Validity and Reliability of Point-of-Care Ultrasound for Detecting Moderate- or High-Grade Carotid Atherosclerosis in an Outpatient Department
Abstract
1. Introduction
2. Materials and Methods
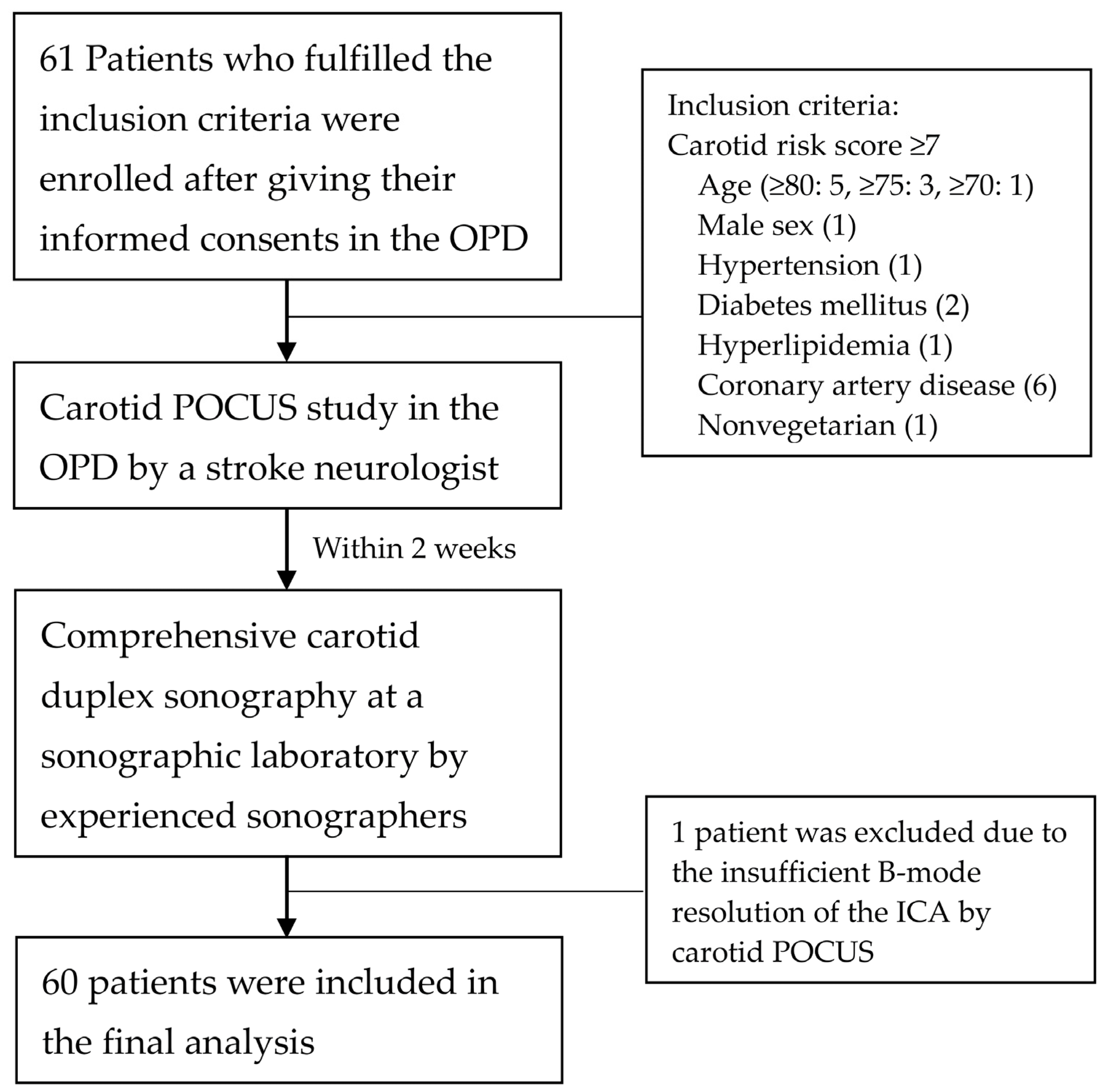
2.1. Design and Participants
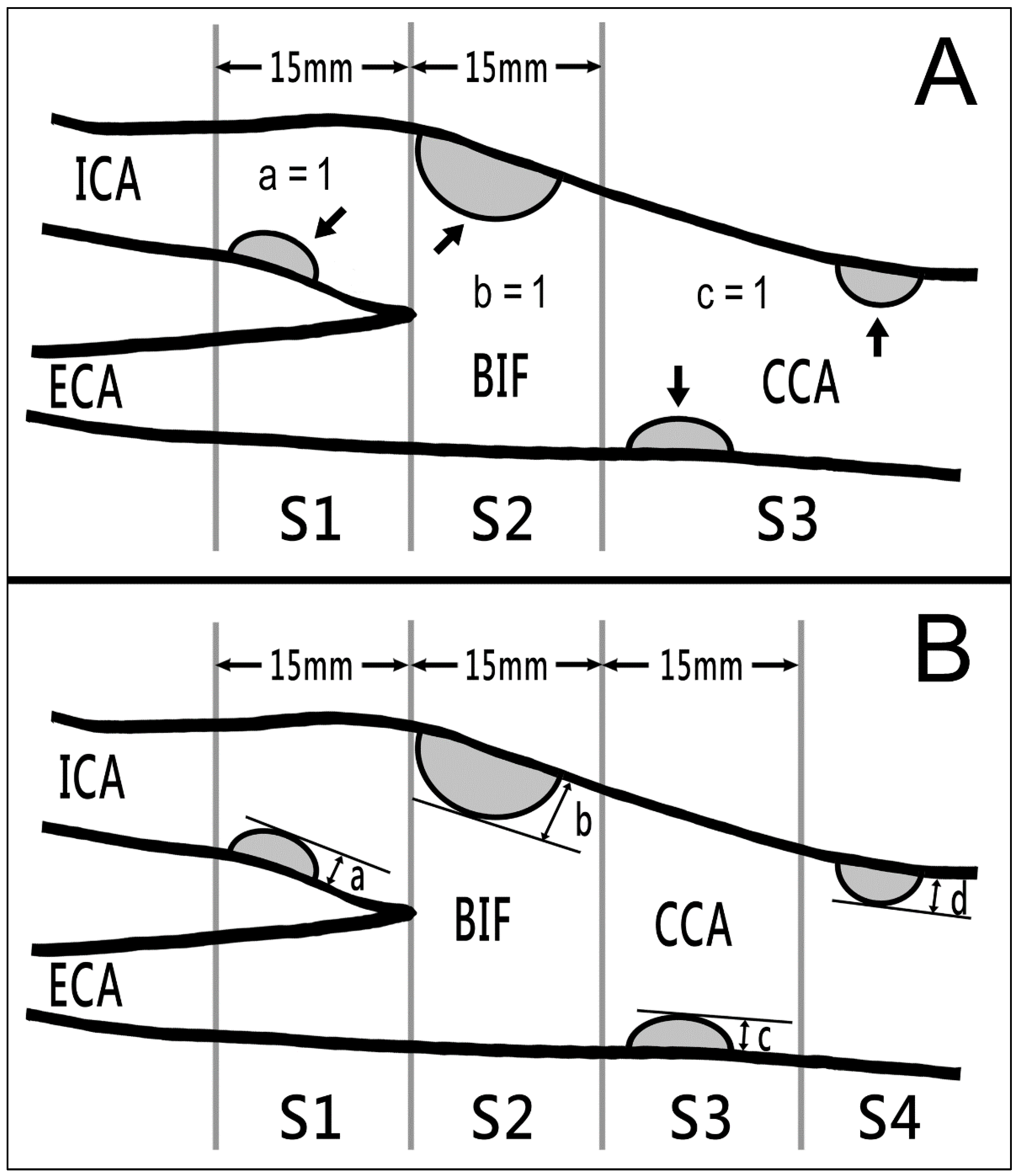
2.2. Instruments and Measurements
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Assessment Reliabilities of Laboratory sCPS and hCPS
3.3. Carotid Sonography Findings
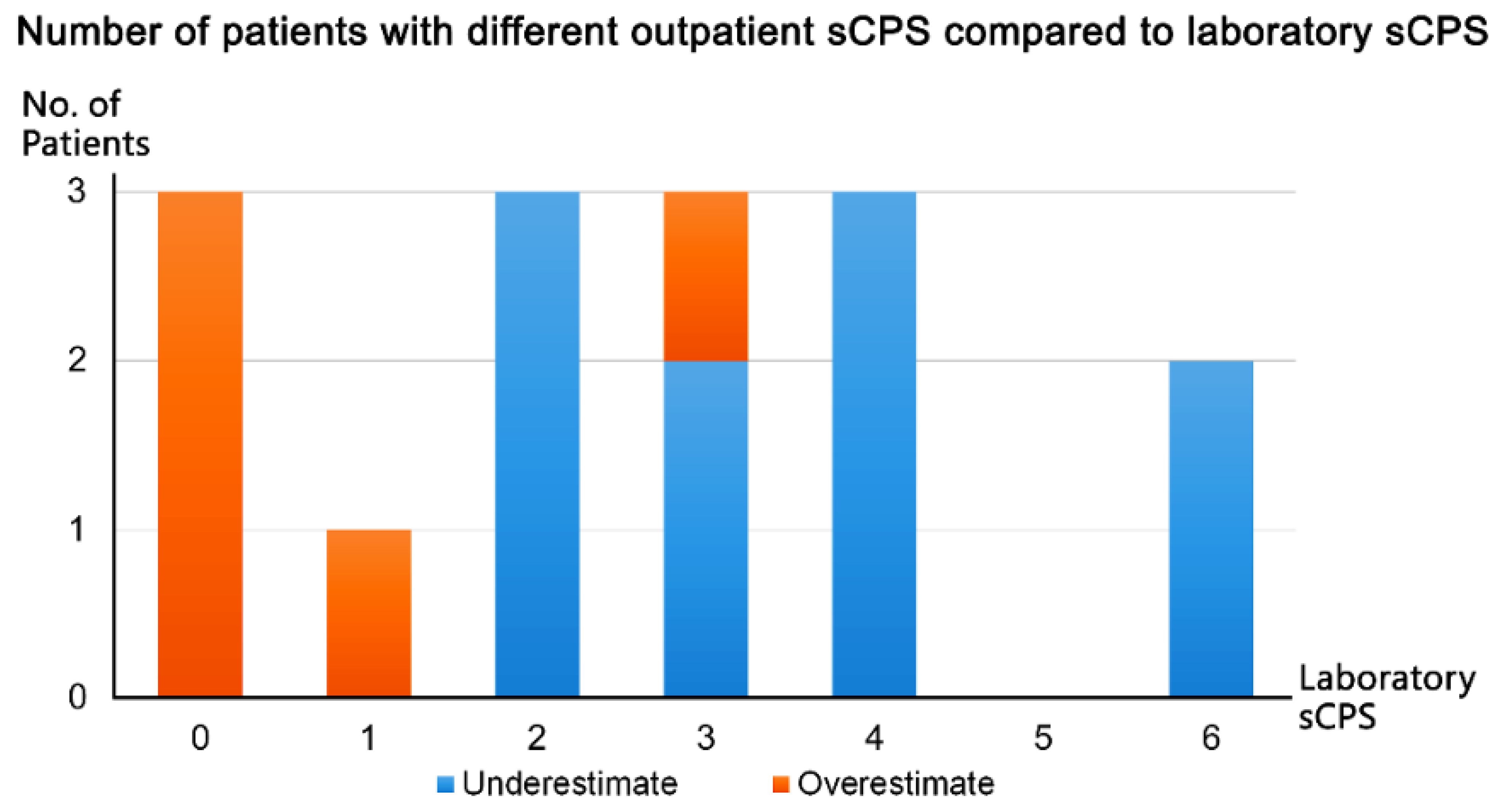
3.4. Agreement and Reliability of Outpatient Carotid POCUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke risk factors, genetics, and prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Mok, Y.; Sang, Y.; Ballew, S.H.; Rebholz, C.M.; Rosamond, W.D.; Heiss, G.; Folsom, A.R.; Coresh, J.; Matsushita, K. American Heart Association’s Life’s Simple 7 at middle age and prognosis after myocardial infarction in later life. J. Am. Heart Assoc. 2018, 7, e007658. [Google Scholar] [CrossRef] [PubMed]
- Bonithon-Kopp, C.; Scarabin, P.Y.; Taquet, A.; Touboul, P.J.; Malmejac, A.; Guize, L. Risk factors for early carotid atherosclerosis in middle-aged French women. Arterioscler Thromb. 1991, 11, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: The BioImage study. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, H.-K.; Kwon, S.U.; Lee, S.-W.; Kim, M.-J.; Park, J.W.; Noh, M.; Han, Y.; Kwon, T.-W.; Cho, Y.-P. Risk of major adverse cardiovascular events in subjects with asymptomatic mild carotid artery stenosis. Sci. Rep. 2018, 8, 4700. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; I Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Spence, J.D.; Eliasziw, M.; DiCicco, M.; Hackam, D.G.; Galil, R.; Lohmann, T. Carotid plaque area: A tool for targeting and evaluating vascular preventive therapy. Stroke 2002, 33, 2916–2922. [Google Scholar] [CrossRef]
