Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Post-Cardiac-Arrest Care
2.3. Data Acquisition
2.3.1. Baseline Characteristics
2.3.2. Gray-White Matter Ratio
2.3.3. Clinical Examination
2.3.4. Serum Biomarkers
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Total Cohort
3.2. Associations between Prognostic Tests and Neurological Outcomes
3.3. Prognostic Performances of Single Prognostic Tests and Combination Strategies
3.4. CART Analysis for Good Neurological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, K.N.; Youn, C.S.; Chae, M.K.; Kim, W.Y.; Lee, B.K.; Lee, D.H.; Jang, T.C.; Lee, J.H.; Choi, Y.H.; et al. Outcome and status of postcardiac arrest care in Korea: Results from the Korean Hypothermia Network prospective registry. Clin. Exp. Emerg. Med. 2020, 7, 250–258. [Google Scholar] [CrossRef]
- Ro, Y.S.; Shin, S.D.; Song, K.J.; Lee, E.J.; Kim, J.Y.; Ahn, K.O.; Chung, S.P.; Kim, Y.T.; Hong, S.O.; Choi, J.A.; et al. A trend in epidemiology and outcomes of out-of-hospital cardiac arrest by urbanization level: A nationwide observational study from 2006 to 2010 in South Korea. Resuscitation 2013, 84, 547–557. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, S.O.; Shin, S.D.; Yang, H.J.; Chung, S.P.; Lee, S.W.; Song, K.J.; Hwang, S.S.; Cho, G.C.; Moon, S.W.; et al. Korean Cardiac Arrest Research Consortium (KoCARC): Rationale, development, and implementation. Clin. Exp. Emerg. Med. 2018, 5, 165–176. [Google Scholar] [CrossRef]
- The Lancet. Out-of-hospital cardiac arrest: A unique medical emergency. Lancet 2018, 391, 911. [Google Scholar] [CrossRef]
- Elmer, J.; Torres, C.; Aufderheide, T.P.; Austin, M.A.; Callaway, C.W.; Golan, E.; Herren, H.; Jasti, J.; Kudenchuk, P.J.; Scales, D.C.; et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016, 102, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Perman, S.M.; Kirkpatrick, J.N.; Reitsma, A.M.; Gaieski, D.F.; Lau, B.; Smith, T.M.; Leary, M.; Fuchs, B.D.; Levine, J.M.; Abella, B.S.; et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit. Care Med. 2012, 40, 719–724. [Google Scholar] [CrossRef]
