Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
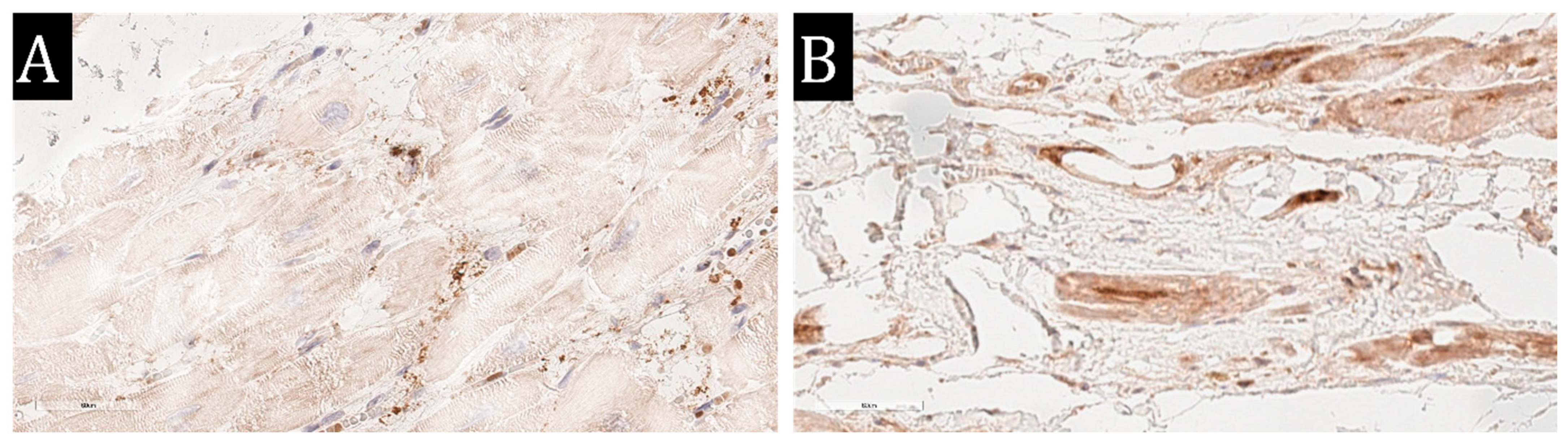
2.2. Histological Examination, Immunohistochemistry, and SARS-CoV-2 RT-ddPCR Test
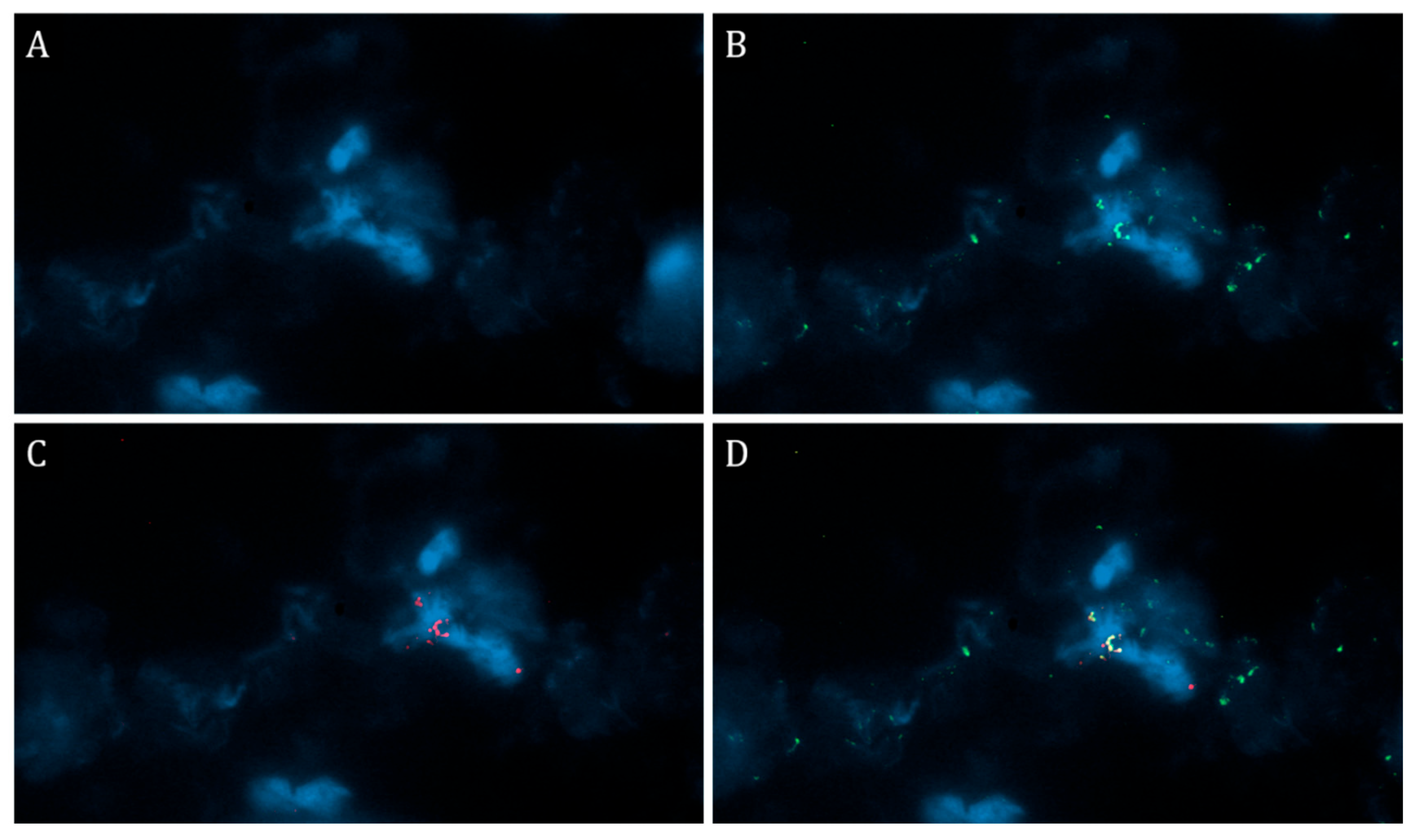
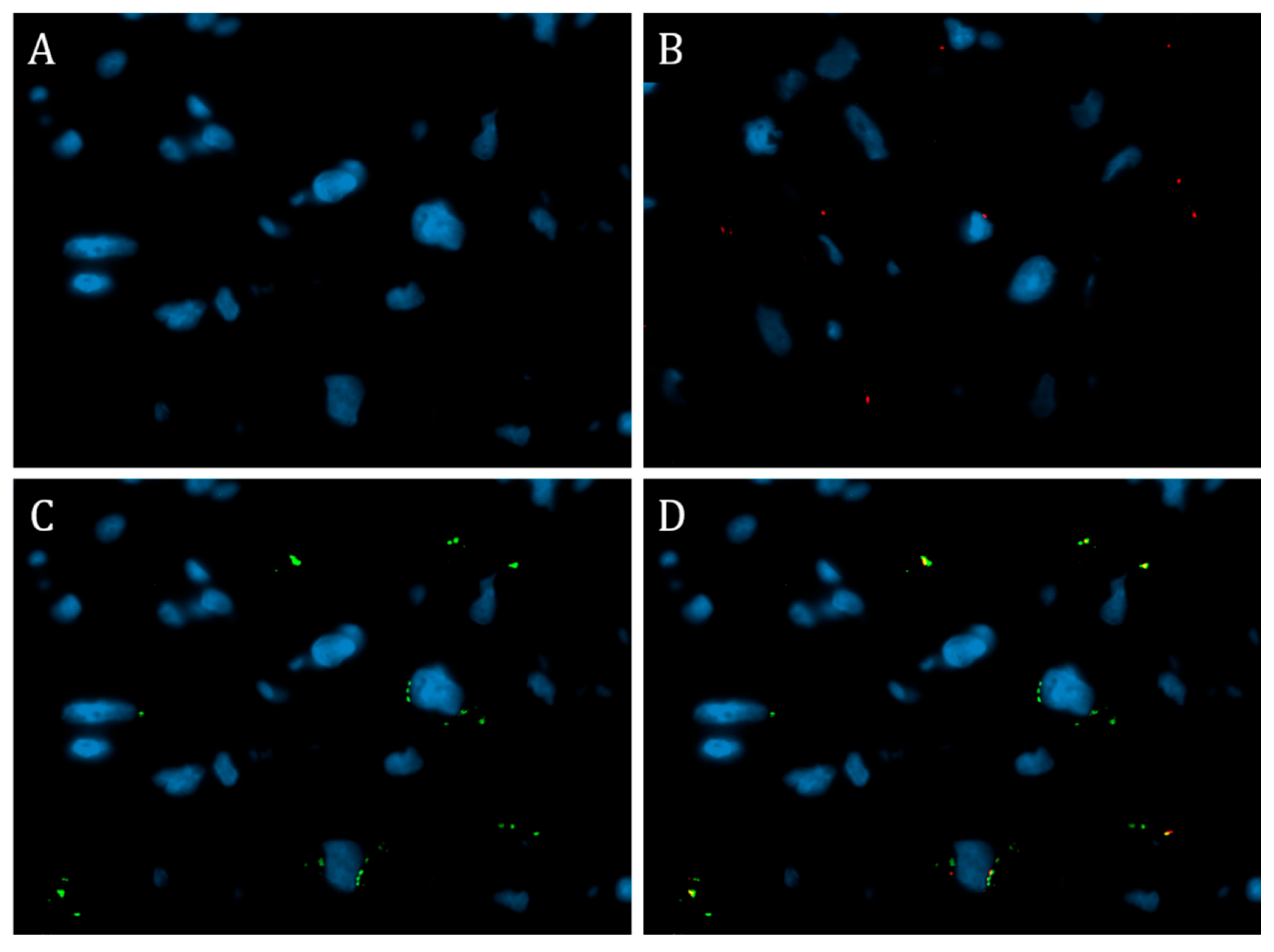
2.3. Immunofluorescence Test
2.4. Statistical Analysis
3. Results
3.1. Subgroup of Subjects with Chronic Lymphocytic Myocarditis
3.2. Subgroup of Subjects without Chronic Lymphocytic Myocarditis
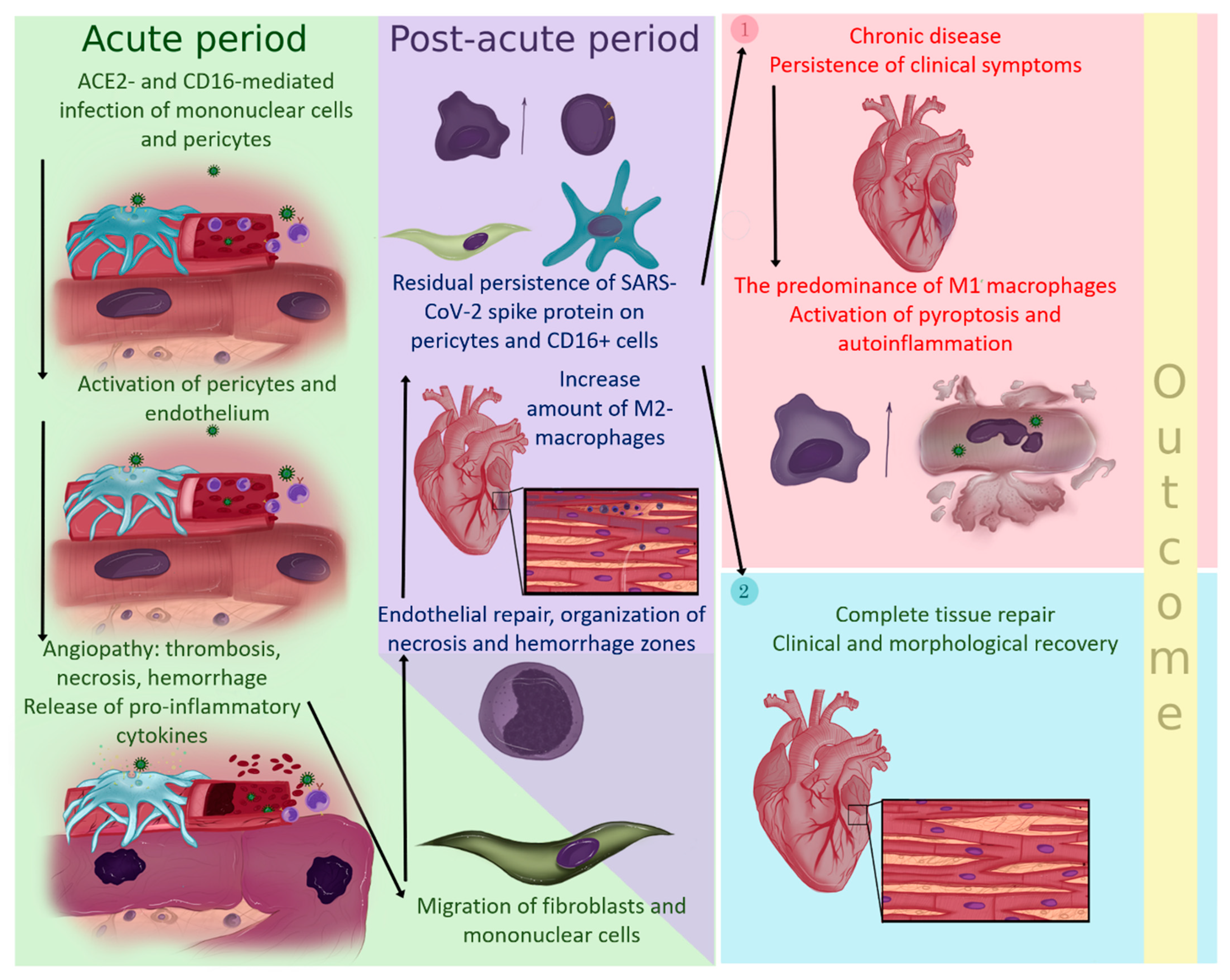
4. Discussion
5. Conclusions
6. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE 2 | angiotensin-converting enzyme 2 |
| AF | atrial fibrillation |
| EMB | endomyocardial biopsy |
| HF | heart failure |
| IFM | immunofluorescence microscopy |
| IHC | immunohistochemistry |
| LVEF | left ventricular ejection fraction |
| PASC | post-acute sequelae of SARS-CoV-2 infection |
| VWF | von Willebrand factor |
References
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors that May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.M.; Kudlay, D.A.; Kudryavtsev, I.V.; Starshinova, A.A.; Yablonsky, P.K.; Shoenfeld, Y. Immunogenetic predictors of severe COVID-19. Vaccines 2021, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Ogungbe, O.; Gilotra, N.A.; Davidson, P.M.; Farley, J.E.; Himmelfarb, C.R.D.; Post, W.S.; Commodore-Mensah, Y. Cardiac