- Spence, J.D. Measurement of carotid plaque burden. JAMA Neurol. 2015, 72, 383–384. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W.; et al. Screening for Asymptomatic Carotid Artery Stenosis: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 476–481. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 7, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart. J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.L.; Chen, P.Y.; Hsu, P.J.; Lin, S.K. Nomogram and Carotid Risk Score for Predicting Moderate or High Carotid Atherosclerosis among Asymptomatic Elderly Recycling Volunteers. Diagnostics 2022, 12, 1407. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Point-of-care ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef]
- Jose, L.; Diaz-Gomez, M.D.; Paul, H.; Mayo, M.D.; Seth, J.; Koenig. Point-of care ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Zhang, H.-J.; Sun, S.-Y.; Wang, L.-Y.; Yan, B.; Liu, C.-Q.; Zhang, W.; Li, X.-J. Relationship of carotid intima-media thickness and duration of vegetarian diet in Chinese male vegetarians. Nutr. Metab. 2011, 8, 63. [Google Scholar] [CrossRef]
- Chen, C.W.; Lin, C.T.; Lin, Y.L.; Lin, T.K.; Lin, C.L. Taiwanese female vegetarians have lower lipoprotein-associated phospholipase A2 compared with omnivores. Yonsei. Med. J. 2011, 52, 13–19. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the society for vascular medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.H.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011): An update on behalf of the advisory board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Hollander, M.; Bots, M.; del Sol, A.I.; Koudstaal, P.; Witteman, J.; Grobbee, D.; Hofman, A.; Breteler, M. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: The Rotterdam study. Circulation 2002, 105, 2872–2877. [Google Scholar] [CrossRef]
