions | 44 (36) | 78 (64) | 17 (14) | 104 (86) | ||
| Age (year) * | 0.59 | 0.64 | ||||
| ≤50 | 17 (39) | 34 (44) | 6 (35) | 43 (41) | ||
| >50 | 27 (61) | 44 (56) | 11 (65) | 61 (59) | ||
| Clinical T stage | 0.17 | <0.001 | ||||
| T1–3 | 35 (80) | 53 (68) | 6 (35) | 81 (78) | ||
| T4 | 9 (20) | 25 (32) | 11 (65) | 23 (22) | ||
| Clinical N stage | 0.29 | 0.007 | ||||
| N0-2 | 40 (91) | 64 (82) | 11 (65) | 93 (89) | ||
| N3 | 4 (9) | 14 (18) | 6 (35) | 11 (11) | ||
| ER status | <0.001 | 1 | ||||
| ER negative | 27 (61) | 14 (18) | 5 (29) | 34 (33) | ||
| ER positive | 17 (39) | 64 (82) | 12 (71) | 70 (67) | ||
| PR status | <0.001 | 0.78 | ||||
| PR negative | 21 (48) | 12 (15) | 4 (24) | 30 (29) | ||
| PR positive | 23 (52) | 66 (85) | 13 (76) | 74 (71) | ||
| HER2 status | <0.001 | 0.45 | ||||
| HER2 negative | 15 (34) | 54 (69) | 11 (65) | 57 (55) | ||
| HER2 positive | 29 (66) | 24 (31) | 6 (35) | 47 (45) | ||
| Ki-67 index | 0.004 | 0.11 | ||||
| <14% | 0 (0) | 13 (17) | 13 (77) | 94 (90) | ||
| ≥14% | 44 (100) | 65 (83) | 4 (23) | 10 (10) | ||
| Molecular subtype | <0.001 | 0.43 | ||||
| Luminal A-like | 0 (0) | 11 (14) | 3 (18) | 6 (6) | ||
| Luminal B-like (HER2 positive) | 16 (36) | 20 (26) | 4 (24) | 32 (31) | ||
| Luminal B-like (HER2 negative) | 11 (25) | 39 (50) | 8 (47) | 43 (41) | ||
| Triple negative | 3 (7) | 4 (5) | 0 (0) | 6 (6) | ||
| HER2-enriched | 14 (32) | 4 (5) | 2 (12) | 17 (16) | ||
| Adjuvant endocrine therapy | 0.68 | 0.045 | ||||
| No | 24 (55) | 37 (47) | 12 (71) | 48 (46) | ||
| Aromatase inhibitors | 10 (23) | 23 (30) | 5 (29) | 28 (27) | ||
| Ovarian function suppression | 10 (23) | 18 (23) | 0 (0) | 28 (27) | ||
| Adjuvant Herceptin | <0.001 | 0.1 | ||||
| Yes | 23 (52) | 16 (21) | 2 (12) | 37 (36) | ||
| No | 21 (48) | 62 (80) | 15 (88) | 67 (64) | ||
| Tumor diameter (cm) | 0.22 | 0.3 | ||||
| Pre-treatment * | 3.96 ± 1.67 | 4.34 ± 1.59 | 4.63 ± 1.93 | 4.10 ± 1.54 | ||
| Parameter | pCR | NonpCR | Total Number | p Value |
|---|---|---|---|---|
| Qualitative parameters pre-NAC | ||||
| Perfusion defect * | 0.02 | |||
| Present | 16 (36) | 46 (59) | 62 (50) | |
| Absent | 28 (64) | 32 (41) | 60 (50) | |
| Radial or penetrating vessels * | 0.50 | |||
| Present | 17 (39) | 35 (45) | 52 (43) | |
| Absent | 27 (61) | 43 (55) | 70 (57) | |
| Quantitative parameters before and during NAC | ||||
| PEAK0 | 57.75 ± 13.79 | 59.54 ± 12.15 | 0.46 | |
| TTP0 | 29.22 ± 8.06 | 31.78 ± 9.98 | 0.19 | |
| RBV0 | 5487.63 ± 2323.33 | 5961.44 ± 2075.39 | 0.25 | |
| RBF0 | 77.34 ± 19.58 | 79.16 ± 17.17 | 0.60 | |
| MTT0 | 68.53 ± 13.96 | 73.99 ± 15.63 | 0.06 | |
| PEAK1 | 45.0 ± 11.51 | 54.59 ± 14.44 | <0.001 | |
