A Novel Diagnostic Imaging Method for the Early Detection of Pancreatic Cancer
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ilic, M.; Ilic, I. Epidemiology of Pancreatic Cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.; Andersson, R.; Ansari, D. The Actual 5-Year Survivors of Pancreatic Ductal Adenocarcinoma Based on Real-World Data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, S.W. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver 2016, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline Resectable Pancreatic Cancer: A Consensus Statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30 Year Anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Patel, C.M.; Sahdev, A.; Reznek, R.H. CT, MRI and PET Imaging in Peritoneal Malignancy. Cancer Imaging 2011, 11, 123–139. [Google Scholar] [CrossRef]
- Leschka, S.; Alkadhi, H.; Wildermuth, S.; Marincek, B. Multi-detector Computed Tomography of Acute Abdomen. Eur. Radiol. 2005, 15, 2435–2447. [Google Scholar] [CrossRef]
- Iafrate, F.; Ciolina, M.; Sammartino, P.; Baldassari, P.; Rengo, M.; Lucchesi, P.; Sibio, S.; Accarpio, F.; Di Giorgio, A.D.; Laghi, A. Peritoneal Carcinomatosis: Imaging with 64-MDCT and 3T MRI with Diffusion-Weighted Imaging. Abdom. Imaging 2012, 37, 616–627. [Google Scholar] [CrossRef]
- Ellis, M.; Powell, J.T.; Greenhalgh, R.M. Limitations of Ultrasonography in Surveillance of Small Abdominal Aortic Aneurysms. Br. J. Surg. 1991, 78, 614–616. [Google Scholar] [CrossRef]
- Saunders, H.M. Ultrasonography of the Pancreas. Probl. Vet. Med. 1991, 3, 583–603. [Google Scholar]
