Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy
Abstract
1. Introduction
2. NVC Findings in COVID-19
3. NVC vs. Other Methods to Evaluate Microcirculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed on 11 March 2023).
- World Health Organization. Therapeutics and COVID-19: Living Guideline, 13 January 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Yelin, D.; Moschopoulos, C.D.; Margalit, I.; Gkrania-Klotsas, E.; Landi, F.; Stahl, J.-P.; Yahav, D. ESCMID Rapid Guidelines for Assessment and Management of Long COVID. Clin. Microbiol. Infect. 2022, 28, 955–972. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Salton, F.; Trotta, L.; Bozzi, C.; Pozzan, R.; Barbieri, M.; Tavano, S.; Lerda, S.; Hughes, M.; Confalonieri, M.; et al. Host-Based Treatments for Severe COVID-19. CIMB 2023, 45, 3102–3121. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Weng, J. Endothelial Dysfunction in COVID-19: An Overview of Evidence, Biomarkers, Mechanisms and Potential Therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Sciaudone, A.; Corkrey, H.; Humphries, F.; Koupenova, M. Platelets and SARS-CoV-2 During COVID-19: Immunity, Thrombosis, and Beyond. Circ. Res. 2023, 132, 1272–1289. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Ruaro, B.; Smith, V.; Sulli, A.; Pizzorni, C.; Tardito, S.; Patané, M.; Paolino, S.; Cutolo, M. Innovations in the Assessment of Primary and Secondary Raynaud’s Phenomenon. Front. Pharmacol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Ruaro, B.; Sulli, A.; Smith, V.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Trombetta, A.C.; Cutolo, M. Advances in nailfold capillaroscopic analysis in systemic sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 122–131. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of Nailfold Capillaroscopy for the Assessment of Patients with Raynaud’s Phenomenon and Systemic Sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef]
- Bernero, E.; Sulli, A.; Ferrari, G.; Ravera, F.; Pizzorni, C.; Ruaro, B.; Zampogna, G.; Alessandri, E.; Cutolo, M. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: Preliminary results. Reumatismo 2013, 65, 186–191. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Smith, V.; Pizzorni, C.; Meroni, M.; Paolino, S.; Cariti, C.; Ruaro, B.; Sulli, A.; Cutolo, M. Quantitative Alterations of Capillary Diameter Have a Predictive Value for Development of the Capillaroscopic Systemic Sclerosis Pattern. J. Rheumatol. 2016, 43, 599–606. [Google Scholar] [CrossRef]