- Bronder, J.; Cho, S.-M.; Geocadin, R.G.; Ritzl, E.K. Revisiting EEG as part of the multidisciplinary approach to post-cardiac arrest care and prognostication: A review. Resusc. Plus 2021, 9, 100189. [Google Scholar] [CrossRef]
- Sandroni, C.; Taccone, F.S. Does early withdrawal of life-sustaining treatment increase mortality after cardiac arrest? Resuscitation 2016, 102, A3–A4. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-Resuscitation Care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W. Targeted temperature management with hypothermia for comatose patients after cardiac arrest. Clin. Exp. Emerg. Med. 2023, 10, 5–17. [Google Scholar] [CrossRef]
- Albaeni, A.; Chandra-Strobos, N.; Vaidya, D.; Eid, S.M. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation 2014, 85, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Wahlster, S.; Danielson, K.; Craft, L.; Matin, N.; Town, J.A.; Srinivasan, V.; Schubert, G.; Carlbom, D.; Kim, F.; Johnson, N.J.; et al. Factors Associated with Early Withdrawal of Life-Sustaining Treatments After Out-of-Hospital Cardiac Arrest: A Subanalysis of a Randomized Trial of Prehospital Therapeutic Hypothermia. Neurocritical Care 2022, 38, 676–687. [Google Scholar] [CrossRef]
- Torbey, M.T.; Selim, M.; Knorr, J.; Bigelow, C.; Recht, L. Quantitative Analysis of the Loss of Distinction Between Gray and White Matter in Comatose Patients after Cardiac Arrest. Stroke 2000, 31, 2163–2167. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; Routledge: London, UK, 2017. [Google Scholar]
- Takahashi, O.; Cook, E.; Nakamura, T.; Saito, J.; Ikawa, F.; Fukui, T. Risk stratification for in-hospital mortality in spontaneous intracerebral haemorrhage: A Classification and Regression Tree Analysis. Qjm Int. J. Med. 2006, 99, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geocadin, R.G.; Callaway, C.W.; Fink, E.L.; Golan, E.; Greer, D.M.; Ko, N.U.; Lang, E.; Licht, D.J.; Marino, B.S.; McNair, N.D.; et al. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e517–e542. [Google Scholar] [CrossRef]