- Engjom, T.; Sangnes, D.A.; Havre, R.F.; Erchinger, F.; Pham, K.D.C.; Haldorsen, I.S.; Gilja, O.H.; Dimcevski, G. Diagnostic Accuracy of Transabdominal Ultrasound in Chronic Pancreatitis. Ultrasound Med. Biol. 2017, 43, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, X.; Li, X.; Ma, L.; Zhao, X.; Yang, X. Ultrasound Combined with Computed Tomography in Pancreatic Cancer. J. BUON 2019, 24, 1562–1567. [Google Scholar] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter Study of Early Pancreatic Cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 Mm) Pancreatic Adenocarcinoma: Analysis of Enhancement Patterns and Secondary Signs with Multiphasic Multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Harinck, F.; Konings, I.C.A.W.; Kluijt, I.; Poley, J.W.; van Hooft, J.E.; van Dullemen, H.M.; Nio, C.Y.; Krak, N.C.; Hermans, J.J.; Aalfs, C.M.; et al. A Multicentre Comparative Prospective Blinded Analysis of EUS and MRI for Screening of Pancreatic Cancer in High-Risk Individuals. Gut 2016, 65, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Robertis, R.D.; Martini, P.T.; Demozzi, E.; Corso, F.D.; Bassi, C.; Pederzoli, P.; D’Onofrio, M. Diffusion-Weighted Imaging of Pancreatic Cancer. World J. Radiol. 2015, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Zukotynski, K.; Kim, C.K. Abdomen: Normal Variations and Benign Conditions Resulting in Uptake on FDG-PET/CT. PET Clin. 2014, 9, 169–183. [Google Scholar] [CrossRef]
- Rijkers, A.P.; Valkema, R.; Duivenvoorden, H.J.; van Eijck, C.H.J. Usefulness of F-18-Fluorodeoxyglucose Positron Emission Tomography to Confirm Suspected Pancreatic Cancer: A Meta-analysis. Eur. J. Surg. Oncol. 2014, 40, 794–804. [Google Scholar] [CrossRef]
- Helmstaedter, L.; Riemann, J.F. Pancreatic Cancer—EUS and Early Diagnosis. Langenbecks Arch. Surg. 2008, 393, 923–927. [Google Scholar] [CrossRef]
