Diagnostic Value of Serum Creatinine and Cystatin-C-Based Indices and Ishii Score in Cancer-Related Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection and Anthropometric Measures
2.3. Assessment of Sarcopenia
2.4. Laboratory Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Association between the CCR, SI, Ishii Score, and Sarcopenia
3.3. Optimal Cut-Off Points of the CCR, SI, and Ishii Scores for Sarcopenia Screening
3.4. Effects of the CCR, SI, and Ishii Scores as Predictors of Sarcopenia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M. Recent Progress in Sarcopenia Research: A Focus on Operationalizing a Definition of Sarcopenia. Curr. Osteoporos. Rep. 2018, 16, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.; Jones, L.; Andersen, J.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle dysfunction in cancer patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef]
- Haiducu, C.; Buzea, A.; Mirea, L.E.; Dan, G.A. The prevalence and the impact of sarcopenia in digestive cancers. A systematic review. Romanian J. Intern. Med. 2021, 59, 328–344. [Google Scholar] [CrossRef]
- Bye, A.; Sjøblom, B.; Wentzel-Larsen, T.; Grønberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Fløtten, F.; Jordhøy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachex Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef]
- Brown, J.C.; Caan, B.J.; Meyerhardt, J.A.; Weltzien, E.; Xiao, J.; Feliciano, E.M.C.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Prado, C.M. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia Sarcopenia Muscle 2018, 9, 664–672. [Google Scholar] [CrossRef]
- Huang, D.-D.; Chen, X.-X.; Chen, X.-Y.; Wang, S.-L.; Shen, X.; Yu, Z.; Zhuang, C.-L. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: A prospective study. J. Cancer Res. Clin. Oncol. 2016, 142, 2347–2356. [Google Scholar] [CrossRef]
- Van der Kroft, G.; Damink, S.W.M.O.; Neumann, U.P.; Lambertz, A. Der Einfluss von Kachexie und Sarkopenie auf das postoperative Outcome [Sarcopenia and Cachexia-associated Risk in Surgery]. Zentralbl. Chir. 2021, 146, 277–282. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Kashani, K.B.; Frazee, E.N.; Kukrálová, L.; Sarvottam, K.; Herasevich, V.; Young, P.M.; Kashyap, R.; Lieske, J.C. Evaluating Muscle Mass by Using Markers of Kidney Function: Development of the Sarcopenia Index. Crit. Care Med. 2017, 45, e23–e29. [Google Scholar] [CrossRef]
- Lien, Y.-H.H. Looking for Sarcopenia Biomarkers. Am. J. Med. 2017, 130, 502–503. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Xie, L.; Hu, S.; Tan, L.; Lei, X.; Luo, X.; Yang, L.; Yang, M. Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J. Cachex Sarcopenia Muscle 2022, 13, 1800–1810. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014, 14, 93–101. [Google Scholar] [CrossRef]