- Handa, N.; Matsumoto, M.; Maeda, H.; Hougaku, H.; Ogawa, S.; Fukunaga, R.; Yoneda, S.; Kimura, K.; Kamada, T. Ultrasonic evaluation of early carotid atherosclerosis. Stroke 1990, 21, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Matsumoto, M.; Maeda, H.; Hougaku, H.; Kamada, T. Ischemic stroke events and carotid atherosclerosis: Results of the Osaka Follow-up Study for Ultrasonographic Assessment of Carotid Atherosclerosis (the OSACA Study). Stroke 1995, 26, 1781–1786. [Google Scholar] [CrossRef]
- Jang, C.W.; Kim, Y.K.; Kim, K.H.; Chiara, A.; Lee, M.S.; Bae, J.H. Predictors for high-risk carotid plaque in asymptomatic Korean population. Cardiovasc. Ther. 2020, 2020, 6617506. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Meesters, A.M.L.; Ten Duis, K.; Banierink, H.; Stirler, V.M.A.; Wouters, P.C.R.; Kraeima, J.; de Vries, J.P.M.; Witjes, M.J.H.; IJpma, F.F.A. What are the interobserver and intraobserver variability of gap and stepoff measurements in acetabular fractures? Clin. Orthop. Relat. Res. 2020, 478, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- van-der-Meer, I.M.; Bots, M.L.; Hofman, A.; del-Sol, A.I.; van-der-Kuip, D.A.; Witteman, J.C. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: The Rotterdam Study. Circulation 2004, 109, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Rosvall, M.; Janzon, L.; Berglund, G.; Engström, G.; Hedblad, B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J. Intern. Med. 2005, 257, 430–437. [Google Scholar] [CrossRef]
- Chien, K.-L.; Su, T.-C.; Jeng, J.-S.; Hsu, H.-C.; Chang, W.-T.; Chen, M.-F.; Lee, Y.-T.; Hu, F.B. Carotid artery intima-media thickness, carotid plaque and coronary heart disease and stroke in Chinese. PLoS ONE 2008, 3, e3435. [Google Scholar] [CrossRef]