- Yasuda, I.; Iwashita, T.; Doi, S.; Nakashima, M.; Moriwaki, H. Role of EUS in the Early Detection of Small Pancreatic Cancer. Dig. Endosc. 2011, 23 (Suppl. S1), 22–25. [Google Scholar] [CrossRef]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of Endoscopic Ultrasonography on Diagnosis of Pancreatic Cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Yokoi, Y.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Honda, G.; Horiguchi, S.; et al. Proposal for Endoscopic Ultrasonography Classification for Small Pancreatic Cancer. Diagnostics 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Debongnie, J.C.; Pauls, C.; Fievez, M.; Wibin, E. Prospective Evaluation of the Diagnostic Accuracy of Liver Ultrasonography. Gut 1981, 22, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.S.; Koduru, P.; Joshi, V.; Saxena, P.; Suzuki, R.; Irisawa, A.; Yamao, K. The Role of Endoscopic Ultrasound in Pancreatic Cancer Screening. Endosc. Ultrasound 2016, 5, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-Guided FNA for Diagnosis of Solid Pancreatic Neoplasms: A Meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Hébert-Magee, S.; Bae, S.; Varadarajulu, S.; Ramesh, J.; Frost, A.R.; Eloubeidi, M.A.; Eltoum, I.A. The Presence of a Cytopathologist Increases the Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology for Pancreatic Adenocarcinoma: A Meta-analysis. Cytopathology 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hawes, R.; Varadarajulu, S. A Meta-analysis Comparing ProCore and Standard Fine-Needle Aspiration Needles for Endoscopic Ultrasound-Guided Tissue Acquisition. Endoscopy 2016, 48, 339–349. [Google Scholar] [CrossRef]