- Admiraal, M.M.; Van Rootselaar, A.; Hofmeijer, J.; Hoedemaekers, C.W.; Van Kaam, C.R.; Msc., H.M.K.; Van Putten, M.J.; Schultz, M.J.; Horn, J. Electroencephalographic reactivity as predictor of neurological outcome in postanoxic coma: A multicenter prospective cohort study. Ann. Neurol. 2019, 86, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Eertmans, W.; Tran, T.M.P.; Genbrugge, C.; Peene, L.; Mesotten, D.; Dens, J.; Jans, F.; De Deyne, C. A prediction model for good neurological outcome in successfully resuscitated out-of-hospital cardiac arrest patients. Scand. J. Trauma, Resusc. Emerg. Med. 2018, 26, 93. [Google Scholar] [CrossRef]
- Sandroni, C.; D’arrigo, S.; Cacciola, S.; Hoedemaekers, C.W.E.; Westhall, E.; Kamps, M.J.A.; Taccone, F.S.; Poole, D.; Meijer, F.J.A.; Antonelli, M.; et al. Prediction of good neurological outcome in comatose survivors of cardiac arrest: A systematic review. Intensiv. Care Med. 2022, 48, 389–413. [Google Scholar] [CrossRef]
- Zellner, T.; Gärtner, R.; Schopohl, J.; Angstwurm, M. NSE and S-100B are not sufficiently predictive of neurologic outcome after therapeutic hypothermia for cardiac arrest. Resuscitation 2013, 84, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Moseby-Knappe, M.; Mattsson-Carlgren, N.; Stammet, P.; Backman, S.; Blennow, K.; Dankiewicz, J.; Friberg, H.; Hassager, C.; Horn, J.; Kjaergaard, J.; et al. Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensiv. Care Med. 2021, 47, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.; Quiroga, D.F.T.; Juan, E.; Novy, J.; White, R.D.; Ben-Hamouda, N.; Britton, J.W.; Oddo, M.; Rabinstein, A.A. Electroencephalography Predicts Poor and Good Outcomes after Cardiac Arrest. Crit. Care Med. 2017, 45, e674–e682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted Temperature Management at 33 °C versus 36 °C after Cardiac Arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseby-Knappe, M.; Pellis, T.; Dragancea, I.; Friberg, H.; Nielsen, N.; Horn, J.; Kuiper, M.; Roncarati, A.; Siemund, R.; Undén, J.; et al. Head computed tomography for prognostication of poor outcome in comatose patients after cardiac arrest and targeted temperature management. Resuscitation 2017, 119, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Metter, R.B.; Rittenberger, J.C.; Guyette, F.X.; Callaway, C.W. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation 2011, 82, 1180–1185. [Google Scholar] [CrossRef] [Green Version]