postacute sequelae symptoms of SARS-CoV-2 in community-dwelling adults: Cross-sectional study. Open Heart 2022, 9, e002084. [Google Scholar] [CrossRef]
- Kozlov, V.A.; Tikhonova, E.P.; Savchenko, A.A.; Kudryavtsev, I.V.; Andronova, N.V.; Anisimova, E.N.; Golovkin, A.S.; Demina, D.V.; Zdzitovetsky, D.E.; Kalinina, Y.u.S.; et al. Clinical immunology. In A Practical Guide for Infectious Disease Specialists; Polikor: Krasnoyarsk, Russia, 2021; 563p. [Google Scholar]
- Savchenko, A.A.; Tikhonova, E.; Kudryavtsev, I.; Kudlay, D.; Korsunsky, I.; Beleniuk, V.; Borisov, A. TREC/KREC Levels and T and B Lymphocyte Subpopulations in COVID-19 Patients at Different Stages of the Disease. Viruses 2022, 14, 646. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Suo, T.; Liu, X.; Feng, J.; Guo, M.; Hu, W.; Guo, D.; Ullah, H.; Yang, Y.; Zhang, Q.; Wang, X.; et al. ddPCR: A more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg. Microbes Infect. 2020, 9, 1259–1268. [Google Scholar] [CrossRef]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on myocarditis. J. Am. Coll. Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization with mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Writing Committee; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K., III; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef]