- Karsenti, D.; Tharsis, G.; Zeitoun, J.D.; Denis, P.; Perrot, B.; Coelho, J.; Bellaiche, G.; Charbit, L.; Hakoune, J.J.; Doumet, S.; et al. Comparison of 20-Gauge Procore® and 22-Gauge Acquire® Needles for EUS-FNB of Solid Pancreatic Masses: An Observational Study. Scand. J. Gastroenterol. 2019, 54, 499–505. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Chang, K.J.; Yamamoto, N.; Hamada, T.; Uchino, R.; Mizuno, S.; Miyabayashi, K.; Yamamoto, K.; Kawakubo, K.; et al. Slow Pull Versus Suction in Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Pancreatic Solid Masses. Dig. Dis. Sci. 2014, 59, 1578–1585. [Google Scholar] [CrossRef]
- Bang, J.Y.; Magee, S.H.; Ramesh, J.; Trevino, J.M.; Varadarajulu, S. Randomized Trial Comparing Fanning with Standard Technique for Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Solid Pancreatic Mass Lesions. Endoscopy 2013, 45, 445–450. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Chang, J.M.; Kan, Q.C.; Chiorean, L.; Ignee, A.; Dietrich, C.F. Endoscopic Ultrasound Elastography: Current Status and Future Perspectives. World J. Gastroenterol. 2015, 21, 13212–13224. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Kikuyama, M.; Satoh, T.; Terada, S.; Kanemoto, H.; Arai, K. Minimally Invasive Ductal Pancreatic Carcinoma without Low Echoic Area on Endoscopic Ultrasound Examination: A Case Report. J. Jpn. Pancreas Soc. 2017, 32, 852–858, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Kalady, M.F.; Peterson, B.; Baillie, J.; Onaitis, M.W.; Abdul-Wahab, O.I.; Howden, J.K.; Jowell, P.S.; Branch, M.S.; Clary, B.M.; Pappas, T.N.; et al. Pancreatic Duct Strictures: Identifying Risk of Malignancy. Ann. Surg. Oncol. 