- Scarpino, M.; Lanzo, G.; Lolli, F.; Carrai, R.; Moretti, M.; Spalletti, M.; Cozzolino, M.; Peris, A.; Amantini, A.; Grippo, A. Neurophysiological and neuroradiological multimodal approach for early poor outcome prediction after cardiac arrest. Resuscitation 2018, 129, 114–120. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.E.; Choi, J.Y.; Gho, Y.-R.; Chae, M.K.; Park, E.J.; Choi, M.H.; Hong, J.M. Useful Computed Tomography Score for Estimation of Early Neurologic Outcome in Post-Cardiac Arrest Patients with Therapeutic Hypothermia. Circ. J. 2017, 81, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Adriaansens, K.; Jewbali, L.; Lemkes, J.; Spoormans, E.; Meuwissen, M.; Blans, M.; van der Harst, P.; Eikemans, B.; Bleeker, G.; Beishuizen, A.; et al. Routine reporting of grey-white matter differentiation in early brain computed tomography in comatose patients after cardiac arrest: A substudy of the COACT trial. Resuscitation 2022, 175, 13–18. [Google Scholar] [CrossRef]
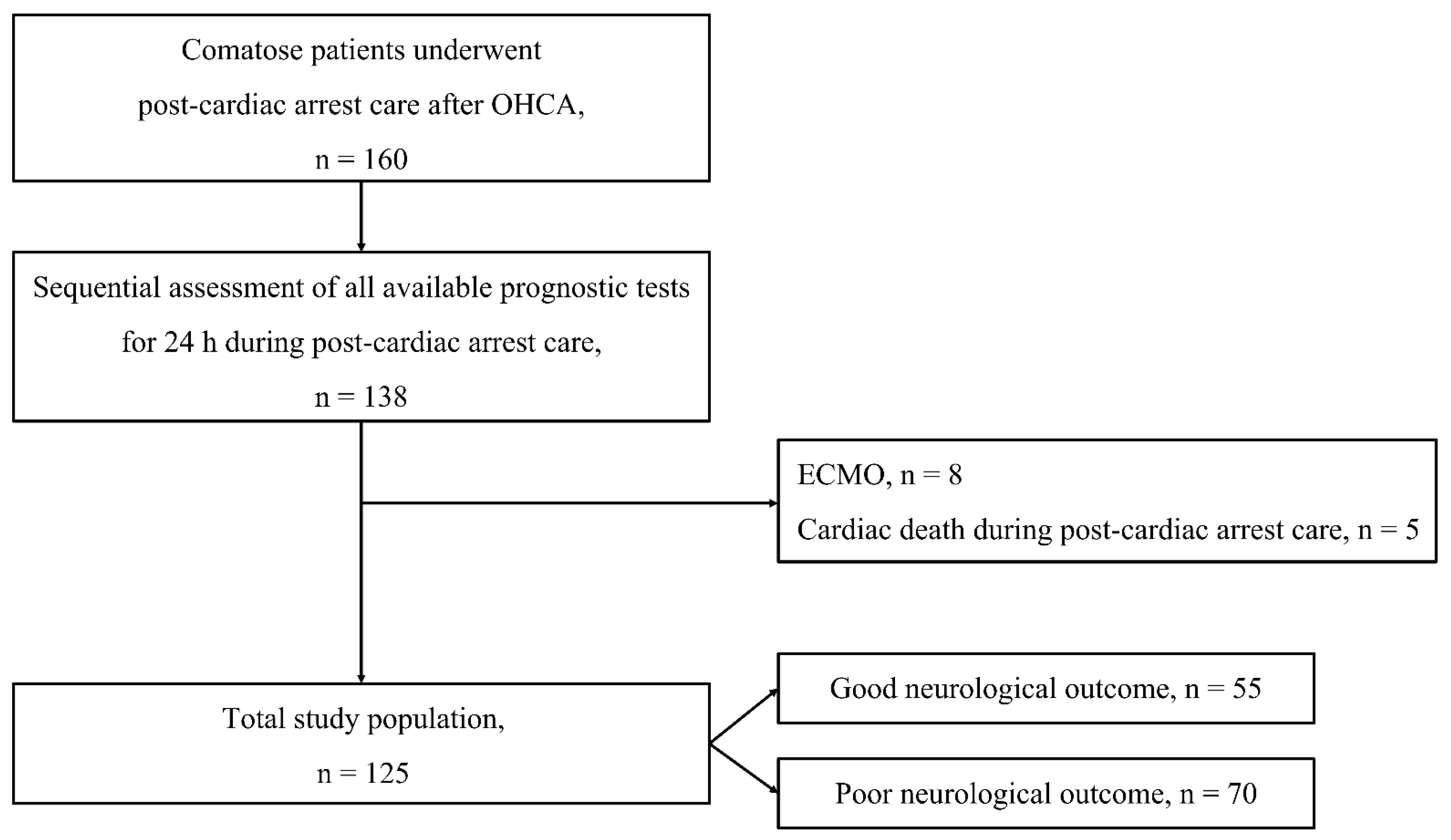
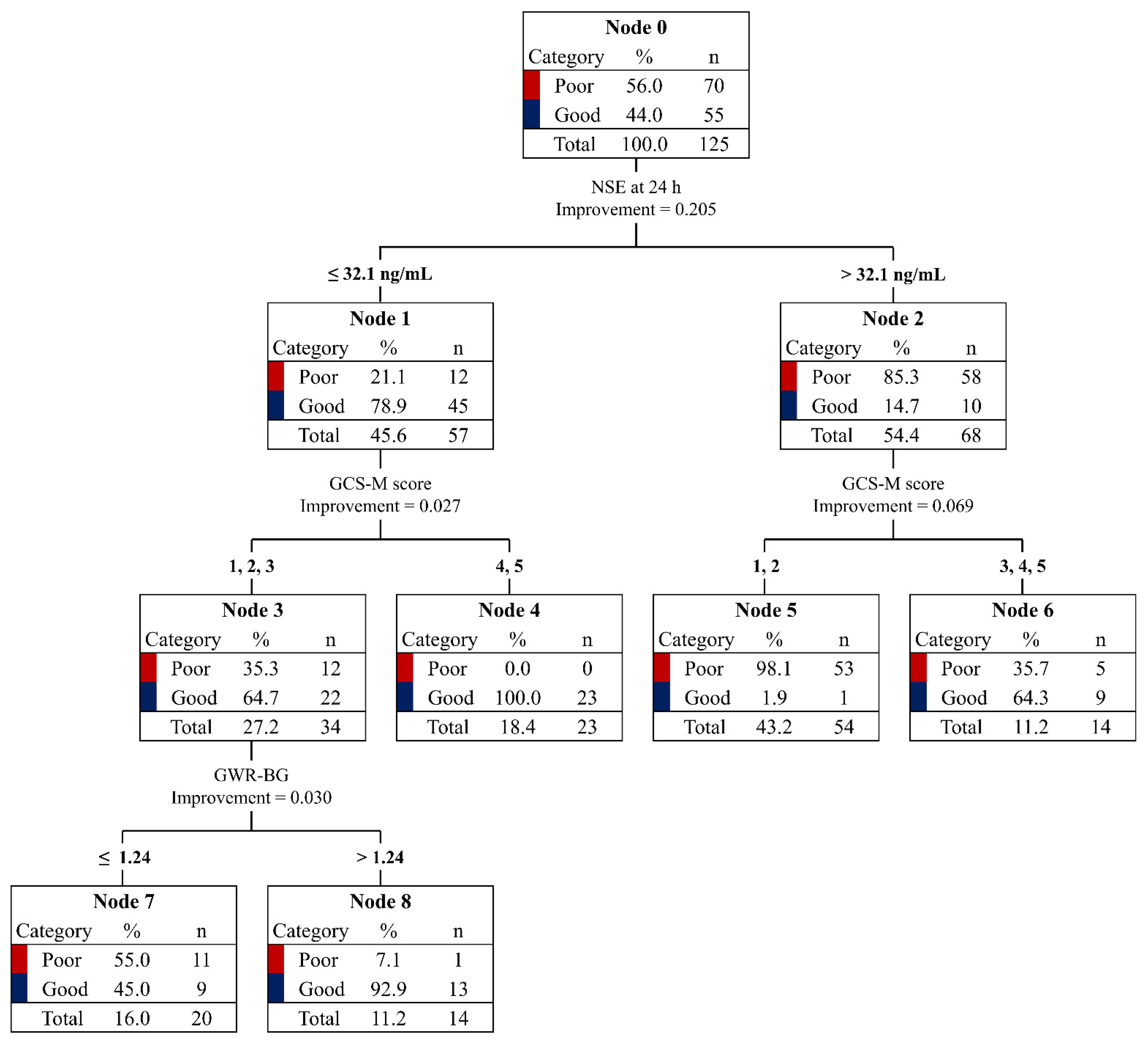
| Variables | Total Patients n = 125 | Good Neurological Outcome n = 55 | Poor Neurological Outcome n = 70 | p |
|---|---|---|---|---|
| Age, years | 58 (42–70) | 59 (45–70) | 58 (40–69) | 0.97 |
| Sex, male | 93 (74.4) | 45 (81.8) | 48 (68.6) | 0.11 |
| Charlson comorbidity index | 2.0 (1.0–4.0) | 2.5 (1.0–4.0) | 2.0 (0.0–4.0) | 0.61 |
| Hypertension | 42 (33.6) | 18 (32.7) | 24 (34.3) | 0.86 |
| Diabetes mellitus | 37 (29.6) | 15 (27.3) | 22 (31.5) | 0.82 |
| Myocardial infarction | 13 (10.4) | 8 (14.5) | 5 (7.1) | 0.18 |
| Cerebrovascular attack | 4 (3.2) | 2 (3.6) | 2 (2.9) | 0.81 |
| Lung disease | 7 (5.6) | 1 (1.8) | 6 (8.6) | 0.10 |
| Renal disease | 19 (15.2) | 8 (14.5) | 11 (15.8) | 0.92 |
