A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection
Abstract
1. Introduction
2. Experimental Design
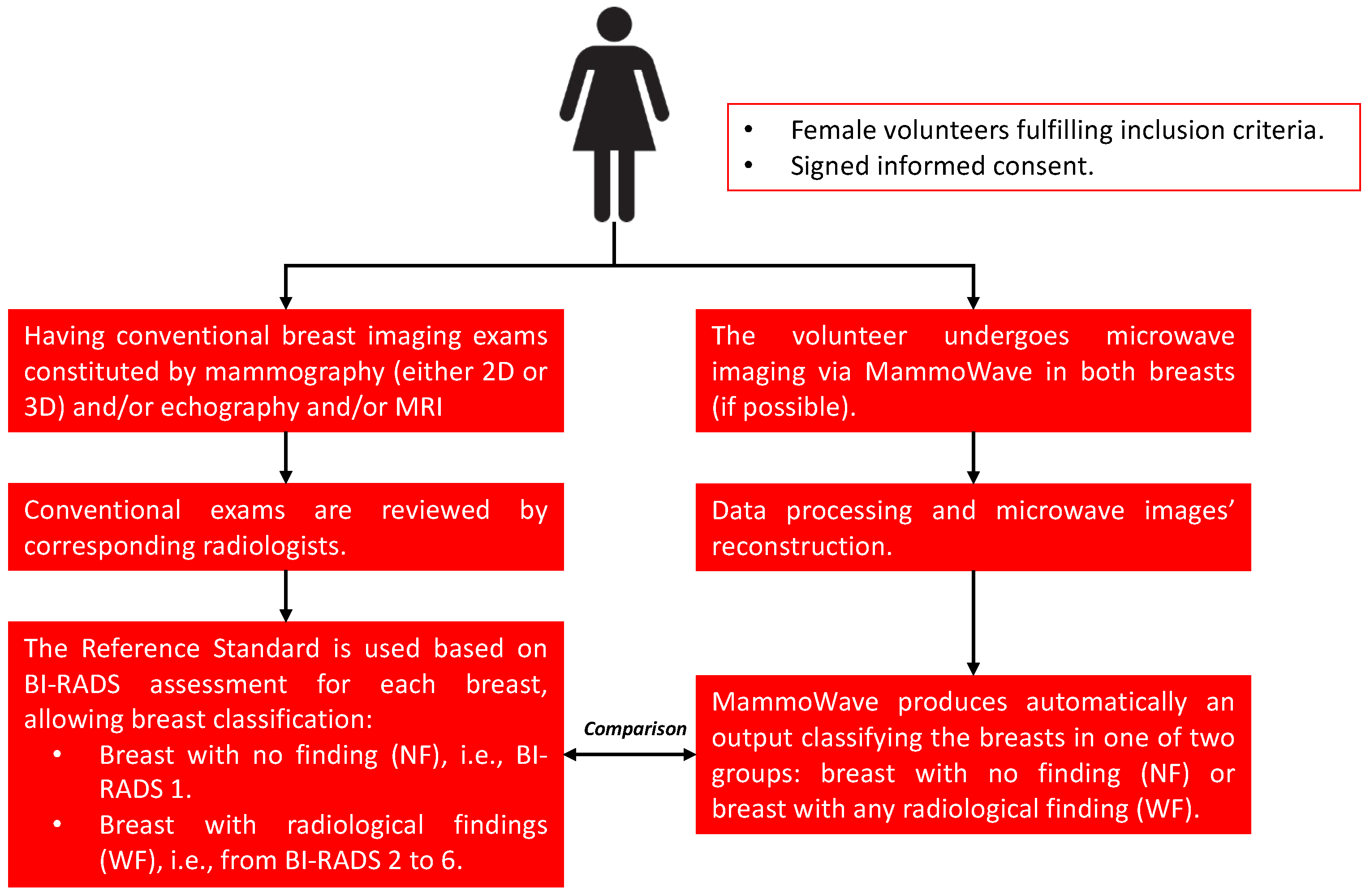
2.1. Clinical Protocol
2.2. Study Design
2.2.1. Inclusion and Exclusion Criteria
- Signed informed consent form;
- Women;
- Adult ≥18 years old;
- Having a radiologist study output (to be considered as the reference standard) obtained using conventional exams within the last month. The conventional exam may be a breast specialist visit and mammography and/or ultrasound and/or magnetic resonance imaging, which will be integrated with the histological one if deemed necessary by the responsible investigator and when available;
- Volunteers willing to comply with the study protocol and recommendations;
- Women with intact breast skin (i.e., without bleeding lesions or scars);
- Women who are participating in another clinical study;
- Women who belong to any vulnerable group (e.g., women with disabilities or impairments);
- Women with implanted electronic devices;
- Women who have undergone a biopsy less than one week before the MammoWave scan;
- Women with breast implants;
- Women with nipple piercings (unless they are removed prior to examination);
- Participation in other studies in the last month before screening;
- Pregnancy or breastfeeding.
