Concordance between Clinical and Pathological T and N Stages in Polish Patients with Head and Neck Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Concordance between Clinical and Pathological T Staging
3.3. Agreement between Clinical and Pathological N Staging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Trojanowski, M. Head and Neck Cancer Prevention Program; Wielkopolskie Centrum Onkologii: Poznań, Poland, 2016. [Google Scholar]
- Pinkas, W.; Jankowski, M.; Wierzba, W. Awareness of Head and Neck Cancers: A 2021 Nationwide Cross-Sectional Survey in Poland. J. Clin. Med. 2022, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef]
- Nguyen-Huu, N.H.; Thilly, N.; Derrough, T.; Sdona, E.; Claudot, F.; Pulcini, C.; Agrinier, N. HPV Policy working group. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine 2020, 38, 1315–1331. [Google Scholar] [CrossRef]
- Shang, C.; Feng, L.; Gu, Y.; Hong, H.; Hong, L.; Hou, J. Impact of Multidisciplinary Team Management on the Survival Rate of Head and Neck Cancer Patients: A Cohort Study Meta-analysis. Front. Oncol. 2021, 11, 630906. [Google Scholar] [CrossRef]
- Nigro, C.L.; Denaro, N.; Merlotti, A.; Merlano, M. Head and neck cancer: Improving outcomes with a multidisciplinary approach. Cancer Manag. Res. 2017, 9, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 727433. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679. [Google Scholar] [CrossRef]
- Shah, J.P.; Montero, P.H. New AJCC/UICC staging system for head and neck, and thyroid cancer. Rev. Med. Clin. Condes. 2018, 29, 397–404. [Google Scholar] [CrossRef]
- Rosen, R.D.; Sapra, A. TNM Classification. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553187/?report=classic (accessed on 13 February 2023).
- Glastonbury, C.M. Critical Changes in the Staging of Head and Neck Cancer. Radiol. Imaging Cancer 2020, 2, e190022. [Google Scholar] [CrossRef]
- Shah, J.P. Staging for Head and Neck Cancer: Purpose, Process and Progress. Indian J. Surg. Oncol. 2018, 9, 116–120. [Google Scholar] [CrossRef]