2004, 11, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Nakahodo, J.; Kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal Parenchymal Atrophy of Pancreas: An Important Sign of Underlying High-Grade Pancreatic Intraepithelial Neoplasia without Invasive Carcinoma, i.e., Carcinoma in Situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Nakahodo, J.; Honda, G.; Horiguchi, S.; Suzuki, M.; Chiba, K.; Tabata, H.; Ome, Y.; Kamisawa, T. Effectiveness of Focal Pancreatic Parenchymal Atrophy in Diagnosing High-Grade Pancreatic Intraepithelial Neoplasia/Carcinoma In Situ. MRAJ 2021, 9, 8. [Google Scholar] [CrossRef]
- Nakahodo, J.; Kikuyama, M.; Fukumura, Y.; Horiguchi, S.I.; Chiba, K.; Tabata, H.; Suzuki, M.; Kamisawa, T. Focal Pancreatic Parenchyma Atrophy Is a Harbinger of Pancreatic Cancer and a Clue to the Intraductal Spreading Subtype. Pancreatology 2022, 22, 1148–1158. [Google Scholar] [CrossRef]
- Toshima, F.; Watanabe, R.; Inoue, D.; Yoneda, N.; Yamamoto, T.; Sasahira, N.; Sasaki, T.; Matsuyama, M.; Minehiro, K.; Tateishi, U.; et al. CT Abnormalities of the Pancreas Associated with the Subsequent Diagnosis of Clinical Stage I Pancreatic Ductal Adenocarcinoma More Than 1 Year Later: A Case-Control Study. AJR 2021, 217, 1353–1364. [Google Scholar] [CrossRef]
- Kameya, S.; Kuno, N.; Kasugai, T. The Diagnosis of Pancreatic Cancer by Pancreatic Juice Cytology. Acta Cytol. 1981, 25, 354–360. [Google Scholar]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of Cytodiagnosis Using Endoscopic Nasopancreatic Drainage for Early Diagnosis of Pancreatic Cancer: Establishing a New Method for the Early Detection of Pancreatic Carcinoma In Situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef]
