Detection of c.375A>G, c.385A>T, c.571C>T, and sedel2 of FUT2 via Real-Time PCR in a Single Tube
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Samples
2.2. Probes and Primers Used in This Study
2.3. Probe Selection for Detection of c.375A>G and c.385A>T by FMCA
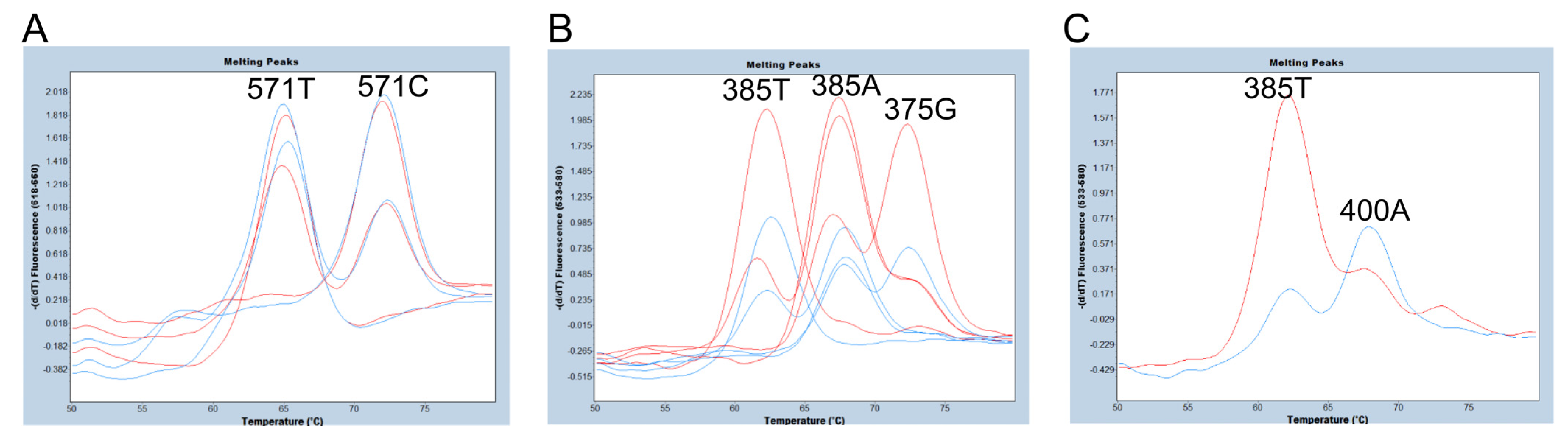
2.4. Evaluation of the Number of Amplicons in Genotyping of c.375A>G, c.385A>T, and c.571C>T of FUT2 via FMCA
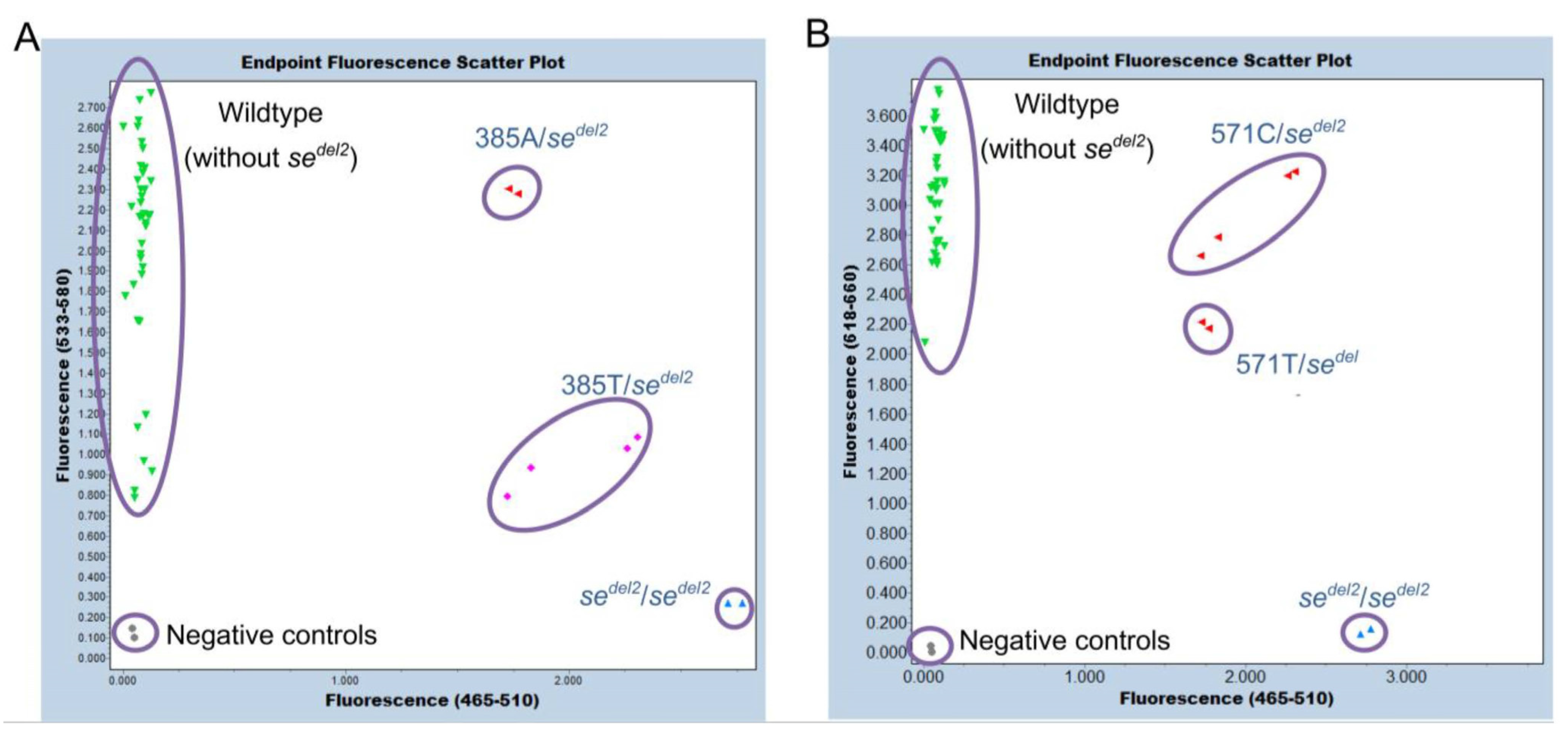
2.5. Detection of c.375A>G, c.385A>T, and c.571C>T via FMCA and Detection of sedel2 via Endpoint Genotyping in a Single Tube
2.6. Real-Time PCR Monitoring, FMCA, and Endpoint Genotyping
3. Results
3.1. Probe Selection for Genotyping of c.375A>G and c.385A>T via FMCA
3.2. Evaluation of the Number of Amplicons in Genotyping of c.375A>G, c.385A>T, and c.571C>T of FUT2 via FMCA
3.3. Genotyping of c.375A>G, c.385A>T, and c.571C>T of FUT2 via FMCA and Endpoint Genotyping of the sedel2 Allele in a Single Tube
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oriol, R.; Danilovs, J.; Hawkins, B.R. A new genetic model proposing that the Se gene is a structural gene closely linked to the H gene. Am. J. Hum. Genet. 1981, 33, 421–431. [Google Scholar] [PubMed]
- Clausen, H.; Hakomori, S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.; Abo, H. Lewis Systems. In Human Blood Groups, 3rd ed.; Daniels, G., Ed.; Wiley-Blackwell: West Sussex, UK, 2013; pp. 11–95. [Google Scholar]
- Kelly, R.J.; Rouquier, S.; Giorgi, D.; Lennon, G.G.; Lowe, J.B. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 1995, 270, 4640–4649. [Google Scholar] [CrossRef] [PubMed]
- Rouquier, S.; Lowe, J.B.; Kelly, R.J.; Fertitta, A.L.; Lennon, G.G.; Giorgi, D. Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J. Biol. Chem. 1995, 270, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Admetlla, A.; Sikora, M.; Laayouni, H.; Esteve, A.; Roubinet, F.; Blancher, A.; Calafell, F.; Bertranpetit, J.; Casals, F. A natural history of FUT2 polymorphism in humans. Mol. Biol. Evol. 2009, 26, 1993–2003. [Google Scholar] [CrossRef]
- Pang, H.; Fujitani, N.; Soejima, M.; Koda, Y.; Islam, M.N.; Islam, A.K.; Kimura, H. Two distinct Alu-mediated deletions of the human ABO-secretor (FUT2) locus in Samoan and Bangladeshi populations. Hum. Mutat. 2000, 16, 274. [Google Scholar] [CrossRef]
- Henry, S.; Mollicone, R.; Lowe, J.B.; Samuelsson, B.; Larson, G. A second nonsecretor allele of the blood group alpha(1,2)fucosyl-transferase gene (FUT2). Vox Sang. 1996, 70, 21–25. [Google Scholar] [CrossRef]