- Nalugo, M.; Schulte, L.J.; Masood, M.F.; Zayed, M.A. Microvascular Angiopathic Consequences of COVID-19. Front. Cardiovasc. Med. 2021, 8, 636843. [Google Scholar] [CrossRef]
- Dolhnikoff, M.; Ferreira Ferranti, J.; de Almeida Monteiro, R.A.; Duarte-Neto, A.N.; Soares Gomes-Gouvêa, M.; Viu Degaspare, N.; Figueiredo Delgado, A.; Montanari Fiorita, C.; Nunes Leal, G.; Rodrigues, R.M.; et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet. Child Adolesc. Health 2020, 4, 790–794. [Google Scholar] [CrossRef]
- Callegari, A.; Klingel, K.; Kelly-Geyer, J.; Berger, C.; Geiger, J.; Knirsch, W. Difficulties in diagnosis of SARS-CoV-2 myocarditis in an adolescent. Swiss Med. Wkly. 2022, 152, w30214. [Google Scholar] [CrossRef]
- Fox, S.E.; Falgout, L.; Vander Heide, R.S. COVID-19 myocarditis: Quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc. Pathol. 2021, 54, 107361. [Google Scholar] [CrossRef]
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M.; et al. Pathological Evidence for SARS-CoV-2 as a Cause of Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 314–325. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Azzawi, M.; Kan, S.W.; Hillier, V.; Yonan, N.; Hutchinson, I.V.; Hasleton, P.S. The distribution of cardiac macrophages in myocardial ischaemia and cardiomyopathy. Histopathology 2005, 46, 314–319. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qian, N.; Xu, J.; Wang, Y. The Roles of Macrophages in Heart Regeneration and Repair After Injury. Front. Cardiovasc. Med. 2021, 8, 744615. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef]
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242. [Google Scholar] [CrossRef]
- Mitrofanova, L.B.; Makarov, I.A.; Runov, A.L.; Vonsky, M.S.; Danilova, I.A.; Sidorin, V.S.; Moiseva, O.M.; Conradi, A.O. Clinical, morphological and molecular biological examination of the myocardium in COVID-19 patients. Russ. J. Cardiol. 2022, 27, 4810. [Google Scholar] [CrossRef]
- Vasichkina, E.; Kofeynikova, O.; Fetisova, S.; Starshinova, A.Y.; Sheyanova, E.; Vershinina, T.; Ryzhkov, A.; Skripnik, A.; Alekseeva, D.; Nechaeva, E.; et al. Severe Course of COVID-19 and Long-COVID-19 in Children: Difficulties in Diagnosis. Life 2023, 13, 781. [Google Scholar] [CrossRef]
- Vasichkina, E.; Alekseeva, D.; Kudryavtsev, I.; Glushkova, A.; Starshinova, A.Y.; Malkova, A.; Kudlay, D.; Starshinova, A. COVID-19 Heart Lesions in Children: Clinical, Diagnostic and Immunological Changes. Int. J. Mol. Sci. 2023, 24, 1147. [Google Scholar] [CrossRef]