| Liver disease | 4 (3.2) | 2 (3.6) | 2 (2.8) | 0.49 |
| Malignancy | 7 (5.6) | 4 (7.3) | 3 (4.3) | 0.50 |
| Cardiac arrest characteristics | ||||
| Witnessed | 75 (60.0) | 48 (87.3) | 27 (38.6) | <0.001 |
| Bystander CPR | 86 (68.8) | 45 (81.8) | 41 (58.6) | 0.005 |
| Shockable rhythm | 40 (32.0) | 33 (60.0) | 7 (10.0) | <0.001 |
| Cardiac etiology | 54 (43.2) | 39 (70.9) | 15 (21.4) | <0.001 |
| No-flow time, min | 1.0 (0.0–13.0) | 1.0 (0.0–2.0) | 9.0 (0.0–23.0) | <0.001 |
| Low-flow time, min | 20.0 (10.3–30.8) | 13.0 (8.0–19.0) | 28.0 (19.8–37.3) | <0.001 |
| SOFA score | 10 (8–12) | 8 (7–11) | 11 (9–12) | 0.62 |
| Time (from the ROSC), hours | ||||
| to targeted temperature | 5.8 (4.6–7.3) | 5.6 (4.5–7.0) | 5.9(4.6–7.5) | 0.88 |
| to perform brain CT | 1.4 (0.9–2.6) | 1.1 (0.8–2.1) | 1.7 (0.9–2.9) | 0.22 |
| to measure GCS-M score | 2.5 (1.5–4.2) | 2.3 (1.1–3.2) | 3.6 (2.2–6.0) | 0.003 |
| CAG performed | 43 (34.4) | 35 (63.6) | 8 (11.4) | <0.001 |
| Abnormal finding | <0.001 | |||
| LM | 2 (4.7) | 2 (5.7) | 0 (0) | |
| LAD | 13 (30.2) | 10 (28.6) | 3 (37.5) | |
| LCx | 9 (20.9) | 7 (20.0) | 2 (25.0) | |
| RCA | 10 (23.3) | 9 (25.7) | 1 (12.5) |
| Prognostic Tests | Total Patients | Good Neurological Outcome n = 55 | Poor Neurological Outcome n = 77 | p |
|---|---|---|---|---|
| GWR-BG | 1.20 (1.13–1.28) | 1.25 (1.20–1.30) | 1.16 (1.05–1.22) | <0.001 |
| GCS-M score | 1 (1–4) | 4 (1–4) | 1 (1–1) | <0.001 |
| NSE level, ng/mL | 35.8 (22.9–110.0) | 24.3 (18.7–31.0) | 83.9 (37.7–251.3) | <0.001 |
| Values | Cut-off | Specificity (95% CI) | Sensitivity (95% CI) | PPV (95% CI) | NPV (95% CI) | TP | FP | TN | FN |
|---|---|---|---|---|---|---|---|---|---|
| GWR-BG | >1.2 | 70.0 (57.9–80.4) | 72.7 (59.0–83.1) | 65.6 (52.3–77.3) | 76.6 (64.3–86.2) | 40 | 21 | 49 | 15 |
| >1.11 | 41.4 (29.8–53.8) | 98.2 (90.3–100.0) | 56.8 (46.3–67.0) | 96.7 (82.8–99.9) | 54 | 41 | 29 | 1 | |
| >1.09 | 38.6 (27.2–51.0) | 100.0 (93.5–100.0) | 56.1 (45.7–66.1) | 100 (87.2–100.0) | 55 | 43 | 27 | 0 | |
| GCS-M score | >2 | 88.6 (78.7–94.9) | 65.6 (51.4–77.8) | 81.8 (67.3–91.8) | 76.5 (65.8–85.2) | 36 | 8 | 62 | 19 |