- Erdogan, T.; Catikkas, N.M.; Oren, M.M.; Kılıc, C.; Karan, M.A.; Bahat, G. Ishii test for screening sarcopenia: Performance in community-dwelling older adults. Aging Clin. Exp. Res. 2022, 34, 785–791. [Google Scholar] [CrossRef]
- Barreto, E.F.; Poyant, J.O.; Coville, H.H.; Dierkhising, R.A.; Kennedy, C.C.; Gajic, O.; Nystrom, E.M.; Takahashi, N.; Moynagh, M.R.; Kashani, K.B. Validation of the sarcopenia index to assess muscle mass in the critically ill: A novel application of kidney function markers. Clin. Nutr. 2019, 38, 1362–1367. [Google Scholar] [CrossRef]
- Kashani, K.; Sarvottam, K.; Pereira, N.L.; Barreto, E.F.; Kennedy, C.C. The sarcopenia index: A novel measure of muscle mass in lung transplant candidates. Clin. Transplant. 2018, 32, e13182. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum 14 creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, T.; Chen, X.; Wang, M.; Jiang, W.; Jia, H. Comparison of the Diagnostic Value of SARC-F and Its Three Modified Versions for Screening Sarcopenia in Chinese Community-Dwelling Older Adults. J. Nutr. Health Aging 2022, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Loeliger, J.; Findlay, M.; Isenring, E.; Baguley, B.J.; Boltong, A.; Butler, A.; Deftereos, I.; Eisenhuth, M.; Fraser, S.F.; et al. Clinical Oncology Society of Australia: Position statement on cancer-related malnutrition and sarcopenia. Nutr. Diet. 2020, 77, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Kashani, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 2016, 8, E305–E311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, K.; Tanaka, A.; Homma, T.; Goto, Y.; Akimoto, K.; Uno, T.; Yoshitaka, U.; Miyata, Y.; Inoue, H.; Ohta, S.; et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin. Nutr. 2021, 40, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Hamaguchi, M.; Hashimoto, Y.; Ushigome, E.; Tanaka, M.; Yamazaki, M.; Fukui, M. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 52–58. [Google Scholar] [CrossRef]
- Xin, C.; Xie, J.; Fan, H.; Sun, X.; Shi, B. Association Between Serum Cystatin C and Thyroid Diseases: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 766516. [Google Scholar] [CrossRef]
- Dikovskaya, M.A.; Russkikh, G.S.; Loktev, K.V.; Johnston, T.P.; Gevorgyan, M.M.; Voronina, N.P.; Chernykh, V.V.; Trunov, A.N.; Korolenko, T.A. Cystatin C and cystatin SN as possible soluble tumor markers in malignant uveal melanoma. Radiol. Oncol. 2022, 56, 83–91. [Google Scholar] [CrossRef]
- Lu, J.-L.; Xu, Q.; Zhu, S.-Q.; Chen, L.; Ding, L.-Y.; Hua, H.-X.; Xu, X.-Y.; Hu, J.-M. Comparison of five sarcopenia screening tools in preoperative patients with gastric cancer using the diagnostic criteria of the European Working Group on Sarcopenia in Older People 2. Nutrition 2022, 95, 111553. [Google Scholar] [CrossRef]