- Vasichkina, E.; Alekseeva, D.; Karev, V.; Podyacheva, E.; Kudryavtsev, I.; Glushkova, A.; Starshinova, A.Y.; Kudlay, D.; Starshinova, A. Cardiac Involvement in Children Affected by COVID-19: Clinical Features and Diagnosis. Diagnostics 2023, 13, 120. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N.; Azzari, C.; Cardinale, F.; Di Mauro, G.; Galli, L.; Gattinara, G.C.; Fainardi, V.; Guarino, A.; Lancella, L.; et al. Italian intersociety consensus on management of long COVID in children. Ital. J. Pediatr. 2022, 48, 42. [Google Scholar] [CrossRef]
- Robinson, F.A.; Mihealsick, R.P.; Wagener, B.M.; Hanna, P.; Poston, M.D.; Efimov, I.R.; Shivkumar, K.; Hoover, D.B. Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1059–H1068. [Google Scholar] [CrossRef]
- Mitrofanova, L.B.; Makarov, I.A.; Gorshkov, A.N.; Runov, A.L.; Vonsky, M.S.; Pisareva, M.M.; Komissarov, A.B.; Makarova, T.A.; Li, Q.; Karonova, T.L.; et al. Comparative Study of the Myocardium of Patients from Four COVID-19 Waves. Diagnostics 2023, 13, 1645. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, R.; Tan, H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int. J. Biol. Sci. 2019, 15, 1345–1357. [Google Scholar] [CrossRef]
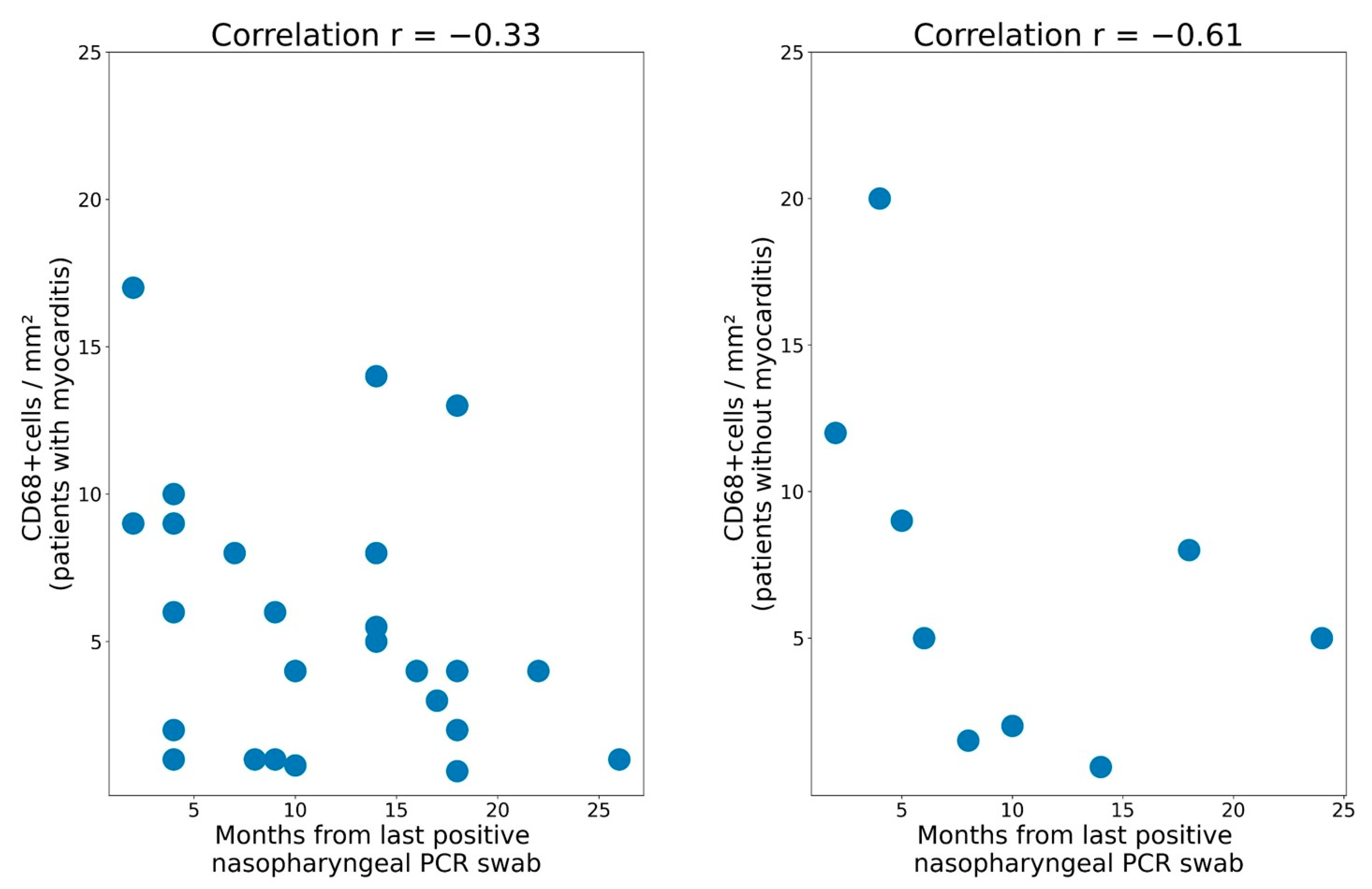