- Patel, S.G.; Shah, J.P. TNM Staging of Cancers of the Head and Neck: Striving for Uniformity Among Diversity. CA Cancer J. Clin. 2005, 55, 242–258. [Google Scholar] [CrossRef] [Green Version]
- Koch, W.M.; Ridge, J.A.; Forastiere, A.; Manola, J. Comparison of Clinical and Pathological Staging in Head and Neck Squamous Cell Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 851–858. [Google Scholar] [CrossRef] [Green Version]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Bangdiwala, S.I.; Shankar, V. The agreement chart. BMC Med. Res. Methodol. 2013, 13, 97. [Google Scholar] [CrossRef] [Green Version]
- Gawełko, J.; Cierpiał-Wolan, M.; Kawecki, A.; Wilk, K.; Pięciak-Kotlarz, D.B.; Sikorski, D. Comparative analysis of the incidence of head and neck cancer in south-eastern Poland and in Poland in the years 1990–2012. Contemp. Oncol. 2017, 21, 77–82. [Google Scholar] [CrossRef] [Green Version]
- López, F.; Mäkitie, A.; de Bree, R.; Franchi, A.; de Graaf, P.; Hernández-Prera, J.C.; Strojan, P.; Zidar, N.; Fležar, M.S.; Rodrigo, J.P.; et al. Qualitative and Quantitative Diagnosis in Head and Neck Cancer. Diagnostics 2021, 11, 1526. [Google Scholar] [CrossRef]
- Choi, N.; Noh, Y.; Lee, E.K.; Chung, M.; Baek, C.-H.; Baek, K.-H.; Jeong, H.-S. Discrepancy between cTNM and pTNM staging of oral cavity cancers and its prognostic significance. J. Surg. Oncol. 2017, 115, 1011–1018. [Google Scholar] [CrossRef]
- Eder-Czembirek, C.; Erlacher, B.; Thurnher, D.; Erovic, B.M.; Selzer, E.; Formanek, M. Comparative analysis of clinical and pathological lymph node staging data in head and neck squamous cell carcinoma patients treated at the General Hospital Vienna. Radiol. Oncol. 2018, 52, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Kreppel, M.; Nazarli, P.; Grandoch, A.; Safi, A.-F.; Zirk, M.; Nickenig, H.-J.; Scheer, M.; Rothamel, D.; Hellmich, M.; Zöller, J.E. Clinical and histopathological staging in oral squamous cell carcinoma–Comparison of the prognostic significance. Oral Oncol. 2016, 60, 68–73. [Google Scholar] [CrossRef]