- Satoh, T.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Muro, H.; Hanada, K. Acute Pancreatitis-Onset Carcinoma In Situ of the Pancreas with Focal Fat Replacement Diagnosed Using Serial Pancreatic-Juice Aspiration Cytologic Examination (SPACE). Clin. J. Gastroenterol. 2017, 10, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Nakahodo, J.; Honda, G.; Suzuki, M.; Horiguchi, S.I.; Chiba, K.; Tabata, H.; Ome, Y.; Uemura, S.I.; Kawamoto, Y.; et al. Pancreatic Duct Epithelial Malignancy Suggested by Large Focal Pancreatic Parenchymal Atrophy in Cystic Diseases of the Pancreas. Pancreatology 2023, 23, 420–428. [Google Scholar] [CrossRef]
- Bhatia, M. Apoptosis of Pancreatic Acinar Cells in Acute Pancreatitis: Is It Good or Bad? J. Cell. Mol. Med. 2004, 8, 402–409. [Google Scholar] [CrossRef]
- Storz, P. Acinar Cell Plasticity and Development of Pancreatic Ductal Adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic Ultrasound Findings and Pathological Features of Pancreatic Carcinoma In Situ. EIO 2019, 7, E585–E593. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Kikuyama, M.; Satoh, T.; Terada, S. Use of Nasopancreatic Drainage for Severe Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Case Series. Intern. Med. 2018, 57, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Kawaguchi, S.; Terada, S.; Satoh, T. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers 2018, 10, 48. [Google Scholar] [CrossRef]
- Furukawa, H.; Iwata, R.; Moriyama, N. Growth Rate of Pancreatic Adenocarcinoma: Initial Clinical Experience. Pancreas 2001, 22, 366–369. [Google Scholar] [CrossRef]
- Nishida, K.; Kaneko, T.; Yoneda, M.; Nakagawa, S.; Ishikawa, T.; Yamane, E.; Nishioka, B.; Miyamoto, Y.; Takano, H.; Yoshikawa, T.; et al. Doubling Time of Serum CA 19–9 in the Clinical Course of Patients with Pancreatic Cancer and Its Significant Association with Prognosis. J. Surg. Oncol. 1999, 71, 140–146. [Google Scholar] [CrossRef]