| Patient’s Parameters | Patients with Myocarditis with the PASC (n = 29) | Patients with Myocarditis in the Comparison Group (n = 33) | Patients without Myocarditis with the PASC (n = 9) | Patients without Myocarditis in the Comparison Group (n = 8) |
|---|---|---|---|---|
| Age, year (50%, 25%, and 75% percentiles) | 35 (32; 44) | 38 (30; 45) | 42 (38; 46) | 41 (36; 48) |
| Clinical suspicion of myocarditis (n, %) | 29 (100%) | 33 (100%) | 9 (100%) | 41 (100%) |
| Hs-cTnT > 100 ng/L (n, %) | 5 (17%) | 7 (21%) | 0 | 1 (13%) |
| Clinic of chronic heart failure (n, %) | 23 (79%) | 27 (82%) | 8 (89%) | 8 (100%) |
| Ventricular rhythm disorders (n, %) | 6 (21%) | 9 (27%) | 3 (33%) | 2 (25%) |
| Association with viral infection (n, %) | 29 (100%) | 30 (91%) | 9 (100%) | 6 (75%) |
| Association with vaccination (n) | 0 | 0 | 0 | 0 |
| Left ventricular ejection fraction at initial examination (n, 25% and 75% percentiles) | 28 (23; 33) % | 26 (20; 34) % | 27 (23; 30) % | 26 (22; 38) % |
| Presence of a clinical phenotype of dilated cardiomyopathy (n, %) | 12 (41%) | 17 (52%) | 7 (78%) | 4 (50%) |
| Immunohistochemical Markers | Patients without Myocarditis (n = 9) | Patients with Myocarditis Total (n = 29) | Patients with Borderline Myocarditis (n = 10) | Patients with Active Myocarditis (n = 19) |
