Cerebral Outflow Discrepancies in Recurrent Benign Paroxysmal Positional Vertigo: Focus on Ultrasonographic Examination
Abstract
1. Introduction
2. Patients and Methods
2.1. Type of Study
2.2. Patients
2.3. Vestibular Assessment
2.4. Vascular Assessment
2.5. Statistical Analysis
2.6. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Von Breven, M.; Seelig, T.; Neuhauser, H.; Lempert, T. Benign paroxysmal positional vertigo predominantly affects the right labyrinth. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1487–1488. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escamez, J.A.; Gamiz, M.J.; Fernandez-Perez, A.; Gomez-Fiñana, M. Long-term outcome and health-related quality of life in benign paroxysmal positional vertigo. Eur. Arch. Otorhinolaryngol. 2005, 262, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Baugh, R.F.; Orvidas, L.; Barrs, D.; Bronston, L.J.; Cass, S.; Chalian, A.A.; Desmond, A.L.; Earll, J.M.; Fife, T.D.; et al. Clinical practice guideline: Benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 2008, 139, S47–S81. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Cogliandolo, C.; Bianchini, C.; Aimoni, C.; Pelucchi, S.; Skarżyński, P.H.; Hatzopoulos, S. Clinical features of benign paroxysmal positional vertigo of the posterior semicircular canal. SAGE Open Med. 2019, 7, 2050312118822922. [Google Scholar] [CrossRef]
- Mandalà, M.; Santoro, G.P.; Awrey, J.; Nuti, D. Vestibular neuritis: Recurrence and incidence of secondary benign paroxysmal positional vertigo. Acta Otolaryngol. 2010, 130, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Yeo, S.G. Clinical analysis of patients with idiopathic sudden sensorineural hearing loss and benign paroxysmal positional vertigo. Acta Otolaryngol. 2013, 133, 439–442. [Google Scholar] [CrossRef]
- Gross, E.M.; Ress, B.D.; Viirre, E.S.; Nelson, J.R.; Harris, J.P. Intractable benign paroxysmal positional vertigo in patients with Meniere’s disease. Laryngoscope 2000, 110, 655–659. [Google Scholar] [CrossRef]
- Zamboni, P.; Sisini, F.; Menegatti, E.; Taibi, A.; Malagoni, A.M.; Morovic, S.; Gambaccini, M. An ultrasound model to calculate the brain blood outflow through collateral vessels: A pilot study. BMC Neurol. 2013, 13, 81. [Google Scholar] [CrossRef]
- Zamboni, P.; Morovic, S.; Menegatti, E.; Viselner, G.; Nicolaides, A.N. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound—Recommendations for a protocol. Int. Angiol. 2011, 30, 571–597. [Google Scholar]
- Zamboni, P.; Menegatti, E.; Pomidori, L.; Morovic, S.; Taibi, A.; Malagoni, A.M.; Cogo, A.L.; Gambaccini, M. Does thoracic pump influence the cerebral venous return? J. Appl. Physiol. 2012, 112, 904–910. [Google Scholar] [CrossRef]
- Zamboni, P. The big idea: Iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J. R. Soc. Med. 2006, 99, 589–593. [Google Scholar] [CrossRef]
- Lee, B.B.; Bergan, J.; Gloviczki, P.; Laredo, J.; Loose, D.A.; Mattassi, R.; Parsi, K.; Villavicencio, J.L.; Zamboni, P.; International Union of Phlebology (IUP). Diagnosis and treatment of venous malformations Consensus Document of the Interntional Union of Phlebology (IUP)—2009. Int. Angiol. 2009, 28, 434–451. [Google Scholar] [PubMed]
- Lee, B.B.; Baumgartner, I.; Berlien, P.; Bianchini, G.; Burrows, P.; Gloviczki, P.; Huang, Y.; Laredo, J.; Loose, D.A.; Markovic, J.; et al. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phle- bology (IUP): Updated 2013. Int. Angiol. 2015, 34, 97–149. [Google Scholar] [PubMed]