| TTP1 | 35.04 ± 15.85 | 30.68 ± 9.31 | 0.18 | |
| RBV1 | 3833.54 ± 1915.79 | 4818.29 ± 2220.50 | 0.009 | |
| RBF1 | 58.47 ± 16.75 | 72.04 ± 21.41 | 0.001 | |
| MTT1 | 64.39 ± 22.39 | 65.24 ± 16.34 | 0.39 | |
| ΔPEAK1 | −0.20 ± 0.22 | −0.07 ± 0.22 | 0.002 | |
| ΔTTP1 | 0.28 ± 0.64 | 0.03 ± 0.39 | 0.02 | |
| ΔRBV1 | −0.26 ± 0.35 | −0.13 ± 0.41 | 0.10 | |
| ΔRBF1 | −0.23 ± 0.24 | −0.07 ± 0.26 | 0.002 | |
| ΔMTT1 | −0.04 ± 0.34 | −0.09 ± 0.26 | 0.42 | |
| PEAK2 | 30.91 ± 9.40 | 45.90 ± 13.79 | <0.001 | |
| TTP2 | 44.52 ± 19.93 | 35.44 ± 12.67 | 0.02 | |
| RBV2 | 2783.98 ± 1278.59 | 4057.81 ± 2072.23 | <0.001 | |
| RBF2 | 39.26 ± 15.53 | 59.40 ± 19.62 | <0.001 | |
| MTT2 | 72.16 ± 22.12 | 66.47 ± 16.98 | 0.23 | |
| ΔPEAK2 | −0.43 ± 0.24 | −0.22 ± 0.27 | <0.001 | |
| ΔTTP2 | 0.68 ± 1.03 | 0.20 ± 0.533 | 0.004 | |
| ΔRBV2 | −0.41 ± 0.35 | −0.27 ± 0.44 | 0.08 | |
| ΔRBF2 | −0.46 ± 0.26 | −0.24 ± 0.28 | <0.001 | |
| ΔMTT2 | 0.11 ± 0.41 | −0.07 ± 0.31 | 0.01 | |
| Tumor diameter during NAC | ||||
| Diameter1 | 25.93 ± 10.88 | 33.34 ± 13.39 | 0.002 | |
| ΔDiameter1 | −0.32 ± 0.13 | −0.23 ± 0.12 | 0.001 | |
| Diameter2 | 20.70 ± 10.49 | 27.61 ± 12.99 | 0.005 | |
| ΔDiameter2 | −0.48 ± 0.19 | −0.37 ± 0.15 | 0.001 |
| Variable | Beta Coefficient | Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|
| After one cycle of NAC | ||||
| PEAK1 | 0.052 | 1.05 | 1.02, 1.09 | 0.004 |
| ΔDiameter1 | −5.541 | 0.004 | 0.00, 0.16 | 0.003 |
| Molecular subtype | 1.813 | 6.13 | 2.05, 18.36 | 0.001 |
| After two cycles of NAC | ||||
| PEAK2 | 0.094 | 1.098 | 1.05, 1.15 | <0.001 |
| ΔTTP2 | −0.76 | 0.47 | 0.23, 0.94 | 0.03 |
| Molecular subtype | 1.409 | 4.09 | 1.31, 12.77 | 0.02 |
| Parameter | Recurrence | Nonrecurrence | Total Number | p Value |
|---|---|---|---|---|
| Quantitative parameters | ||||
| PEAK0 | 58.52 ± 10.71 | 59.11 ± 12.99 | 0.86 | |
| TTP0 | 36.67 ± 8.20 | 29.86 ± 9.26 | 0.005 | |
| RBV0 | 5525.26 ± 1636.11 | 5807.69 ± 2243.84 | 0.62 | |
| RBF0 | 75.95 ± 16.57 | 78.84 ± 18.35 | 0.54 | |
| MTT0 | 71.52 ± 12.51 | 71.85 ± 15.52 | 0.93 | |
| Qualitative parameters | ||||
| Perfusion defect | 0.006 | |||
| Present | 14 (82) | 48 (46) | 62 (51) | |
| Absent | 3 (18) | 56 (54) | 59 (49) | |
| Radial or penetrating vessels | 0.50 | |||
| Present | 6 (35) | 46 (44) | 52 (43) | |
| Absent | 11 (65) | 58 (56) | 69 (57) |
| Characteristic | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
| Age (year) | 0.99 | 0.95, 1.03 | 0.53 | |||
| Clinical T stage | 0.001 | 0.002 | ||||
| T1~3 | Reference category | Reference category | ||||
| T4 | 5.38 | 1.99, 14.57 | 4.75 | 1.75, 12.87 | ||
| Clinical N stage | 0.01 | 0.02 | ||||
| N0~2 | Reference category | Reference category | ||||