|---|---|---|---|---|
| CD3 + cells/mm2 | 4.0 (3.0; 5.0) | 19.0 (13.0; 27.0) | 11.5 (8.0; 12.25) | 20.0 (16.5; 31.0) |
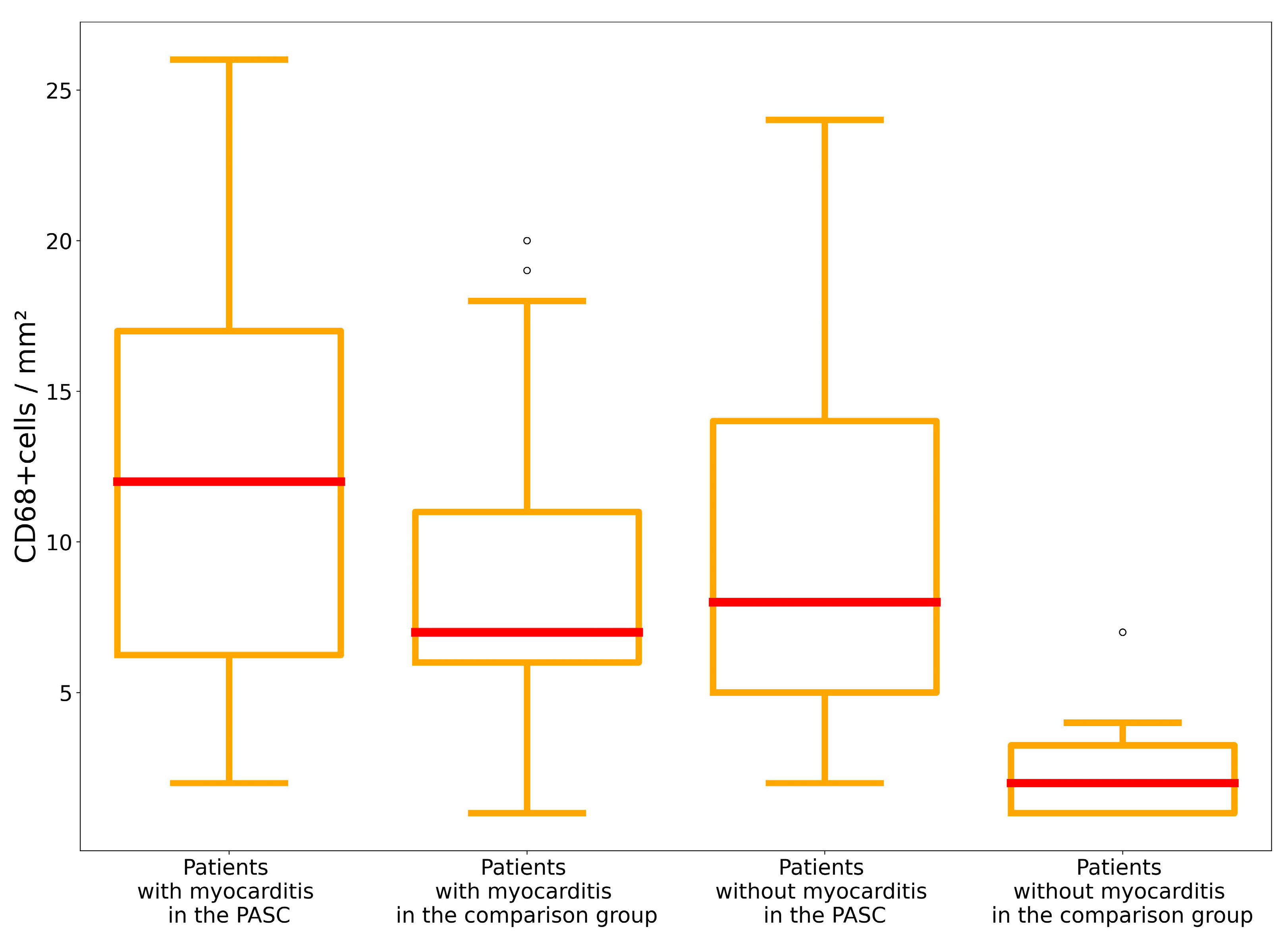
| CD68 + cells/mm2 | 8.0 (5.0; 14.0) | 11,5 (8.75; 14.5) | 11.0 (8.25; 15.5) | 12.0 (10.0; 17.5) |
| HLA-DR (score) | 1.0 (0.0; 3.0) | 3.0 (2.75; 4.0) | 3.0 (2.25; 4.0) | 3.0 (3.0; 4.0) |
| MHCI (score) | 4.0 (4.0; 4.0) | 4.0 (4.0; 4.0) | 4.0 (4.0; 4.0) | 4.0 (4.0; 4.0) |
| C1q (score) | 1.0 (1.0; 2.0) | 3.0 (2.0; 4.0) | 2.0 (2.0; 3.0) | 3.0 (2.0; 4.0) |
| VP1-EntV in vessels, score | 0.0 (0.0; 0.0) | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 1.0 (0.0; 1.5) |
| VP1-EntV in cardiomyocytes, score | 0.0 (0.0; 0.0) | 2.0 (1.0; 2.0) | 1.5 (1.0; 2.0) | 2.0 (1.0; 2.0) |
| VWF in vessels, score | 1.0 (1.0; 1.75) | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 1.0 (1.0; 1.5) |
| VWF in cardiomyocytes, score | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) |
| Ang1 in vessels, score | 4.0 (4.0; 4.0) | 3.0 (2.0; 4.0) | 4.0 (3.0; 4.0) | 2.0 (1.0; 2.5) |
| Ang1 in cardiomyocytes, score | 0.0 (0.0; 0.75) | 3.0 (2.0; 4.0) | 2.0 (2.0; 3.0) | 3.0 (2.0; 4.0) |
| VEGF in vessels, score | 2.5 (1.25; 3.75) | 3.0 (3.0; 4.0) | 3.0 (3.0; 3.0) | 4.0 (3.0; 4.0) |
| VEGF in cardiomyocytes, score | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) |