- Prati, P.; Tosetto, A.; Casaroli, M.; Bignamini, A.; Canciani, L.; Bornstein, N.; Prati, G.; Touboul, P. Carotid plaque morphology improves stroke risk prediction: Usefulness of a new ultrasonographic score. Cerebrovasc. Dis. 2011, 31, 300–304. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Luo, B.; Zhang, Y. The predictive value of carotid ultrasonography with cardiovascular risk factors—A “SPIDER” promoting atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 706490. [Google Scholar] [CrossRef]
- Polak, J.F.; Shemanski, L.; O’Leary, D.H.; Lefkowitz, D.; Price, T.R.; Savage, P.J.; Brant, W.E.; Reid, C. Hypoechoic plaque at US of the carotid artery: An independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology 1998, 208, 649–654. [Google Scholar] [CrossRef]
- Yang, F.Y.; Hsu, P.R.; Lin, S.K. Reduced internal carotid artery flow in color-coded carotid duplex sonography. Acta Neurol. Taiwan. 2016, 25, 136–147. [Google Scholar]
- Näslund, U.; Ng, N.; Lundgren, A.; Fhärm, E.; Grönlund, C.; Johansson, H.; Lindahl, B.; Lindahl, B.; Lindvall, K.; Nilsson, S.K.; et al. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): A pragmatic, open-label, randomised controlled trial. Lancet 2019, 393, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Hackam, D.G. Treating arteries instead of risk factors: A paradigm change in management of atherosclerosis. Stroke 2010, 41, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Weinreich, A.; Mucha, S.; Saur, D.; Pelz, J.O. Evaluation of the interrater and intermethod agreement of the German multiparametric ultrasound criteria for the grading of internal carotid artery stenosis. Neuroradiology 2021, 63, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Davidsen, A.H.; Schirmer, H.; Melbye, H.; Spigt, M.; Aviles-Solis, J.C. Interrater and intrarater agreement on heart murmurs. Scand. J. Prim. Health Care 2022, 40, 491–497. [Google Scholar] [CrossRef]