2.2.2. Primary and Secondary Objectives
2.2.3. Number of Participants
2.2.4. Participating Centres
2.2.5. Recruitment and Data Collection
- Age;
- Assessed breast (right or left);
- Qualitative breast density according to the Breast Imaging-Reporting and Data System (BI-RADS): A for entirely fatty breasts, B for breasts with scattered areas of fibroglandular density, C for heterogeneous dense breasts, and D for extremely dense breasts [21];
- Radiologists’ conventional study (mammographic and/or echography and/or magnetic resonance imaging) will be collected using BI-RADS notation. This will allow for classifying breasts as NF when the BI-RADS score is 1 and as WF breasts when the BI-RADS score is different from 1, with 2 being for benign lesions, 3 being for follow-up lesions, 4 being for suspicious lesions, 5 being for a highly suspicious lesion, likely to be breast cancer, and 6 being for lesions with confirmed cancer diagnosis [21];
- Histological output will also be collected (when available) using standard classifications;
- MammoWave final output will be collected as NF (breast without relevant findings) or WF (breast with findings);
- Satisfaction questionnaires related to MammoWave use.
2.2.6. Study’s Ethical Conduct
2.2.7. Participants’ Privacy and Confidentiality
3. Materials and Equipment
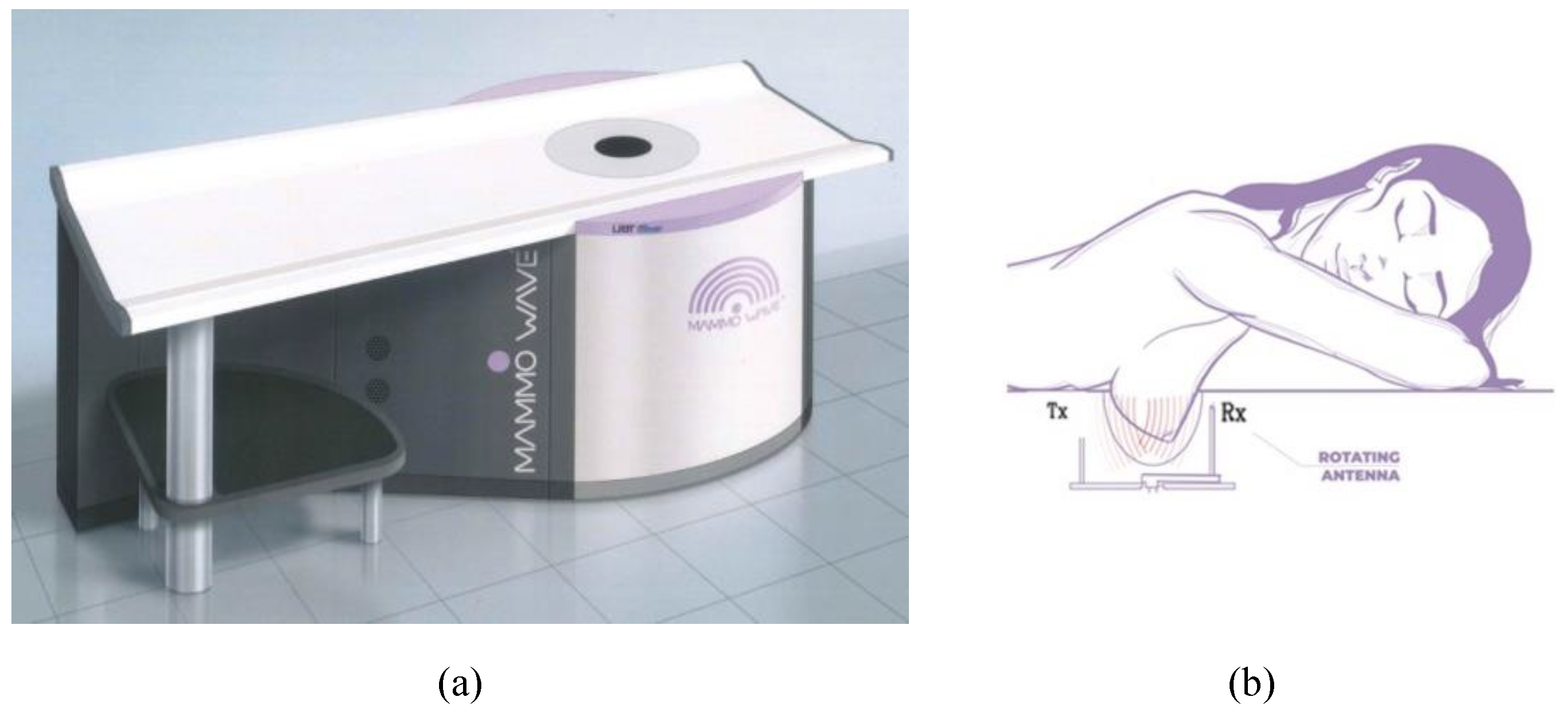
3.1. MammoWave Device and Imaging Algorithm
3.2. Previous Clinical Studies
4. Detailed Procedure
4.1. Microwave Images Analysis
- MAX = Maximum value of the image;
- MIN = Minimum value of the image;
- MEA = Mean value of the image;
- MED = Median value of the image;
- VAR = Variance of the image;
- MAD0 = Mean absolute deviation of the image;
- MAD1 = Median absolute deviation of the image;
- M2AVG = (MAX)/(MEA);