- Bilge, T.Ü.R.K.; Kaya, K.S.; Ünsal, Ö.; Tetik, F.; Çelebi, İ.; Bozkurt, G.; Coşkun, B.U. What is the consistency between clinical and pathological staging in tongue cancer? Tr-ENT 2018, 28, 173–180. [Google Scholar]
- Biron, V.L.; AO’Connell, D.; Seikaly, H. The impact of clinical versus pathological staging in oral cavity carcinoma—A multi-institutional analysis of survival. J. Otolaryngol. Head Neck Surg. 2013, 42, 28. [Google Scholar] [CrossRef] [Green Version]
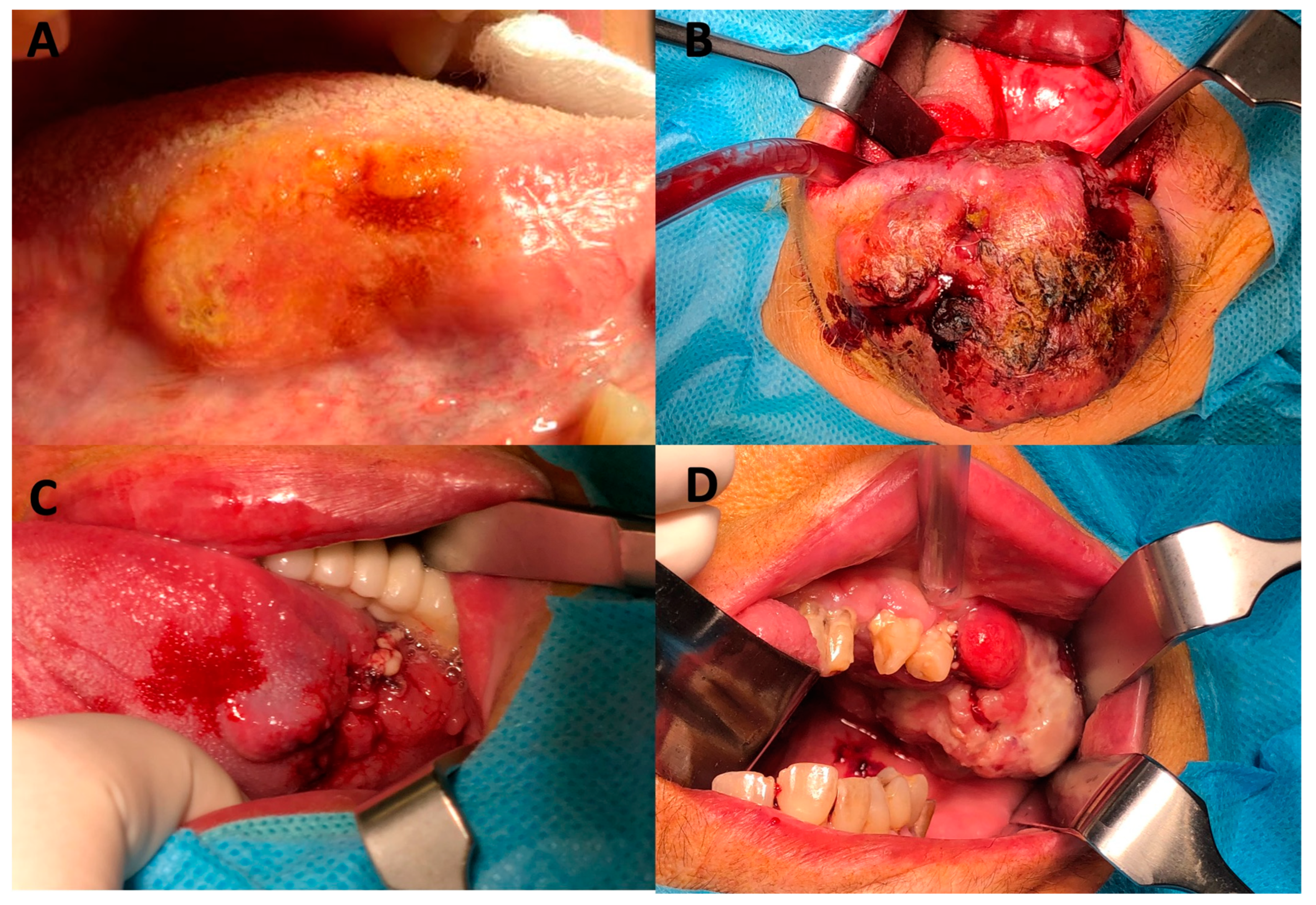

| Total Cohort | T-Stage Cohort | N-Stage Cohort | |
|---|---|---|---|
| Characteristic | n = 203 | n = 182 | n = 53 |
| Age, years | 64 (56, 73) | 64 (57, 74) | 64 (59, 67) |
| Women | 84 (42) | 72 (40) | 21 (40) |
| Histological type | |||
| Squamous cell carcinoma | 155 (76) | 148 (81) | 49 (92) |
| Other | 48 (24) | 34 (19) | 4 (8) |
| Grade | |||
| G1 | 13 (9) | 13 (9) | 0 |
| G2 | 112 (78) | 110 (78) | 38 (86) |
| G3 | 19 (13) | 18 (13) | 6 (14) |
| Pathological M stage | |||
| Mx | 126 (62) | 109 (59) | 31 (58) |
| M0 | 75 (37) | 72 (40) | 22 (42) |
| M1 | 2 (1.0) | 1 (1) | 1 (2) |
| Location * | |||
| Tongue | 74 (36) | 70 (38) | 32 (60) |
| Floor of mouth | 65 (32) | 63 (35) | 33 (62) |
| Mandible | 29 (14) | 27 (15) | 14 (26) |
| Lip | 29 (14) | 28 (15) | 4 (8) |
| Gum | 26 (13) | 24 (13) | 8 (15) |
| Maxilla | 18 (9) | 18 (10) | 0 (0) |
| Palette | 16 (8) | 15 (8) | 2 (4) |
| Cheek | 16 (8) | 12 (7) | 8 (15) |
| Other | 29 (14) | 24 (13) | 2 (4) |
| Surgery type | |||
| Resection | 196 (97) | 176 (97) | 52 (100) |
| Biopsy | 6 (3) | 5 (3) | 1 (2) |
| Nodes dissection | |||
| SND | 84 (41) | 78 (43) | 33 (62) |
| RND | 55 (27) | 53 (29) | 31 (58) |
| MRND | 35 (17) | 35 (19) | 20 (38) |
| ERND | 4 (2) | 3 (2) | 2 (4) |
| Adjuvant treatment | |||
| None | 137 (68) | 121 (66) | 24 (45) |
| Radiation | 57 (28) | 54 (30) | 25 (47) |
| Radiation and chemotherapy | 7 (3) | 6 (3) | 3 (6) |