- Suttie, R.; Woo, M.Y.; Park, L.; Nemnom, M.J.; Stotts, G.; Perry, J.J. Can emergency physicians perform carotid artery point-of-care ultrasound to detect stenosis in patients with TIA and stroke? A pilot study. West J. Emerg. Med. 2020, 21, 626–632. [Google Scholar] [CrossRef]
- Saxhaug, L.M.; Graven, T.; Olsen, Ø.; Kleinau, J.O.; Skjetne, K.; Ellekjær, H.; Dalen, H. Reliability and agreement of point-of-care carotid artery examinations by experts using hand-held ultrasound devices in patients with ischaemic stroke or transitory ischaemic attack. Open Heart 2022, 9, e001917. [Google Scholar] [CrossRef]



| Items | Score |
|---|---|
| Age | |
| <70 years | 0 |
| 70–74 years | 1 |
| 75–79 years | 3 |
| ≥80 years | 5 |
| Male sex | 1 |
| Hypertension | 1 |
| Diabetes mellitus | 2 |
| Hyperlipidemia | 1 |
| Coronary artery disease | 6 |
| Nonvegetarian | 1 |
| Total score | 0–17 |
| Characteristics | Total (n = 60) | Male (n = 20) | Female (n = 40) | p Value |
|---|---|---|---|---|
| Clinical features | 0.670 | |||
| Dementia | 39 (65.1%) | 13 (65%) | 26 (65%) | |
| Dizziness/vertigo | 6 (10.0%) | 2 (10%) | 4 (10%) | |
| Parkinson’s disease | 5 (8.3%) | 1 (5%) | 4 (10%) | |
| Hypertension | 5 (8.3%) | 1 (5%) | 4 (10%) | |
| Neuromuscular disorder | 5 (8.3%) | 3 (15%) | 2 (5%) | |
| Risk factors | ||||
| Age | 81.9 (79.7–85.2) | 81.5 (77.9–84.2) | 82.0 (80.3–87.0) | 0.233 |
| ≥90 years | 7 (11.7%) | 1 (5%) | 6 (15%) | |
| 85–89 years | 9 (15.02%) | 2 (10%) | 7 (18%) | |
| 80–84 years | 29 (48.3%) | 11 (55%) | 18 (45%) | |
| 75–79 years | 12 (20.0%) | 3 (15%) | 9 (22%) | |
| 70–74 years | 2 (3.3%) | 2 (10%) | 0 (0%) | |
| <70 years | 1 (1.7%) | 1 (5%) | 0 (0%) | |
| Male sex | 20 (33%) | - | - | - |
| Hypertension | 52 (87%) | 17 (85%) | 35 (88%) | >0.999 |