- ROS1 = (MAX-MIN)/(MEA-MIN);
- ROS2 = (MAX-MIN)/(MED-MIN).
4.2. Statistical Analysis
4.3. Retrospective Analysis
5. Expected Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, T.A.; Guraya, S.S. Breast Cancer Screening Programs: Review of Merits, Demerits, and Recent Recommendations Practiced across the World. J. Microsc. Ultrastruct. 2017, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.-H.; Yen, A.M.-F.; Fann, J.C.-Y.; Gordon, P.; Chen, S.L.-S.; Chiu, S.Y.-H.; Hsu, C.-Y.; Chang, K.-J.; Lee, W.-C.; Yeoh, K.G.; et al. Clarifying the Debate on Population-Based Screening for Breast Cancer with Mammography: A Systematic Review of Randomized Controlled Trials on Mammography with Bayesian Meta-Analysis and Causal Model. Medicine 2017, 96, e5684. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; Hannigan, A.; O’Brien, K.M. Mortality Reductions Due to Mammography Screening: Contemporary Population-Based Data. PLoS ONE 2017, 12, e0188947. [Google Scholar] [CrossRef] [PubMed]
- Castellano, C.R.; Aguilar Angulo, P.M.; Hernández, L.C.; González-Carrato, P.S.-C.; González, R.G.; Alvarez, J.; Chacón, J.I.; Ruiz, J.; Fuentes Guillén, M.Á.; Gutiérrez Ávila, G. Breast Cancer Mortality after Eight Years of an Improved Screening Program Using Digital Breast Tomosynthesis. J. Med. Screen 2021, 28, 456–463. [Google Scholar] [CrossRef] [PubMed]
- European Guidelines on Breast Cancer Screening and Diagnosis: Screening Ages and Frequencies. Available online: https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines/screening-ages-and-frequencies (accessed on 20 May 2023).
- Breast Cancer Screening, U.S. Preventive Services Task Force Draft Recommendation Statement. Available online: https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults (accessed on 20 May 2023).