- Mansueto, N.; Rotondo, C.; Corrado, A.; Cantatore, F.P. Nailfold Capillaroscopy: A Comprehensive Review on Common Findings and Clinical Usefulness in Non-Rheumatic Disease. J. Med. Investig. 2021, 68, 6–14. [Google Scholar] [CrossRef]
- Antonios, T.F.T.; Singer, D.R.J.; Markandu, N.D.; Mortimer, P.S.; MacGregor, G.A. Structural Skin Capillary Rarefaction in Essential Hypertension. Hypertension 1999, 33, 998–1001. [Google Scholar] [CrossRef]
- Ruaro, B.; Baratella, E.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Busca, A.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics 2021, 11, 1960. [Google Scholar] [CrossRef]
- Natalello, G.; De Luca, G.; Gigante, L.; Campochiaro, C.; De Lorenzis, E.; Verardi, L.; Paglionico, A.; Petricca, L.; Martone, A.M.; Calvisi, S.; et al. Nailfold Capillaroscopy Findings in Patients with Coronavirus Disease 2019: Broadening the Spectrum of COVID-19 Microvascular Involvement. Microvasc. Res. 2021, 133, 104071. [Google Scholar] [CrossRef]
- Sulli, A.; Secchi, M.E.; Pizzorni, C.; Cutolo, M. Scoring the Nailfold Microvascular Changes during the Capillaroscopic Analysis in Systemic Sclerosis Patients. Ann. Rheum. Dis. 2008, 67, 885–887. [Google Scholar] [CrossRef]
- Sambataro, D.; Sambataro, G.; Zaccara, E.; Maglione, W.; Polosa, R.; Afeltra, A.M.; Vitali, C.; Del Papa, N. Nailfold Videocapillaroscopy Micro-Haemorrhage and Giant Capillary Counting as an Accurate Approach for a Steady State Definition of Disease Activity in Systemic Sclerosis. Arthritis Res. Ther. 2014, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Soldano, S.; Smith, V.; Paolino, S.; Contini, P.; Montagna, P.; Pizzorni, C.; Casabella, A.; Tardito, S.; Sulli, A.; et al. Correlation between circulating fibrocytes and dermal thickness in limited cutaneous systemic sclerosis patients: A pilot study. Rheumatol. Int. 2019, 39, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Monticone, G.; Colonna, L.; Palermi, G.; Bono, R.; Puddu, P. Quantitative Nailfold Capillary Microscopy Findings in Patients with Acrocyanosis Compared with Patients Having Systemic Sclerosis and Control Subjects. J. Am. Acad. Dermatol. 2000, 42, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Ingegnoli, F.; Gualtierotti, R.; Lubatti, C.; Zahalkova, L.; Meani, L.; Boracchi, P.; Zeni, S.; Fantini, F. Feasibility of Different Capillaroscopic Measures for Identifying Nailfold Microvascular Alterations. Semin. Arthritis Rheum. 2009, 38, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Etehad Tavakol, M.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B.-E. Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? BioMed Res. Int. 2015, 2015, 974530. [Google Scholar] [CrossRef]