| Diabetes mellitus | 20 (33%) | 5 (25%) | 15 (38%) | 0.395 |
| Hyperlipidemia | 31 (52%) | 9 (45%) | 22 (55%) | 0.586 |
| Coronary artery disease | 16 (27%) | 7 (35%) | 9 (23%) | 0.359 |
| Nonvegetarian | 52 (87%) | 18 (90%) | 34 (85%) | 0.707 |
| Carotid risk score | 8.0 (7.0–10.0) | 8.0 (7.0–13.0) | 8.0 (7.0–10.0) | 0.519 |
| 7–8 | 35 (58.3%) | 11 (55%) | 24 (60%) | |
| 9–12 | 17 (28.3%) | 4 (20%) | 13 (33%) | |
| >12 | 8 (13.3%) | 5 (25%) | 3 (7%) |
| Intraobserver | Interobserver | |||
|---|---|---|---|---|
| Assessment | ICC (95% CI) | Weighted Kappa (95% CI) | ICC (95% CI) | Weighted Kappa (95% CI) |
| sCPS | 0.958 (0.914 to 0.979) | 0.934 (0.842 to 1.000) | 0.939 (0.877 to 0.970) | 0.844 (0.743 to 0.945) |
| hCPS | 0.974 (0.946 to 0.987) | 0.897 (0.823 to 0.972) | 0.970 (0.939 to 0.986) | 0.830 (0.753 to 0.907) |
| Total (n = 60) | Male (n = 20) | Female (n = 40) | p Value | |
|---|---|---|---|---|
| Carotid risk score | 8.0 (7.0–10.0) | 8.0 (7.0–13.0) | 8.0 (7.0–10.0) | 0.519 |
| Carotid risk probability (%) | 63.7 (50.5–75.8) | 63.7 (58.6–87.9) | 61.2 (50.5–75.8) | 0.137 |
| Outpatient sCPS | 2.0 (1.0–3.5) | 3.0 (1.5–3.0) | 2.0 (1.0–4.0) | 0.719 |
| Laboratory sCPS | 2.0 (1.5–4.0) | 3.0 (1.5–4.1) | 2.0 (1.5–4.0) | 0.555 |
| Laboratory hCPS | 5.0 (3.1–9.0) | 5.9 (2.9–8.9) | 4.3 (3.1–9.0) | 0.742 |
| hCPS ≤ 5 (n = 30) | hCPS > 5 (n = 30) | p Value | |
|---|---|---|---|
| Carotid risk score | 8.0 (7.0–10.0) | 8.0 (7.0–12.0) | 0.529 |
| Carotid risk probability (%) | 58.6 (50.5–75.8) | 63.7 (58.6–88.9) | 0.221 |
| sCPS at outpatient clinic | 1.0 (1.0–2.0) | 3.5 (3.0–5.0) | <0.001 |
| sCPS at laboratory | 1.5 (0.0–2.0) | 4.0 (3.0–5.0) | <0.001 |
| hCPS at laboratory | 3.1 (1.4–3.7) | 9.0 (6.9–11.5) | <0.001 |
| Age | 81.9 (80.1–87.7) | 81.9 (78.7–84.4) | 0.506 |
| Male sex | 8 (27%) | 12 (40%) | 0.412 |