- Assi, H.A.; Khoury, K.E.; Dbouk, H.; Khalil, L.E.; Mouhieddine, T.H.; El Saghir, N.S. Epidemiology and Prognosis of Breast Cancer in Young Women. J. Thorac. Dis. 2013, 5 (Suppl. S1), S2–S8. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Arao, R.F.; Sprague, B.L.; Lee, J.M.; Buist, D.S.M.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Tosteson, A.N.A.; Rauscher, G.H.; et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017, 283, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.K.; Lee, S.J.; Schechter, C.B.; Kerlikowske, K.; Alagoz, O.; Berry, D.; Buist, D.S.M.; Cevik, M.; Chisholm, G.; de Koning, H.J.; et al. Benefits, Harms, and Costs for Breast Cancer Screening After US Implementation of Digital Mammography. J. Natl. Cancer Inst. 2014, 106, dju092. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Pappas, M.; Cantor, A.; Griffin, J.; Daeges, M.; Humphrey, L. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 256. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.M.A.; Fanizzi, A.; Losurdo, L.; Bellotti, R.; Tangaro, S.; La Forgia, D.; Didonna, V.; Massafra, R.; Tamborra, P.; Moschetta, M.; et al. Hough Transform for Clustered Microcalcifications Detection in Full-Field Digital Mammograms. In Proceedings of the Applications of Digital Image Processing XL, San Diego, CA, USA, 7–10 August 2017; Tescher, A.G., Ed.; SPIE: Bellingham, WA, USA, 2017; p. 41. [Google Scholar]
- Joy, J.E.; Penhoet, E.E.; Petitti, D.B. Saving Women’s Lives; National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-09213-5. [Google Scholar]
- Nikolova, N. Microwave Imaging for Breast Cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of Microwaves Techniques for Breast Cancer Detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef] [PubMed]
- Vispa, A.; Sani, L.; Paoli, M.; Bigotti, A.; Raspa, G.; Ghavami, N.; Caschera, S.; Ghavami, M.; Duranti, M.; Tiberi, G. UWB Device for Breast Microwave Imaging: Phantom and Clinical Validations. Measurement 2019, 146, 582–589. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Alvarez Sánchez-Bayuela, D.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast Lesion Detection through MammoWave Device: Empirical Detection Capability Assessment of Microwave Images’ Parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef] [PubMed]
- Sani, L.; Vispa, A.; Ghavami, N.; Loretoni, R.; Duranti, M.; Sanchez-Bayuela, D.A.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Empirical Assessment of Breast Lesion Detection Capability through an Innovative Microwave Imaging Device. In Proceedings of the 15th European Conference on Antennas and Propagation, EuCAP 2021, Dusseldorf, Germany, 22–26 March 2021. [Google Scholar]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 Explanation and Elaboration: Guidance for Protocols of Clinical Trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [PubMed]
- Deep Oscillatory Neural Networks Computing and Learning through the Dynamics of RF Neurons Interconnected by RF Spintronic Synapses. Available online: https://cordis.europa.eu/project/id/101017098 (accessed on 20 May 2023).