- Lu, J.-L.; Xu, X.-Y.; Chen, L.; Ding, L.-Y.; Hu, J.-M.; Li, W.-Y.; Zhu, S.-Q.; Xu, Q. The Predictive Values of Five Sarcopenia Screening Tools on Clinical Outcomes Following Surgery in Patients with Gastric Cancer: A Prospective Cohort Study. J. Nutr. Health Aging 2022, 26, 259–265. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Chen, H.; Chu, A.; Song, G.; Cui, Y. Accuracy and prognostic ability of the SARC-F questionnaire and Ishii’s score in the screening of sarcopenia in geriatric inpatients. Braz. J. Med. Biol. Res. 2019, 52, e8204. [Google Scholar] [CrossRef]
- Yao, R.; Yao, L.; Yuan, C.; Gao, B.-L. Accuracy of Calf Circumference Measurement, SARC-F Questionnaire, and Ishii’s Score for Screening Stroke-Related Sarcopenia. Front. Neurol. 2022, 13, 880907. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual Energy X-Ray Absorptiometry Body Composition Reference Values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef] [Green Version]




| Variable | Clinical Practice | EWGSOP2 Cut-Off Points | AWGS2019 Cut-Off Points |
|---|---|---|---|
| Muscle strength | HGS | Male < 27 kg, female < 16 kg | Male < 28 kg, female < 18 kg |
| Chair stand test | >15 s for five rises | >12 s for five rises | |
| Muscle quantity | ASM/height2 | Male < 7.0 kg/m2, female < 5.5 kg/m2 | DXA: male < 7.0 kg/m2, female < 5.4 kg/m2 |
| BIA: male < 7.0 kg/m2, female < 5.7 kg/m2 | |||
| Physical performance | 6 m pace | ≤0.8 m/s | ≤1.0 m/s |
| SPPB | ≤8 score | ≤9 score |
| Variables | All Patients | Non-Sarcopenia | Sarcopenia | p |
|---|---|---|---|---|
| N (%) | 215 (100) | 112 (52.1) | 103 (47.9) | N/A |
| Age (years) | 60.5 (17, 80) | 58 (17, 76) | 66 (44, 80) | <0.001 |
| Sex | ||||
| Male, N (%) | 117 (54.4) | 44 (37.6) | 73 (62.4) | <0.001 |
| Female, N (%) | 98 (45.6) | 68 (69.4) | 30 (30.6) | |
| Diagnosis | ||||
| Digestive system cancer, N (%) | 96 (44.7) | 42 (43.8) | 54 (56.2) | 0.028 |
| Others, N (%) | 119 (55.3) | 70 (58.8) | 49 (41.2) | |
| Tumor stage | ||||
| Ⅲ, N (%) | 91 (42.3) | 54 (59.3) | 37 (40.7) | 0.068 |
| Ⅳ, N (%) | 124 (57.7) | 58 (46.8) | 66 (53.2) | |
| BMI(kg/m2) | 22.03 ± 3.25 | 23.83 ± 2.84 | 20.08 ± 2.45 | <0.001 |
| HGS(kg) | 23.2 ± 6.52 | 24.6 ± 6.60 | 21.7 ± 6.10 | 0.01 |
| CC (cm) | 31.5 ± 3.01 | 33.1 ± 2.65 | 29.8 ± 2.35 | <0.001 |
| Slow gait speed (<1.0 m/s), N (%) | 90 (41.8) | 24 (26.7) | 66 (73.3) | <0.001 |
| SMI (kg/m2) | 6.35 ± 0.75 | 6.67 ± 0.71 | 6.0 ± 0.64 | <0.001 |
| Serum Cr (mg/dL) | 0.67 ± 0.15 | 0.67 ± 0.16 | 0.66 ± 0.15 | 0.836 |
| Serum CysC (mg/L) | 0.99 ± 0.18 | 0.95 ± 0.17 | 1.04 ± 0.19 | <0.001 |
| eGFRCysC (mL/min.1.73 m2) | 90.6 ± 19.73 | 95.0 ± 18.76 | 85.9 ± 19.75 | 0.01 |