| NSE level, ng/mL | ≤32 | 82.9 (72.0–90.8) | 81.8 (69.1–90.9) | 78.9 (66.1–88.6) | 85.3 (74.6–92.7) | 45 | 12 | 58 | 10 |
| ≤58.9 | 58.6 (46.2–70.2) | 98.2 (90.3–100.0) | 65.1 (53.8–75.2) | 97.6 (87.4–99.9) | 54 | 29 | 41 | 1 | |
| ≤85.3 | 51.4 (39.2–63.6) | 100.0 (93.5–100.0) | 61.8 (50.9–71.9) | 100.0 (90.3–100.0) | 55 | 34 | 36 | 0 |
| Values | AUC (95% CI) | p-Value a | Specificity (95% CI) | Sensitivity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| GWR-BG + GCS-M score | 0.89 (0.82–0.94) | * | 37.1 (25.9–49.5) | 98.2 (90.3–100.0) | 55.1 (44.7–65.2) | 96.3 (81.0–99.9) | ||
| GWR-BG + NSE level | 0.90 (0.83–0.94) | 0.85 | * | 71.4 (59.4–81.6) | 98.2 (90.3–100.0) | 73.0 (61.4–82.6) | 98.0 (89.6–100.0) | |
| GCS-M score + NSE level | 0.93 (0.87–0.97) | 0.15 | 0.20 | * | 72.9 (60.9–82.8) | 98.2 (90.3–100.0) | 74.0 (62.4–83.5) | 98.1 (89.7–100.0) |
| GWR-BG + GCS-M score + NSE level | 0.96 (0.90–0.98) | 0.002 | 0.005 | 0.09 | 77.1 (65.6–86.3) | 98.2 (90.3–100.0) | 77.1 (65.6–86.3) | 98.2 (90.3–100.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, J.S.; You, Y.; Min, J.H.; Jeong, W.; Ahn, H.J.; In, Y.N.; Cho, Y.C.; Lee, I.H.; Lee, J.K.; et al. Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care. Diagnostics 2023, 13, 2174. https://doi.org/10.3390/diagnostics13132174
Lee S, Park JS, You Y, Min JH, Jeong W, Ahn HJ, In YN, Cho YC, Lee IH, Lee JK, et al. Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care. Diagnostics. 2023; 13(13):2174. https://doi.org/10.3390/diagnostics13132174
Chicago/Turabian StyleLee, Sunghyuk, Jung Soo Park, Yeonho You, Jin Hong Min, Wonjoon Jeong, Hong Joon Ahn, Yong Nam In, Yong Chul Cho, In Ho Lee, Jae Kwang Lee, and et al. 2023. "Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care" Diagnostics 13, no. 13: 2174. https://doi.org/10.3390/diagnostics13132174
APA StyleLee, S., Park, J. S., You, Y., Min, J. H., Jeong, W., Ahn, H. J., In, Y. N., Cho, Y. C., Lee, I. H., Lee, J. K., & Kang, C. (2023). Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care. Diagnostics, 13(13), 2174. https://doi.org/10.3390/diagnostics13132174