- Karbalaie, A.; Emrani, Z.; Fatemi, A.; Etehadtavakol, M.; Erlandsson, B.-E. Practical Issues in Assessing Nailfold Capillaroscopic Images: A Summary. Clin. Rheumatol. 2019, 38, 2343–2354. [Google Scholar] [CrossRef]
- Karahan, S.; Aydin, K.; Cetinkaya, A.; Sirakaya, H.A. Nailfold Videocapillaroscopy in Patients with COVID-19-Associated Pneumonia in Intensive Care Units. J. Coll. Phys. Surg. Pak. 2022, 32, 455–460. [Google Scholar] [CrossRef]
- Rosei, C.A.; Gaggero, A.; Famà, F.; Malerba, P.; Chiarini, G.; Nardin, M.; Brami, V.; Rossini, C.; Coschignano, M.A.; Porteri, E.; et al. Skin Capillary Alterations in Patients with Acute SarsCoV2 Infection. J. Hypertens. 2022, 40, 2385–2393. [Google Scholar] [CrossRef]
- Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://www.cdc.gov/mis/mis-c/hcp_cstecdc/index.html (accessed on 15 April 2023).
- Andrade, L.E.C.; Gabriel, A.; Assad, R.L.; Ferrari, A.J.L.; Atra, E. Panoramic Nailfold Capillaroscopy: A New Reading Method and Normal Range. Semin. Arthritis Rheum. 1990, 20, 21–31. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Accardo, S. Nailfold Videocapillaroscopy Assessment of Microvascular Damage in Systemic Sclerosis. J. Rheumatol. 2000, 27, 155–160. [Google Scholar]
- Çakmak, F.; Demirbuga, A.; Demirkol, D.; Gümüş, S.; Torun, S.H.; Kayaalp, G.K.; Ömeroglu, R.E.; Somer, A.; Uysalol, M.; Yıldız, R.; et al. Nailfold Capillaroscopy: A Sensitive Method for Evaluating Microvascular Involvement in Children with SARS-CoV-2 Infection. Microvasc. Res. 2021, 138, 104196. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Medsger, T.A., Jr. Raynaud’s Phenomenon: A Proposal for Classification. Clin. Exp. Rheumatol. 1992, 10, 485–488. [Google Scholar]
- Sulli, A.; Gotelli, E.; Bica, P.F.; Schiavetti, I.; Pizzorni, C.; Aloè, T.; Grosso, M.; Barisione, E.; Paolino, S.; Smith, V.; et al. Detailed Videocapillaroscopic Microvascular Changes Detectable in Adult COVID-19 Survivors. Microvasc. Res. 2022, 142, 104361. [Google Scholar] [CrossRef]
- Damiani, E.; Carsetti, A.; Casarotta, E.; Scorcella, C.; Domizi, R.; Adrario, E.; Donati, A. Microvascular Alterations in Patients with SARS-CoV-2 Severe Pneumonia. Ann. Intensive Care 2020, 10, 60. [Google Scholar] [CrossRef]
- Scorcella, C.; Damiani, E.; Domizi, R.; Pierantozzi, S.; Tondi, S.; Carsetti, A.; Ciucani, S.; Monaldi, V.; Rogani, M.; Marini, B.; et al. MicroDAIMON Study: Microcirculatory Daily Monitoring in Critically Ill Patients: A Prospective Observational Study. Ann. Intensive Care 2018, 8, 64. [Google Scholar] [CrossRef]