| Chemotherapy | 2 (1) | 1 (1) | 1 (2) |
| Plastic surgery | |||
| Local | 121 (60) | 106 (58) | 30 (57) |
| Other | 58 (28) | 56 (31) | 19 (36) |
| None | 24 (12) | 20 (11) | 4 (7) |
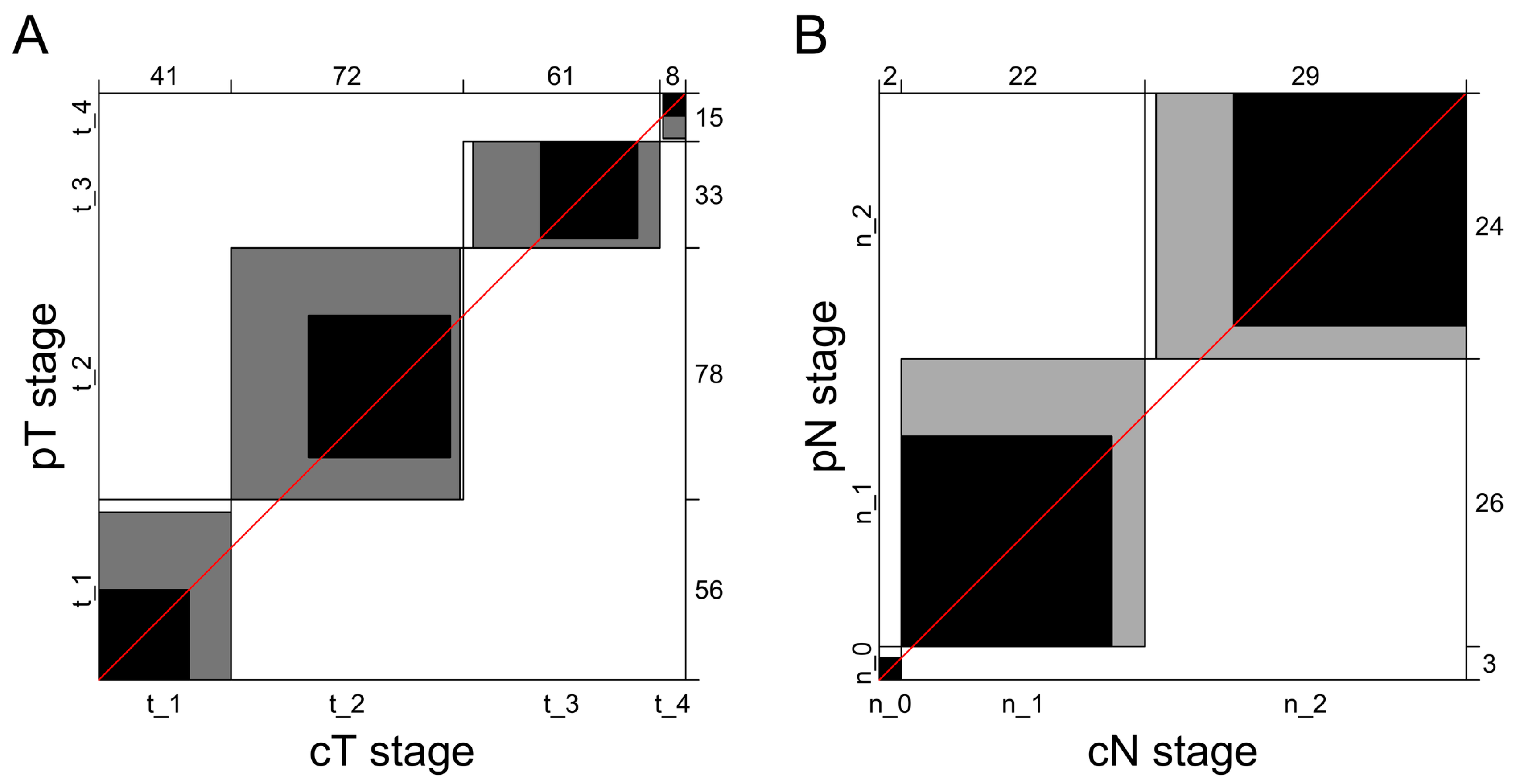
| Pathological Stage | Total, n (%) | cT1, n | cT2, n | cT3, n | cT4, n | Correct Diagnosis, n (%) | Underdiagnosis, n (%) | Overdiagnosis, n (%) |
|---|---|---|---|---|---|---|---|---|
| pT1 | 56 (30.8) | 28 | 24 | 3 | 1 | 28 (50) | 0 (0) | 28 (50) |
| pT2 | 78 (42.9) | 13 | 44 | 21 | 0 | 44 (56.4) | 13 (16.7) | 21 (26.9) |
| pT3 | 33 (18.1) | 0 | 3 | 30 | 0 | 30 (90.9) | 3 (9.1) | 0 (0) |
| pT4 | 15 (8.2) | 0 | 1 | 7 | 7 | 7 (46.7) | 8 (53.3) | 0 (0) |
| Pathological Stage | Total, n (%) | cN0, n | cN1, n | cN2, n | Correct Diagnosis, n (%) | Underdiagnosis, n (%) | Overdiagnosis, n (%) |
|---|---|---|---|---|---|---|---|
| pN0 | 3 (5.7) | 2 | 0 | 1 | 2 (66.7) | 0 (0) | 1 (33.3) |
| pN1 | 26 (49.0) | 0 | 19 | 7 | 19 (73.1) | 0 (0) | 7 (26.9) |
| pN2 | 24 (45.3) | 0 | 3 | 21 | 21 (87.5) | 3 (12.5) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chloupek, A.; Kania, J.; Jurkiewicz, D. Concordance between Clinical and Pathological T and N Stages in Polish Patients with Head and Neck Cancers. Diagnostics 2023, 13, 2202. https://doi.org/10.3390/diagnostics13132202
Chloupek A, Kania J, Jurkiewicz D. Concordance between Clinical and Pathological T and N Stages in Polish Patients with Head and Neck Cancers. Diagnostics. 2023; 13(13):2202. https://doi.org/10.3390/diagnostics13132202
Chicago/Turabian StyleChloupek, Aldona, Joanna Kania, and Dariusz Jurkiewicz. 2023. "Concordance between Clinical and Pathological T and N Stages in Polish Patients with Head and Neck Cancers" Diagnostics 13, no. 13: 2202. https://doi.org/10.3390/diagnostics13132202
APA StyleChloupek, A., Kania, J., & Jurkiewicz, D. (2023). Concordance between Clinical and Pathological T and N Stages in Polish Patients with Head and Neck Cancers. Diagnostics, 13(13), 2202. https://doi.org/10.3390/diagnostics13132202