- Yu, L.C.; Chu, C.C.; Chan, Y.S.; Chang, C.Y.; Twu, Y.C.; Lee, H.L.; Lin, M. Polymorphism and distribution of the Secretor alpha(1,2)-fucosyltransferase gene in various Taiwanese populations. Transfusion 2001, 41, 1279–1284. [Google Scholar] [CrossRef]
- Chang, J.G.; Ko, Y.C.; Lee, J.C.; Chang, S.J.; Liu, T.C.; Shih, M.C.; Peng, C.T. Molecular analysis of mutations and polymorphisms of the Lewis secretor type alpha(1,2)-fucosyltransferase gene reveals that Taiwan aborigines are of Austronesian derivation. J. Hum. Genet. 2002, 47, 60–65. [Google Scholar] [CrossRef]
- Koda, Y.; Ishida, T.; Tachida, H.; Wang, B.; Pang, H.; Soejima, M.; Soemantri, A.; Kimura, H. DNA sequence variation of the human ABO-secretor locus ( FUT2) in New Guinean populations: Possible early human migration from Africa. Hum. Genet. 2003, 113, 534–541. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Real-time PCR-based detection of the Alu-mediated deletion of FUT2 (se(del2)). Leg. Med. 2022, 54, 101986. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Estimation of Lewis Blood Group Status by Fluorescence Melting Curve Analysis in Simultaneous Genotyping of c.385A>T and Fusion Gene in FUT2 and c.59T>G and c.314C>T in FUT3. Diagnostics 2023, 13, 931. [Google Scholar] [CrossRef]
- Schmid, C.W.; Jelinek, W.R. The Alu family of dispersed repetitive sequences. Science 1982, 216, 1065–1070. [Google Scholar] [CrossRef]
- Sen, S.K.; Han, K.; Wang, J.; Lee, J.; Wang, H.; Callinan, P.A.; Dyer, M.; Cordaux, R.; Liang, P.; Batzer, M.A. Human genomic deletions mediated by recombination between Alu elements. Am. J. Hum. Genet. 2006, 79, 41–53. [Google Scholar] [CrossRef]
- Kim, S.; Cho, C.S.; Han, K.; Lee, J. Structural Variation of Alu Element and Human Disease. Genom. Inform. 2016, 14, 70–77. [Google Scholar] [CrossRef]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]
- El Housni, H.; Heimann, P.; Parma, J.; Vassart, G. Single-nucleotide polymorphism genotyping by melting analysis of dual-labeled probes: Examples using factor V Leiden and prothrombin 20210A mutations. Clin. Chem. 2003, 49, 1669–1672. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Z.; Liao, Y.; Chen, X.; Zhang, Y.; Li, Q. Multiplex fluorescence melting curve analysis for mutation detection with dual-labeled, self-quenched probes. PLoS ONE 2011, 6, e19206. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Xu, J.X.; Wang, M.M.; Hu, D.; Xie, F.; Huang, D.; Chen, J.; Yang, T.; Zhang, J.; Song, F.; et al. Single probe PCR melting curve analysis MTHFR C677T SNP sites. Anal. Biochem. 2021, 619, 114102. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Li, B.; Xie, J.; Liu, J.; Zhang, Z. Single nucleotide polymorphism genotyping of ALDH2 gene based on asymmetric PCR and fluorescent probe-mediated melting curves. Anal. Biochem. 2021, 642, 114509. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Detection of the weak-secretor rs1047781 (385A>T) single nucleotide polymorphism using an unlabeled probe high-resolution melting-based method. Electrophoresis 2021, 42, 1362–1365. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Simultaneous genotyping of three major Se enzyme inactivating SNPs of FUT2 based on a triplex probe-based fluorescence melting-curve analysis. Clin. Chim. Acta 2022, 530, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hippman, C.; Nislow, C. Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. J. Pers. Med. 2019, 9, 40. [Google Scholar] [CrossRef]
- Wittwer, C.T. High-resolution DNA melting analysis: Advancements and limitations. Hum. Mutat. 2009, 30, 857–859. [Google Scholar] [CrossRef]
- Kimura, R.; Ohashi, J.; Matsumura, Y.; Nakazawa, M.; Inaoka, T.; Ohtsuka, R.; Osawa, M.; Tokunaga, K. Gene flow and natural selection in oceanic human populations inferred from genome-wide SNP typing. Mol. Biol. Evol. 2008, 25, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Wollstein, A.; Lao, O.; Becker, C.; Brauer, S.; Trent, R.J.; Nurnberg, P.; Stoneking, M.; Kayser, M. Demographic history of Oceania inferred from genome-wide data. Curr. Biol. 2010, 20, 1983–1992. [Google Scholar] [CrossRef]
- Choin, J.; Mendoza-Revilla, J.; Arauna, L.R.; Cuadros-Espinoza, S.; Cassar, O.; Larena, M.; Ko, A.M.; Harmant, C.; Laurent, R.; Verdu, P.; et al. Genomic insights into population history and biological adaptation in Oceania. Nature 2021, 592, 583–589. [Google Scholar] [CrossRef]
- Henry, S.; Mollicone, R.; Fernandez, P.; Samuelsson, B.; Oriol, R.; Larson, G. Molecular basis for erythrocyte Le(a+ b+) and salivary ABH partial-secretor phenotypes: Expression of a FUT2 secretor allele with an A-->T mutation at nucleotide 385 correlates with reduced alpha(1,2) fucosyltransferase activity. Glycoconj. J. 1996, 13, 985–993. [Google Scholar] [CrossRef]
- Condemi, S.; Mazieres, S.; Faux, P.; Costedoat, C.; Ruiz-Linares, A.; Bailly, P.; Chiaroni, J. Blood groups of Neandertals and Denisova decrypted. PLoS ONE 2021, 16, e0254175. [Google Scholar] [CrossRef]