- Carsetti, A.; Damiani, E.; Casarotta, E.; Scorcella, C.; Domizi, R.; Montomoli, J.; Gasparri, F.; Gabbanelli, V.; Pantanetti, S.; Carozza, R.; et al. Sublingual Microcirculation in Patients with SARS-CoV-2 Undergoing Veno-Venous Extracorporeal Membrane Oxygenation. Microvasc. Res. 2020, 132, 104064. [Google Scholar] [CrossRef]
- do Espírito Santo, D.A.; Lemos, A.C.B.; Miranda, C.H. In Vivo Demonstration of Microvascular Thrombosis in Severe COVID-19. J. Thromb. Thrombolysis 2020, 50, 790–794. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary Post-Mortem Findings in a Series of COVID-19 Cases from Northern Italy: A Two-Centre Descriptive Study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Kanoore Edul, V.S.; Caminos Eguillor, J.F.; Ferrara, G.; Estenssoro, E.; Siles, D.S.P.; Cesio, C.E.; Dubin, A. Microcirculation Alterations in Severe COVID-19 Pneumonia. J. Crit. Care 2021, 61, 73–75. [Google Scholar] [CrossRef]
- Radic, M.; Thomas, J.; McMillan, S.; Frech, T. Does Sublingual Microscopy Correlate with Nailfold Videocapillaroscopy in Systemic Sclerosis? Clin. Rheumatol. 2021, 40, 2263–2266. [Google Scholar] [CrossRef]
- Zharkikh, E.V.; Loktionova, Y.I.; Fedorovich, A.A.; Gorshkov, A.Y.; Dunaev, A.V. Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics 2023, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, V.; Cutolo, M.; De Keyser, F.; Decuman, S.; Ruaro, B.; Sulli, A.; Deschepper, E.; Smith, V. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann. Rheum. Dis. 2016, 75, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Ruaro, B.; Cutolo, M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2059–2061. [Google Scholar] [CrossRef]
- Borrelli, E.; Parravano, M.; Sacconi, R.; Costanzo, E.; Queques, L.; Vella, G.; Bandello, F.; Querques, G. Guidelines on Optical Coherence Tomography Angiography Imaging: 2020 Focused Update. Ophthalmol. Ther. 2020, 9, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Esen, E.; Tas, D.A.; Sizmaz, S.; Turk, I.; Unal, I.; Demircan, N. Evaluating Choroidal Characteristics in Systemic Sclerosis Using Enhanced Depth Imaging Optical Coherence Tomography. Ocul. Immunol. Inflamm. 2016, 25, 356–362. [Google Scholar] [CrossRef]
- Fang, X.; Yu, S.; Peng, Y.; Huang, B.; Kang, M.; Xiong, J.; Luo, T.; Wu, R.; Shao, Y. The Function of Retinal Thickness and Microvascular Alterations in the Diagnosis of Systemic Sclerosis. BioMed Res. Int. 2023, 2023, 1805938. [Google Scholar] [CrossRef]