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Ghavami, N.; Tiberi, G.; Edwards, D.J.; Monorchio, A. UWB Microwave Imaging of Objects With Canonical Shape. IEEE Trans. Antennas Propag. 2012, 60, 231–239. [Google Scholar] [CrossRef]
- Sani, L.; Ghavami, N.; Vispa, A.; Paoli, M.; Raspa, G.; Ghavami, M.; Sacchetti, F.; Vannini, E.; Ercolani, S.; Saracini, A.; et al. Novel Microwave Apparatus for Breast Lesions Detection: Preliminary Clinical Results. Biomed. Signal. Process. Control. 2019, 52, 257–263. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Ghavami, N.; Sanchez-Bayuela, D.A.; Badia, M.; Bigotti, A.; Raspa, G.; Castellano, C.R.; Ghavami, M.; Tiberi, G. MammoWave Breast Imaging Device: A Procedure for Device’s Characterization Via Phantom Measurements and Subsequent Clinical Trials’ Preliminary Results. In Proceedings of the 2021 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; pp. 483–486. [Google Scholar]
- Sohani, B.; Tiberi, G.; Ghavami, N.; Ghavami, M.; Dudley, S.; Rahmani, A. Microwave Imaging for Stroke Detection: Validation on Head-Mimicking Phantom. In Proceedings of the 2019 PhotonIcs & Electromagnetics Research Symposium-Spring (PIERS-Spring), Rome, Italy, 17–20 June 2019; pp. 940–948. [Google Scholar]
- Ghavami, N.; Sánchez-Bayuela, D.Á.; Sani, L.; Vispa, A.; Bigotti, A.; Badia, M.; Papini, L.; Raspa, G.; Rana, S.P.; Castellano, C.R.; et al. MammoWave Breast Imaging Device: An International and Multicentric Clinical Investigation. In Proceedings of the 17th European Conference on Antennas and Propagation, EuCAP 2023, Florence, Italy, 26–31 March 2023; pp. 1–5. [Google Scholar]
- Papini, L.; Badia, M.; Sani, L.; Rana, S.P.; Sánchez-Bayuela, D.Á.; Vispa, A.; Bigotti, A.; Raspa, G.; Ghavami, N.; Castellano, C.R.; et al. Breast Cancer Detection using Machine Learning Approaches on Microwave-based Data. In Proceedings of the 17th European Conference on Antennas and Propagation, EuCAP 2023, Florence, Italy, 26–31 March 2023; pp. 1–5. [Google Scholar]
- Moskowitz, M.; Feig, S.; Cole-Beuglet, C.; Fox, S.; Haberman, J.; Libshitz, H.; Zermeno, A. Evaluation of new imaging procedures for breast cancer: Proper process. AJR 1983, 140, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. MARIA M4: Clinical Evaluation of a Prototype Ultrawideband Radar Scanner for Breast Cancer Detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef] [PubMed]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA® M5: A Multicentre Clinical Study to Evaluate the Ability of the Micrima Radio-Wave Radar Breast Imaging System (MARIA®) to Detect Lesions in the Symptomatic Breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer—Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2022, 29, S211–S222. [Google Scholar] [CrossRef] [PubMed]

| Visit 1 | |
|---|---|
| Signed written informed consent | X |
| Demography | X |
| Medical History | X |
| Inclusion/exclusion criteria | X |
| Qualitative breast density assessment | X |
| Standard breast evaluation | X |
| MammoWave examination | X |
| Satisfaction questionnaire (after MammoWave examination) | X |
| Adverse event (if any) | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez Sánchez-Bayuela, D.; Ghavami, N.; Romero Castellano, C.; Bigotti, A.; Badia, M.; Papini, L.; Raspa, G.; Palomba, G.; Ghavami, M.; Loretoni, R.; et al. A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection. Diagnostics 2023, 13, 2100. https://doi.org/10.3390/diagnostics13122100
Álvarez Sánchez-Bayuela D, Ghavami N, Romero Castellano C, Bigotti A, Badia M, Papini L, Raspa G, Palomba G, Ghavami M, Loretoni R, et al. A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection. Diagnostics. 2023; 13(12):2100. https://doi.org/10.3390/diagnostics13122100
Chicago/Turabian StyleÁlvarez Sánchez-Bayuela, Daniel, Navid Ghavami, Cristina Romero Castellano, Alessandra Bigotti, Mario Badia, Lorenzo Papini, Giovanni Raspa, Gianmarco Palomba, Mohammad Ghavami, Riccardo Loretoni, and et al. 2023. "A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection" Diagnostics 13, no. 12: 2100. https://doi.org/10.3390/diagnostics13122100
APA StyleÁlvarez Sánchez-Bayuela, D., Ghavami, N., Romero Castellano, C., Bigotti, A., Badia, M., Papini, L., Raspa, G., Palomba, G., Ghavami, M., Loretoni, R., Calabrese, M., Tagliafico, A., & Tiberi, G. (2023). A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection. Diagnostics, 13(12), 2100. https://doi.org/10.3390/diagnostics13122100