| SARS-CoV-2 spike protein in infiltrate cells, score | 2.0 (1.0; 2.0) | 1.0 (0.0; 1.25) | 0.0 (0.0; 1.0) | 1.0 (0.0; 1.25) |
| SARS-CoV-2 spike protein in endothelium, score | 1.0 (0.0; 1.0) | 0.0 (0.0; 1.0) | 0.0 (0.0; 1.0) | 0.0 (0.0; 1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.; Mayrina, S.; Makarova, T.; Karonova, T.; Starshinova, A.; Kudlay, D.; Mitrofanova, L. Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies. Diagnostics 2023, 13, 2212. https://doi.org/10.3390/diagnostics13132212
Makarov I, Mayrina S, Makarova T, Karonova T, Starshinova A, Kudlay D, Mitrofanova L. Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies. Diagnostics. 2023; 13(13):2212. https://doi.org/10.3390/diagnostics13132212
Chicago/Turabian StyleMakarov, Igor, Sofya Mayrina, Taiana Makarova, Tatiana Karonova, Anna Starshinova, Dmitry Kudlay, and Lubov Mitrofanova. 2023. "Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies" Diagnostics 13, no. 13: 2212. https://doi.org/10.3390/diagnostics13132212
APA StyleMakarov, I., Mayrina, S., Makarova, T., Karonova, T., Starshinova, A., Kudlay, D., & Mitrofanova, L. (2023). Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies. Diagnostics, 13(13), 2212. https://doi.org/10.3390/diagnostics13132212