- Soysal, G.G.; Kimyon, S.; Mete, A.; Güngör, K. Evaluation of the retina with optical coherence tomography angiography (OCTA) in patients with coronavirus (COVID-19) infection. J. Fr. Ophtalmol. 2023, in press. [Google Scholar] [CrossRef]
- Sodhi, P.K.; Arora, R.; Kumar, S.; Jaisingh, K.R.A.T.; Rao, K.C.; Chhabra, K.; Saxena, S.; Manchanda, V.; Sharma, S. Optical Coherence Tomography Angiography Parameters of the Retina in SARS-CoV-2 Recovered Subjects. Cureus 2023, 15, e33548. [Google Scholar] [CrossRef]
- Özbaş, M.; Demirayak, B.; Vural, A.; Karabela, Y.; Yigit, F.U. Investigation of Retinal Alterations in Patients Recovered from COVID-19: A Comparative Study. Ocul. Immunol. Inflamm. 2023, 31, 252–256. [Google Scholar] [CrossRef]
- Karkhur, S.; Chauhan, K.; Soni, D.; Sharma, B.; Yadav, N.; Banerjee, L.; Nyodu, R.; Verma, S. Optical coherence tomography-based assessment of macular vessel density, retinal layer metrics and sub-foveal choroidal thickness in COVID-19 recovered patients. Indian J. Ophthalmol. 2023, 71, 385–395. [Google Scholar] [CrossRef]
- Beyoğlu, A.; Küçüködük, A.; Meşen, A.; Aksoy, M.; Kaya, E.; Dağhan, B. Investigation of changes in retinal vascular parameters and choroidal vascular index values during the early recovery period of COVID-19: The COVID-OCTA study. Photodiagnosis Photodyn Ther. 2023, 42, 103338. [Google Scholar] [CrossRef]
- Rizzoni, D.; Mengozzi, A.; Masi, S.; Agabiti Rosei, C.; De Ciuceis, C.; Virdis, A. New Noninvasive Methods to Evaluate Microvascular Structure and Function. Hypertension 2022, 5, 874–886. [Google Scholar] [CrossRef]
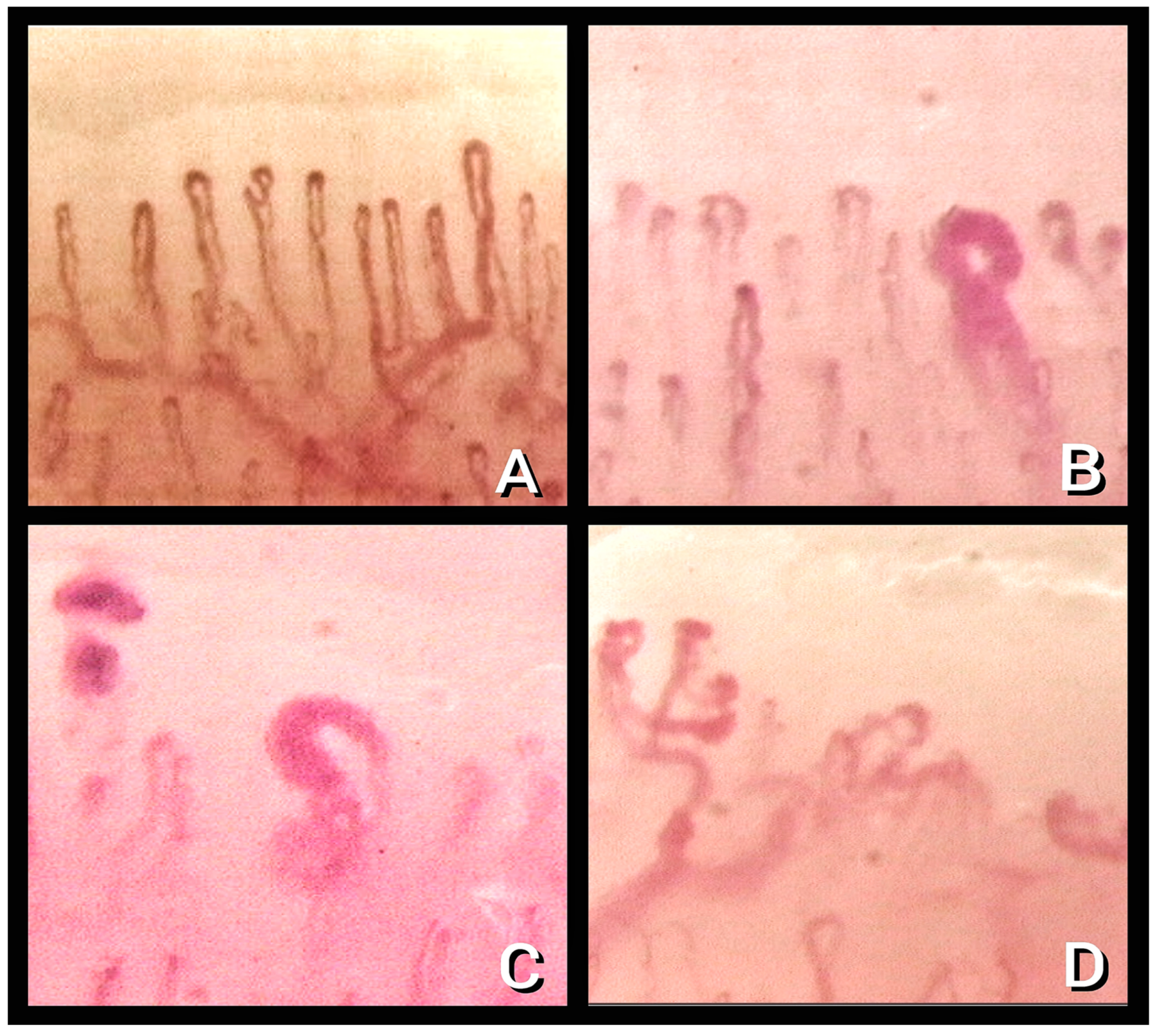
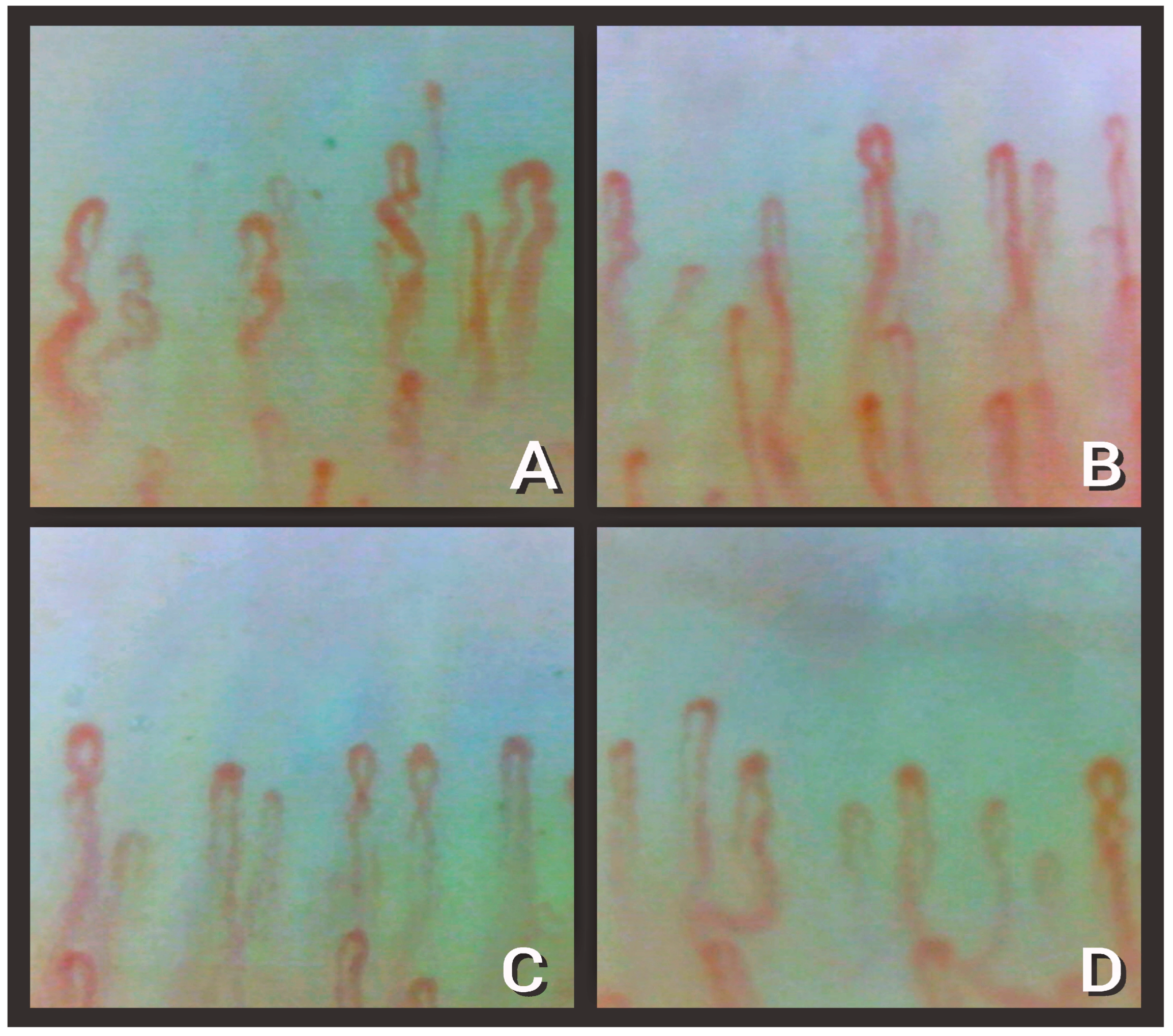
| Alteration | Definition | Recovered Subgroups (n = 28) | Discharged Subgroup (n = 54) |
|---|---|---|---|
| 1.Enlarged capillary [22] | Increased capillary diameter (homogeneous or irregular) > 20 μm and < 50 μm | 14.3% | 85.2% |
| 2. Giant capillary [22] | Homogeneously enlarged loops (diameter ≥ 50 μm) | 0% | 0% |
| 3. Hemosiderin deposit [23] | Dark mass due to micro-hemorrhage or microthrombosis | 46.4% | 11.1% |
| 4. Lower capillary density [15] | Fewer than 9 capillaries per millimeter | 25% | 34% |
| 5. Microvascular derangement [22] | Irregular capillary distribution and orientation and heterogeneity of the loops of the same finger | 50% | 46.3% |
| 6. Capillary ramifications and bizarre morphology [22] | Branching, bushy, or coiled capillaries, often originated from a single normal-sized capillary | 28.6% | 24.1% |
| 7. Meandering capillary [24] | Limbs crossing upon themselves or with each other more than twice | 0% | 81.4% |
| 8. Sludge flow [24] | Markedly slowed or discontinuous flow inside the capillary at the dynamic evaluation at the time of examination | 78.6% | 40.7% |