- Loureiro Tonini, M.A.; Pires Goncalves Barreira, D.M.; Bueno de Freitas Santolin, L.; Bondi Volpini, L.P.; Gagliardi Leite, J.P.; Le Moullac-Vaidye, B.; Le Pendu, J.; Cruz Spano, L. FUT2, Secretor Status and FUT3 Polymorphisms of Children with Acute Diarrhea Infected with Rotavirus and Norovirus in Brazil. Viruses 2020, 12, 1084. [Google Scholar] [CrossRef]
- Nishida, N.; Sugiyama, M.; Kawai, Y.; Naka, I.; Iwamoto, N.; Suzuki, T.; Suzuki, M.; Miyazato, Y.; Suzuki, S.; Izumi, S.; et al. Genetic association of IL17 and the importance of ABO blood group antigens in saliva to COVID-19. Sci. Rep. 2022, 12, 3854. [Google Scholar] [CrossRef]
- Moslemi, C.; Saekmose, S.; Larsen, R.; Brodersen, T.; Didriksen, M.; Hjalgrim, H.; Banasik, K.; Nielsen, K.R.; Bruun, M.T.; Dowsett, J.; et al. A large cohort study of the effects of Lewis, ABO, 13 other blood groups, and secretor status on COVID-19 susceptibility, severity, and long COVID-19. Transfusion 2023, 63, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Kraft, P.; Selhub, J.; Giovannucci, E.L.; Thomas, G.; Hoover, R.N.; Chanock, S.J.; Hunter, D.J. Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat. Genet. 2008, 40, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.P.; Jones, M.R.; Taylor, K.D.; Marciante, K.; Yan, X.; Dubinsky, M.; Ippoliti, A.; Vasiliauskas, E.; Berel, D.; Derkowski, C.; et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum. Mol. Genet. 2010, 19, 3468–3476. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.S.; Alakulppi, N.; Paavola-Sakki, P.; Kurppa, K.; Halme, L.; Farkkila, M.; Turunen, U.; Lappalainen, M.; Kontula, K.; Kaukinen, K.; et al. Association study of FUT2 (rs601338) with celiac disease and inflammatory bowel disease in the Finnish population. Tissue Antigens 2012, 80, 488–493. [Google Scholar] [CrossRef]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, D.; Zheng, S.; Guo, M.; Lin, X.; Jiang, Y. Association of Ulcerative Colitis with FUT2 and FUT3 Polymorphisms in Patients from Southeast China. PLoS ONE 2016, 11, e0146557. [Google Scholar] [CrossRef]
- Mottram, L.; Wiklund, G.; Larson, G.; Qadri, F.; Svennerholm, A.M. FUT2 non-secretor status is associated with altered susceptibility to symptomatic enterotoxigenic Escherichia coli infection in Bangladeshis. Sci. Rep. 2017, 7, 10649. [Google Scholar] [CrossRef]