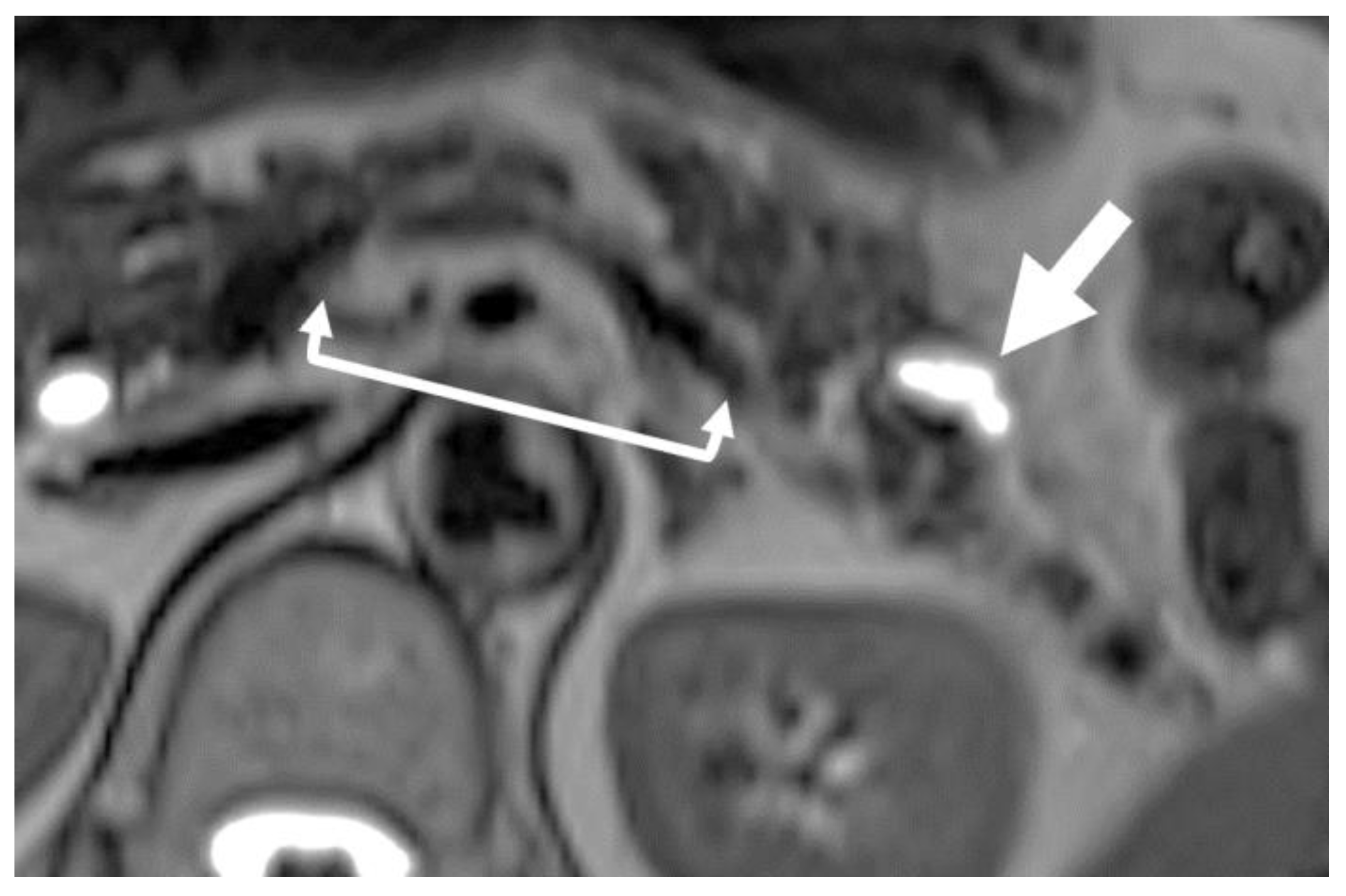
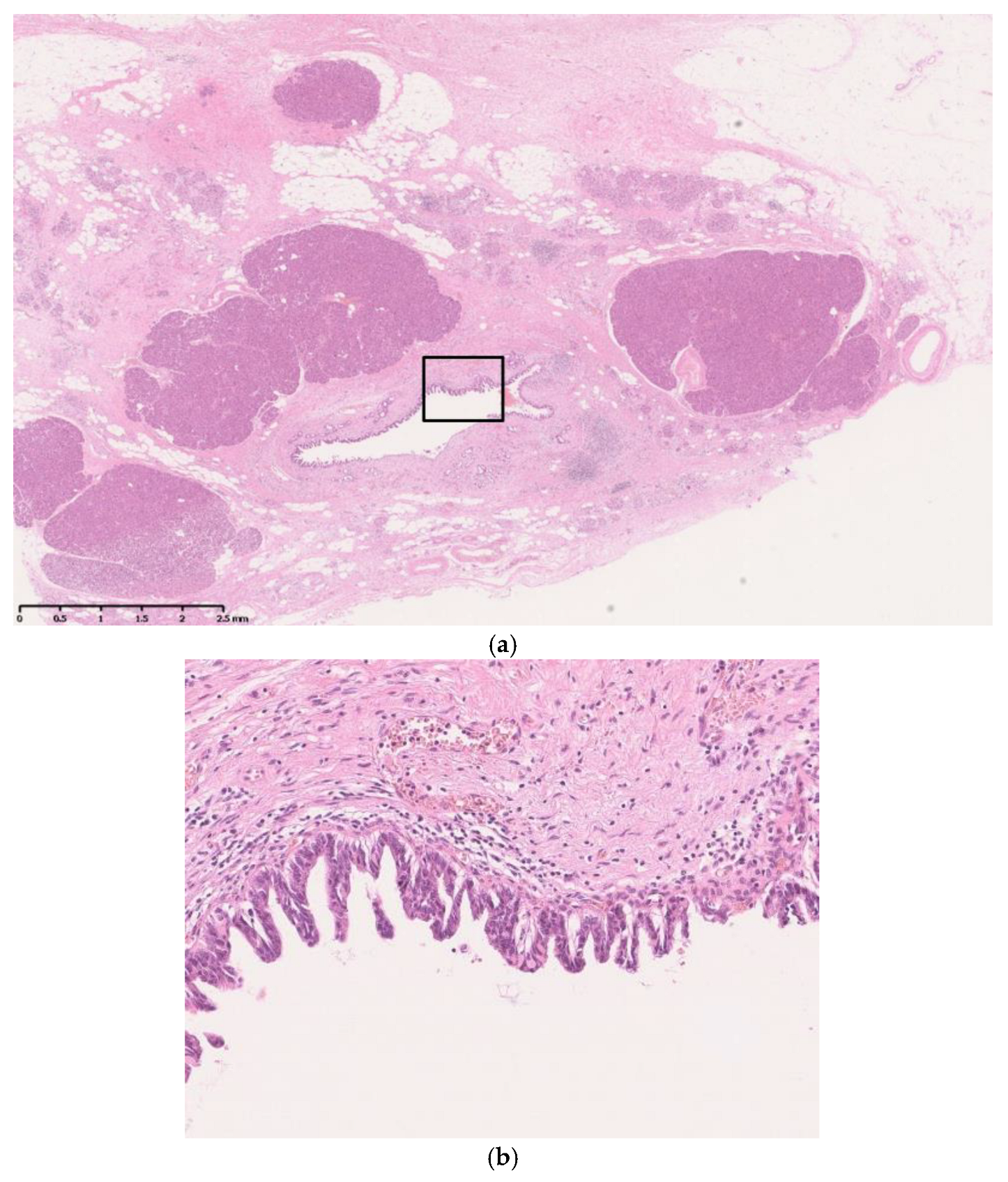

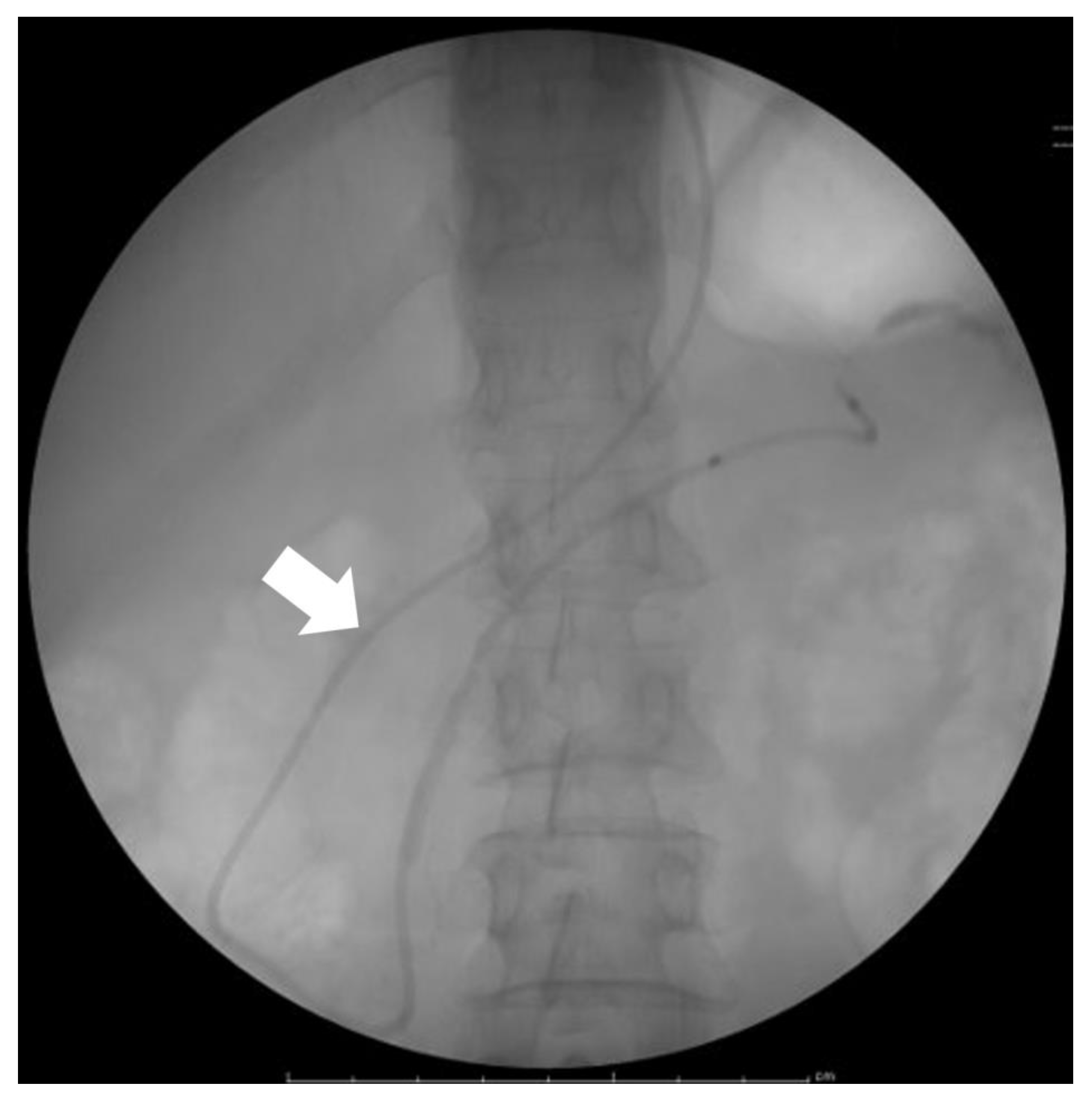
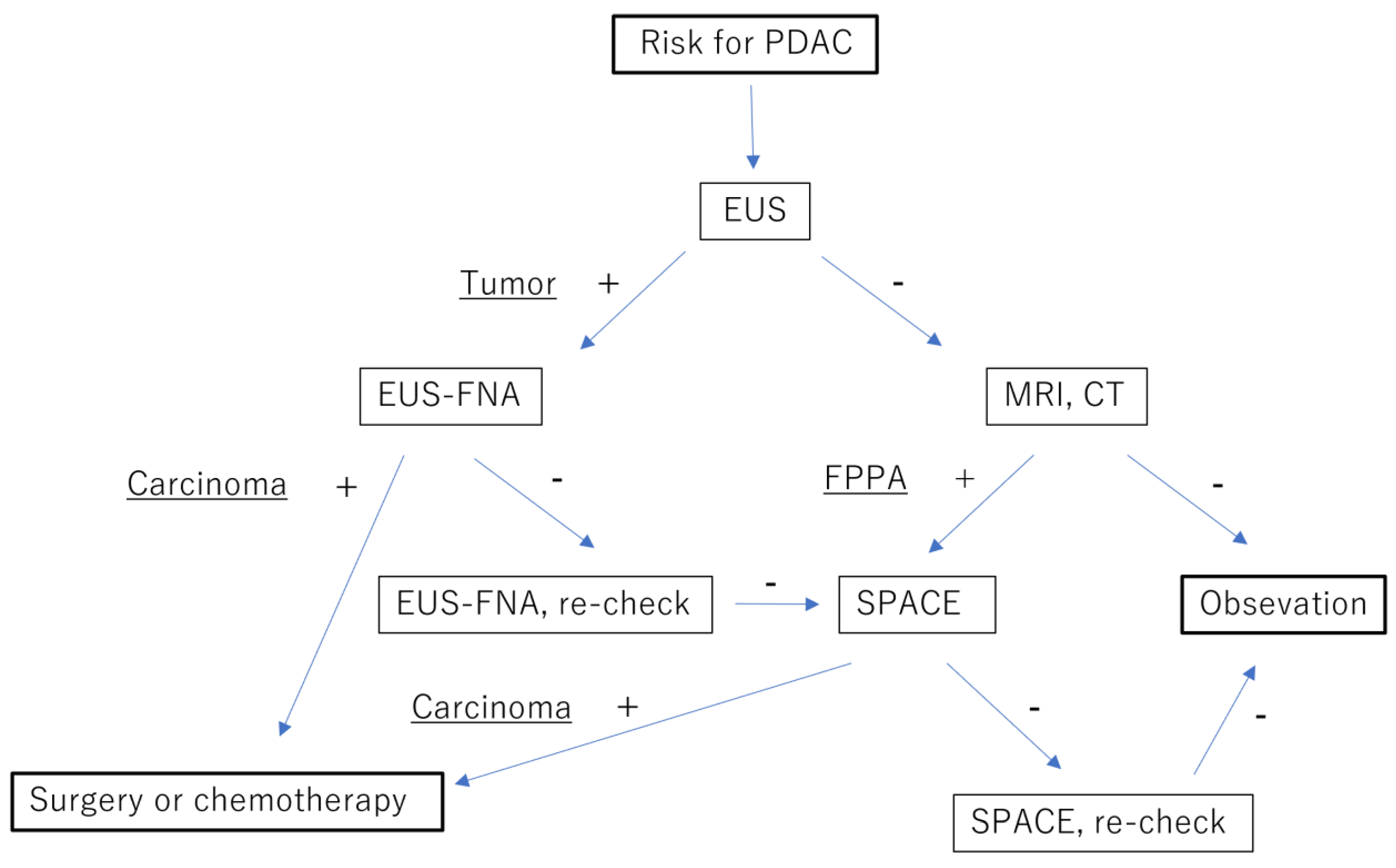
| Advantage | Disadvantage | Commonly Detectable Tumor Size | |
|---|---|---|---|
| US | Easy Low cost | Poor detection of the pancreas and pancreatic lesions | >10 mm (Location limited) |
| CT | Easy Observation of the entire pancreas | Radiation exposure Poor detection of small PDACs | >20 mm |
| MRI | Easy Observation of the entire pancreas | Poor detection of small PDACs | >10 mm |
| PET | Easy Observation of the entire pancreas | Radiation exposure Poor detection of small PDACs Expensive | >10 mm |
| EUS | Detection of very small PDACs Obtaining tissue for histopathological diagnosis | Invasive Technical difficulty | >5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuyama, M. A Novel Diagnostic Imaging Method for the Early Detection of Pancreatic Cancer. Diagnostics 2023, 13, 2080. https://doi.org/10.3390/diagnostics13122080
Kikuyama M. A Novel Diagnostic Imaging Method for the Early Detection of Pancreatic Cancer. Diagnostics. 2023; 13(12):2080. https://doi.org/10.3390/diagnostics13122080
Chicago/Turabian StyleKikuyama, Masataka. 2023. "A Novel Diagnostic Imaging Method for the Early Detection of Pancreatic Cancer" Diagnostics 13, no. 12: 2080. https://doi.org/10.3390/diagnostics13122080
APA StyleKikuyama, M. (2023). A Novel Diagnostic Imaging Method for the Early Detection of Pancreatic Cancer. Diagnostics, 13(12), 2080. https://doi.org/10.3390/diagnostics13122080