| CCR | 67.8 ± 0.82 | 70.9 ± 12.46 | 64.4 ± 10.68 | <0.001 |
| SI | 50.98 ± 10.83 | 54.09 ± 11.36 | 47.59 ± 9.12 | <0.001 |
| Ishii score | 113.6 ± 34.73 | 93.5 ± 27.82 | 135.4 ± 27.63 | <0.001 |
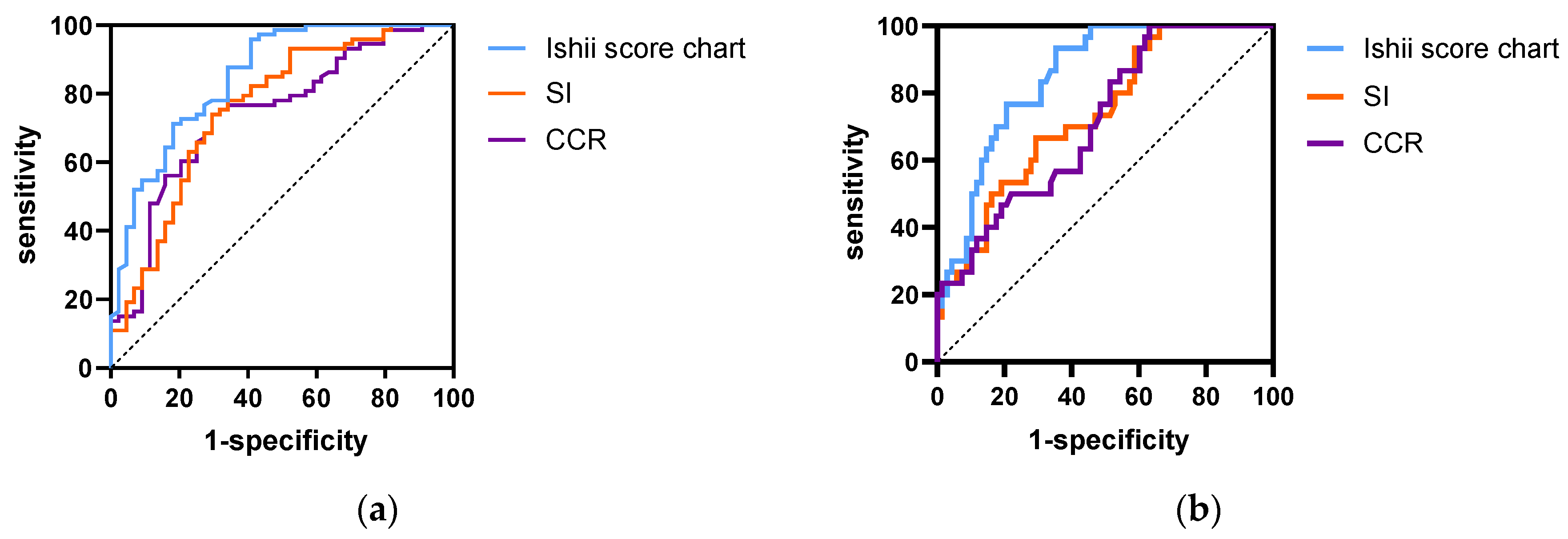
| Screening Tools | AUC% (95%CI) | Cut-Off Value (Points) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden Index |
|---|---|---|---|---|---|---|---|
| CCR | |||||||
| Male | 0.743 (0.65–0.836) | 71.95 | 74 | 70.5 | 80.6 | 62 | 0.445 |
| Female | 0.714 (0.61–0.818) | 66.5 | 86.7 | 45.6 | 41.27 | 88.57 | 0.323 |
| SI | |||||||
| Male | 0.758 (0.665–0.852) | 55.34 | 74 | 70.5 | 80.6 | 62 | 0.445 |
| Female | 0.737 (0.635–0.839) | 44.4 | 66.7 | 70.6 | 50 | 82.76 | 0.373 |
| Ishii score | |||||||
| Male | 0.833 (0.751–0.909) | 102.3 | 93.2 | 59.1 | 79.07 | 83.87 | 0.523 |
| Female | 0.849 (0.775–0.932) | 98.3 | 93.3 | 64.7 | 52.83 | 95.6 | 0.580 |
| Variables 1 | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR(95%CI) | p Value | OR(95%CI) | p Value | OR(95%CI) | p Value | |
| Sex | 4.509 (1.64–12.38) | 0.003 | 5.013 (1.80–13.96) | 0.002 | 1.480 (0.63–3.47) | 0.367 |
| Age (years) | 1.073 (1.03–1.12) | 0.001 | 1.044 (0.99–1.09) | 0.056 | 1.020 (0.97–1.07) | 0.421 |
| Diagnosis | 0.639 (0.28–1.46) | 0.286 | 0.677 (0.30–1.54) | 0.351 | 0.728 (0.31–1.69) | 0.460 |
| Tumor stage | 0.801 (0.36–1.81) | 0.594 | 0.828 (0.37–1.87) | 0.649 | 0.376 (0.15–0.92) | 0.032 |
| BMI | 0.624 (0.54–0.73) | <0.001 | 0.617 (0.53–0.72) | <0.001 | 0.667 (0.57–0.79) | <0.001 |
| Albumin (g/L) | 1.005 (0.92–1.10) | 0.91 | 1.004 (0.92–1.10) | 0.936 | 0.999 (0.91–1.09) | 0.974 |
| CCR | 0.922 (0.89–0.96) | <0.001 | - | - | - | - |
| SI | - | - | 0.905 (0.86–0.95) | <0.001 | - | - |
| Ishii | - | - | - | - | 1.043(1.02–1.06) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Wang, X.; Mao, T.; Li, J. Diagnostic Value of Serum Creatinine and Cystatin-C-Based Indices and Ishii Score in Cancer-Related Sarcopenia. Diagnostics 2023, 13, 2179. https://doi.org/10.3390/diagnostics13132179
Ding L, Wang X, Mao T, Li J. Diagnostic Value of Serum Creatinine and Cystatin-C-Based Indices and Ishii Score in Cancer-Related Sarcopenia. Diagnostics. 2023; 13(13):2179. https://doi.org/10.3390/diagnostics13132179
Chicago/Turabian StyleDing, Liming, Xingyu Wang, Tiantao Mao, and Jibin Li. 2023. "Diagnostic Value of Serum Creatinine and Cystatin-C-Based Indices and Ishii Score in Cancer-Related Sarcopenia" Diagnostics 13, no. 13: 2179. https://doi.org/10.3390/diagnostics13132179
APA StyleDing, L., Wang, X., Mao, T., & Li, J. (2023). Diagnostic Value of Serum Creatinine and Cystatin-C-Based Indices and Ishii Score in Cancer-Related Sarcopenia. Diagnostics, 13(13), 2179. https://doi.org/10.3390/diagnostics13132179