- Zamboni, P.; Galeotti, R.; Menegatti, E.; Malagoni, A.M.; Gianesini, S.; Bartolomei, I.; Mascoli, F.; Salvi, F. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J. Vasc. Surg. 2009, 50, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Tessari, M.; Mazzoli, M.; Tavoni, V.; Sisini, F.; Aimoni, C.; Stomeo, F.; Menegatti, E.; Pelucchi, S.; Zamboni, P. Cerebral Inflow and Outflow Discrepancies in Severe Sudden Sensorineural Hearing Loss. Curr. Neurovasc. Res. 2018, 15, 220–225. [Google Scholar] [CrossRef]
- Tessari, M.; Ciorba, A.; Mueller, L.O.; Zhang, Q.; Cristini, M.; Menegatti, E.; Mazzoli, M.; Pelucchi, S.; Toro, E.F. Jugular valve function and petrosal sinuses pressure: A computational model applied to sudden sensorineural hearing loss. Veins Lymphat. 2017, 6. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Scicchitano, P.; Gesualdo, M.; Cortese, F.; Zito, A.; Manca, F.; Boninfante, B.; Recchia, P.; Leogrande, D.; Viola, D.; et al. Idiopathic sudden sensorineural hearing loss and Ménière syndrome: The role of cerebral venous drainage. Clin. Otolaryngol. 2018, 43, 230–239. [Google Scholar] [CrossRef]
- Alpini, D.; Bavera, P.M.; Di Berardino, F.; Barozzi, S.; Cesarani, A. Bilateral sudden sensorineural hearing loss and chronic venous cerebrospinal insufficiency: A case report. Phlebology 2013, 28, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Alpini, D.C.; Bavera, P.M.; Hahn, A.; Mattei, V. Chronic cerebrospinal venous insufficiency (CCSVI) in Meniere disease. Case or Cause. Sci. Med. 2013, 4, 9–15. [Google Scholar]
- Filipo, R.; Ciciarello, F.; Attanasio, G.; Mancini, P.; Covelli, E.; Agati, L.; Fedele, F.; Viccaro, M. Chronic cerebrospinal venous insufficiency in patients with Ménière’s disease. Aur. Arch. Otorhinolaryngol. 2015, 272, 77–82. [Google Scholar] [CrossRef]
- Di Berardino, F.; Alpini, D.C.; Bavera, P.M.; Cecconi, P.; Farabola, M.; Mattei, V.; Ambrosetti, U.; Cesarani, A. Chronic cerebrospinal venous insufficiency in Ménière disease. Phlebology 2015, 30, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, G.; Cagnoni, L.; Masci, E.; Ciciarello, F.; Diaferia, F.; Bruno, A.; Greco, A.; De Vincentiis, M. Chronic cerebrospinal venous insufficiency as a cause of inner ear diseases. Acta Otolaryngol. 2017, 37, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, G.; Califano, L.; Bruno, A.; Giugliano, V.; Ralli, M.; Martellucci, S.; Milella, C.; de Vincentiis, M.; Russo, F.Y.; Greco, A. Chronic cerebrospinal venous insufficiency and menière’s disease: Interventional versus medical therapy. Laryngoscope 2020, 130, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Head Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef]
- Sfakianaki, I.; Binos, P.; Karkos, P.; Dimas, G.G.; Psillas, G. Risk Factors for Recurrence of Benign Paroxysmal Positional Vertigo. A Clinical Review. J. Clin. Med. 2021, 10, 4372. [Google Scholar] [CrossRef]
- Lugli, M.; Morelli, M.; Guerzoni, S.; Maleti, O. The hypothesis of patho- physiological correlation between chronic cerebrospinal venous insufficiency and multiple sclerosis: Rationale of treatment. Phlebology 2012, 27, 178–186. [Google Scholar] [CrossRef]
- Brott, T.G.; Halperin, J.L.; Abbara, S.; Bacharach, J.M.; Barr, J.D.; Bush, R.L.; Cates, C.U.; Creager, M.A.; Fowler, S.B.; Friday, G.; et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc. Med. 2011, 16, 35–77. [Google Scholar]
- Simka, M.; Hubbard, D.; Siddiqui, A.H.; Dake, M.D.; Sclafani, S.J.; Al-Omari, M.; Eisele, C.G.; Haskal, Z.J.; Ludyga, T.; Miloševič, Z.V.; et al. International Society for Neurovascular Disease. Catheter venography for the assessment of internal jugular veins and azygous vein: Position statement by expert panel of the International Society for Neurovascular Disease. Vasa 2013, 42, 168–176. [Google Scholar] [CrossRef]