| N3 | 3.495 | 1.29, 9.49 | 3.39 | 1.25, 9.19 | ||
| ER status | 0.87 | |||||
| ER negative | Reference category | |||||
| ER positive | 0.92 | 0.32, 2.60 | ||||
| PR status | 0.76 | |||||
| PR negative | Reference category | |||||
| PR positive | 0.84 | 0.27, 2.58 | ||||
| HER2 status | 0.51 | |||||
| HER2 negative | Reference category | |||||
| HER2 positive | 1.395 | 0.52, 3.77 | ||||
| Ki-67 index | 0.12 | |||||
| >14 | 2.42 | 0.79, 7.43 | ||||
| ≤14 | Reference category | |||||
| Molecular subtype | 0.39 | |||||
| Luminal | Reference category | |||||
| Triple negative and HER2-enriched | 0.53 | 0.12, 2.31 | ||||
| Adjuvant endocrine therapy | 0.05 | |||||
| Yes | 0.35 | 0.12, 0.995 | ||||
| No | Reference category | |||||
| Adjuvant Herceptin | 0.08 | |||||
| Yes | Reference category | |||||
| No | 0.27 | 0.06, 1.19 | ||||
| Tumor diameter | 1.02 | 0.99, 1.05 | 0.19 | |||
| CEUS parameters pre-NAC | ||||||
| perfusion defect | 0.01 | |||||
| Present | 0.20 | 0.06, 0.71 | ||||
| Absent | Reference category | |||||
| Radial or penetrating vessels | 0.50 | |||||
| Present | 1.41 | 0.52, 3.81 | ||||
| Absent | Reference category | |||||
| PEAK0 | 0.99 | 0.96, 1.03 | 0.82 | |||
| TTP0 | 1.06 | 1.02, 1.095 | 0.004 | 1.06 | 1.01, 1.11 | 0.02 |
| RBV0 | 1.00 | 1.00, 1.00 | 0.60 | |||
| RBF0 | 0.99 | 0.97, 1.02 | 0.50 | |||
| MTT0 | 0.99 | 0.97, 1.03 | 0.94 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Zhou, L.; Li, H.; Wang, L.; Li, F.; Yin, W.; Wang, Y.; Jiang, L.; Lu, J. Multiparametric Contrast-Enhanced Ultrasound in Early Prediction of Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival in Breast Cancer. Diagnostics 2023, 13, 2378. https://doi.org/10.3390/diagnostics13142378
Wan C, Zhou L, Li H, Wang L, Li F, Yin W, Wang Y, Jiang L, Lu J. Multiparametric Contrast-Enhanced Ultrasound in Early Prediction of Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival in Breast Cancer. Diagnostics. 2023; 13(14):2378. https://doi.org/10.3390/diagnostics13142378
Chicago/Turabian StyleWan, Caifeng, Liheng Zhou, Hongli Li, Lin Wang, Fenghua Li, Wenjin Yin, Yaohui Wang, Lixin Jiang, and Jinsong Lu. 2023. "Multiparametric Contrast-Enhanced Ultrasound in Early Prediction of Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival in Breast Cancer" Diagnostics 13, no. 14: 2378. https://doi.org/10.3390/diagnostics13142378
APA StyleWan, C., Zhou, L., Li, H., Wang, L., Li, F., Yin, W., Wang, Y., Jiang, L., & Lu, J. (2023). Multiparametric Contrast-Enhanced Ultrasound in Early Prediction of Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival in Breast Cancer. Diagnostics, 13(14), 2378. https://doi.org/10.3390/diagnostics13142378