- Santos-Cortez, R.L.P.; Chiong, C.M.; Frank, D.N.; Ryan, A.F.; Giese, A.P.J.; Bootpetch Roberts, T.; Daly, K.A.; Steritz, M.J.; Szeremeta, W.; Pedro, M.; et al. FUT2 Variants Confer Susceptibility to Familial Otitis Media. Am. J. Hum. Genet. 2018, 103, 679–690. [Google Scholar] [CrossRef]
- Colston, J.M.; Francois, R.; Pisanic, N.; Penataro Yori, P.; McCormick, B.J.J.; Olortegui, M.P.; Gazi, M.A.; Svensen, E.; Ahmed, M.M.M.; Mduma, E.; et al. Effects of Child and Maternal Histo-Blood Group Antigen Status on Symptomatic and Asymptomatic Enteric Infections in Early Childhood. J. Infect. Dis. 2019, 220, 151–162. [Google Scholar] [CrossRef] [PubMed]



| Primer Sequences | Position of FUT2 | Position of SEC1P | Differences with SEC1P | Amplicon Length |
|---|---|---|---|---|
| Detection of 375A>G, 385A>T | ||||
| FUT2-337F: 5′-TGGCAGAACTACCACCTGAA-3′ | 337–356 bp | 379–398 bp | 0 | 76 bp |
| FUT2-412R: 5′-CGGTGAAGCGGACGTACT-3′ | 395–412 bp | 437–454 bp | 6 | |
| 385-probe: 5′-HEX GGAGGAATACCGCCACATCCCGGGG-BHQ1-3′ | 369–393 bp | 411–435 bp | 1 | |
| 375-probe: 5′-HEX GGAGGAGTACCGCCACATCCCGGGG-BHQ1-3′ | 369–393 bp | 411–435 bp | 0 | |
| Detection of 571T>C | 539–556 bp | 581–598 bp | 5 | |
| FUT2-531F: 5′-GGCCGGGCACCTTTGTAG-3′ | 598–617 bp | 640–659 bp | 5 | 79 bp |
| FUT2-617R: 5′-ACCACCCCCTTCCACACTTT-3′ | 558–582 bp | 600–624 bp | 3 | |
| 571C-probe: 5′-Cy5-GGTCCATGTTCGCCGAGGGGACTAT-BHQ2-3′ | 539–556 bp | 581–598 bp | 5 | |
| Detection of 375A>G, 385A>T, 571T>C | ||||
| FUT2-337F: 5′-TGGCAGAACTACCACCTGAA-3′ | 337–356 bp | 379–398 bp | 0 | 281 bp |
| FUT2-617R: 5′-ACCACCCCCTTCCACACTTT-3′ | 598–617 bp | 640–659 bp | 5 | |
| 375-probe and 571-probe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soejima, M.; Koda, Y. Detection of c.375A>G, c.385A>T, c.571C>T, and sedel2 of FUT2 via Real-Time PCR in a Single Tube. Diagnostics 2023, 13, 2022. https://doi.org/10.3390/diagnostics13122022
Soejima M, Koda Y. Detection of c.375A>G, c.385A>T, c.571C>T, and sedel2 of FUT2 via Real-Time PCR in a Single Tube. Diagnostics. 2023; 13(12):2022. https://doi.org/10.3390/diagnostics13122022
Chicago/Turabian StyleSoejima, Mikiko, and Yoshiro Koda. 2023. "Detection of c.375A>G, c.385A>T, c.571C>T, and sedel2 of FUT2 via Real-Time PCR in a Single Tube" Diagnostics 13, no. 12: 2022. https://doi.org/10.3390/diagnostics13122022
APA StyleSoejima, M., & Koda, Y. (2023). Detection of c.375A>G, c.385A>T, c.571C>T, and sedel2 of FUT2 via Real-Time PCR in a Single Tube. Diagnostics, 13(12), 2022. https://doi.org/10.3390/diagnostics13122022