| Hypertension | 26 (87%) | 26 (87%) | >0.999 |
| Diabetes mellitus | 7 (23%) | 13 (43%) | 0.170 |
| Hyperlipidemia | 13 (43%) | 18 (60%) | 0.302 |
| Coronary artery disease | 8 (27%) | 8 (27%) | >0.999 |
| Nonvegetarian diet | 25 (83%) | 27 (90%) | 0.707 |
| Carotid Risk Score | Outpatient sCPS | Laboratory sCPS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | R2 | p | Coefficient | R2 | p | Coefficient | R2 | p | |
| Outpatient sCPS | 0.223 | 0.130 | 0.005 | - | - | - | - | - | - |
| Laboratory sCPS | 0.215 | 0.104 | 0.012 | 1.03 | 0.916 | <0.001 | - | - | - |
| Laboratory hCPS | 0.438 | 0.070 | 0.042 | 2.449 | 0.833 | <0.001 | 2.314 | 0.861 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-L.; Chen, P.-Y.; Hsu, P.-J.; Lin, S.-K. Validity and Reliability of Point-of-Care Ultrasound for Detecting Moderate- or High-Grade Carotid Atherosclerosis in an Outpatient Department. Diagnostics 2023, 13, 1952. https://doi.org/10.3390/diagnostics13111952
Chang W-L, Chen P-Y, Hsu P-J, Lin S-K. Validity and Reliability of Point-of-Care Ultrasound for Detecting Moderate- or High-Grade Carotid Atherosclerosis in an Outpatient Department. Diagnostics. 2023; 13(11):1952. https://doi.org/10.3390/diagnostics13111952
Chicago/Turabian StyleChang, Wan-Ling, Pei-Ya Chen, Po-Jen Hsu, and Shinn-Kuang Lin. 2023. "Validity and Reliability of Point-of-Care Ultrasound for Detecting Moderate- or High-Grade Carotid Atherosclerosis in an Outpatient Department" Diagnostics 13, no. 11: 1952. https://doi.org/10.3390/diagnostics13111952
APA StyleChang, W.-L., Chen, P.-Y., Hsu, P.-J., & Lin, S.-K. (2023). Validity and Reliability of Point-of-Care Ultrasound for Detecting Moderate- or High-Grade Carotid Atherosclerosis in an Outpatient Department. Diagnostics, 13(11), 1952. https://doi.org/10.3390/diagnostics13111952