| 9. Pericapillary edema [25] | Foggy appearance around capillaries due to fluid buildup | 100% | 40.7% |
| 10. Subpapillary plexus visibility [26] | Large and linked arrangement of vessels under the distal row due to enlargement and congestion of venules and capillaries related to persistent opening of arteriovenous anastomoses | 3.6% | 11.1% |
| 11. Avascular area [27] | Distance > than 500 μm between two adjacent capillary loops from the distal rows | 0% | 5.6% |
| 12. Empty dermal papilla [21] | One or more missing capillaries at the expected place inside dermal papilla, which does not reach the extent to define an avascular area | 0% | 22.2% |
| Alteration | Definition |
|---|---|
| 1. Capillary morphology [28] | Normal, serpentine, or branched |
| 2. Capillary loop diameter [27] | μm diameter at the apical margin of a capillary loop |
| 3. Low capillary density [27] | Number of capillaries in a 1 mm lengthof the distal row of each finger |
| 4. Enlarged capillaries or capillary dilatation [22] | Capillary diameter 20–50 μm |
| 5. Giant capillaries [22] | Capillary diameter > 50 μm |
| 6. Avascular area [27] | Distance between two capillary loops > 500 μm |
| 7. Microaneurysms | Irregular enlargement and circumscribed increase of the capillary loop diameter |
| 8. Microhemorrhages [22] | Hemosiderin deposits with red and/or black images in the distal areas |
| Alteration | Natalello et al. [21] | Karahan et al. [29] | Rosei et al. [30] | Sulli et al. [36] |
|---|---|---|---|---|
| 54 hospitalized vs. 28 discharged | 38 patients in ICU 29 patients healthy | 22 patients during and after infection | 61 post-COVID (34 mild and 27 severe) vs. 30 healthy patients and 31 patients with RP | |
| Capillary morphology [22] | No statistically significative findings | Serpentine (56% in COVID-19, 20.7% in healthy, p < 0.001) Normal (37.5% in COVID-19, 79.5% in healthy, p < 0.001) | Not considered | Not considered |
| Dilatated capillaries [22] | 61% in all patients, with 14.3% in acute phase group and 28% in discharged group (p < 0.001) | 43.8% in COVID-19 vs. 6.9% in healthy control (p = 0.001) | Not considered | Present, but not statistically significative |
| Giant capillaries [22] | Not found (0% in all groups) | Found, but not statistically significative | Not considered | Not found (0% in all groups) |
| Avascular areas [27] | Not found | Not found | Not considered | Not found |
| Microaneurysm [29] | Not considered | 62.5% in COVID-19 vs. 10.3% in healthy control (p < 0.001) | Not considered | Not considered |
| Hemosiderin deposit [22,23] | 19% in all patients, with 46.4% in acute phase group and 11.1% in discharged group (p < 0.001) | Considered only as sign of microhemorrhages (listed next) | Separately considered microthrombosis and microhemorrhages | Considered only as sign of microhemorrhages (listed next) |
| Microthrombosis [23] | 7.3% in all patients, with 17.9% in acute phase group and 1.9% in discharged group (p = 0.016) | Not considered | Found in 32% patients, not detected after 3 months | Not considered |
| Microhemorrhages [23] | 13% in all patients, with 28.6% in acute phase group and 9.3% in discharged group (p = 0.027) | 21.9% in COVID-19 vs. 0% in healthy control (p < 0.001) | Found in 36% of patients, not detected after 3 months | 32.4% in patients with moderate COVID-19; 22.2% in patients with severe COVID-19 64.5% in PRP group 46.7% in control group (p univariate 0.005) |
| Capillary density | Lower than 9/mm in 50% in all patients, with 25% in acute phase group and 63% in discharged group (p = 0.002) | 6.41 ± 1.21/mm in COVID-19 vs. 8.55 ± 1.12/mm in healthy group | Statistically significant reduction in capillary density after 3 months from the acute infection | 8.44 ± 0.75/mm in moderate COVID 8.22 ± 1.15/mm in severe COVID 8.74 ± 0.68/mm in PRP group 9.30 ± 0.53/mm in control group (p univariate < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondini, L.; Confalonieri, P.; Pozzan, R.; Ruggero, L.; Trotta, L.; Lerda, S.; Hughes, M.; Bellan, M.; Confalonieri, M.; Ruaro, B.; et al. Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy. Diagnostics 2023, 13, 1905. https://doi.org/10.3390/diagnostics13111905
Mondini L, Confalonieri P, Pozzan R, Ruggero L, Trotta L, Lerda S, Hughes M, Bellan M, Confalonieri M, Ruaro B, et al. Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy. Diagnostics. 2023; 13(11):1905. https://doi.org/10.3390/diagnostics13111905
Chicago/Turabian StyleMondini, Lucrezia, Paola Confalonieri, Riccardo Pozzan, Luca Ruggero, Liliana Trotta, Selene Lerda, Michael Hughes, Mattia Bellan, Marco Confalonieri, Barbara Ruaro, and et al. 2023. "Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy" Diagnostics 13, no. 11: 1905. https://doi.org/10.3390/diagnostics13111905
APA StyleMondini, L., Confalonieri, P., Pozzan, R., Ruggero, L., Trotta, L., Lerda, S., Hughes, M., Bellan, M., Confalonieri, M., Ruaro, B., Salton, F., & Tavano, S. (2023). Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy. Diagnostics, 13(11), 1905. https://doi.org/10.3390/diagnostics13111905