- Neuhauser, H.K. Epidemiology of vertigo. Curr. Opin. Neurol. 2007, 20, 40–46. [Google Scholar] [CrossRef]
- Alawneh, K.Z.; Raffee, L.A.; Oqlat, A.A.; Oglat, A.A.; Al Qawasmeh, M.; Ali, M.K.; Okour, A.M.; Al-Mistarehi, A.H. The utility of brain CT scan modality in the management of dizziness at the emergency department: A retrospective single-center study. Ann. Med. Surg. 2021, 64, 102220. [Google Scholar] [CrossRef]
- Narozny, W.S.H. Zarys Otoneurologii; Medical Education: Warsaw, Poland, 2018; Volume I. [Google Scholar]
- Li, S.; Wang, Z.; Liu, Y.; Cao, J.; Zheng, H.; Jing, Y.; Han, L.; Ma, X.; Xia, R.; Yu, L. Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2022, 101, NP112–NP134. [Google Scholar] [CrossRef]
- Picciotti, P.M.; Lucidi, D.; De Corso, E.; Meucci, D.; Sergi, B.; Paludetti, G. Comorbidities and Recurrence of Benign Paroxysmal Positional Vertigo: Personal Experience. Int. J. Audiol. 2016, 55, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Franco, V.; Cuesta, P.; Aldama, P.; Alvarez, M.J.; Méndez, J.C. Recurrence of benign paroxysmal positional vertigo. Otol. Neurotol. 2012, 33, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Von Brevern, M.; Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-Toker, D. Benign paroxysmal positional vertigo: Diagnostic criteria. J. Vestib. Res. 2015, 25, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Power, L.; Murray, K.; Szmulewicz, D.J. Characteristics of assessment and treatment in Benign Paroxysmal Positional Vertigo (BPPV). J. Vestib. Res. 2020, 30, 55–62. [Google Scholar] [CrossRef]
- Ismail, E.I.; Morgan, A.E.; Abdeltawwab, M.M. Home particle repositioning maneuver to prevent the recurrence of posterior canal BPPV. Auris Nasus Larynx 2018, 45, 980–984. [Google Scholar] [CrossRef]
- Li, J.C.; Li, C.J.; Epley, J.; Weinberg, L. Cost-effective management of benign positional vertigo using canalith repositioning. Otolaryngol. Head Neck Surg. 2000, 122, 334–339. [Google Scholar] [CrossRef]
- Whang, H.; Yu, D.; Song, N.; Yin, S. Delayed diagnosis and treatment of benign paroxysmal positional vertigo associated with current practice. Eur. Arch. Otorhinolaryngol. 2014, 271, 261–264. [Google Scholar] [CrossRef]
- Oghalai, J.S.; Manolidis, S.; Barth, J.L.; Stewart, M.G.; Jenkins, H.A. Unrecognized benogn paroxysmal positional vertigo in elderly patients. Otolaryngol. Head Neck Surg. 2000, 122, 630–634. [Google Scholar] [CrossRef]
- Akula, S.; Reddy, L.S.; Kiran, A.S.; Suresh, A.M. Clinical Study of BPPV and the Effectiveness of Canalolith Repositioning Manoeuvre in Subjects of BPPV. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 96–102. [Google Scholar] [CrossRef]
- Balatsouras, D.G.; Koukoutsis, G.; Aspris, A.; Fassolis, A.; Moukos, A.; Economou, N.C.; Katotomichelakis, M. Benign Paroxysmal Positional Vertigo Secondary to Mild Head Trauma. Ann. Otol. Rhinol. Laryngol. 2017, 126, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, S.U.; Kim, J.S. Prevention of recurrent benign paroxysmal positional vertigo with vitamin D supplementation: A meta-analysis. J. Neurol. 2022, 269, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, A.; İnanç, Y. Evaluation of BPPV with vertebral artery values. Neuropsychiatr. Dis. Treat. 2018, 14, 1975–1979. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Zhang, H.; Xu, Y.; Fu, S.; Yu, M.; Ji, P. Evaluation of vertebrobasilar artery changes in patients with benign paroxysmal positional vertigo. Neuroreport 2013, 24, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Filograna Pignatelli, G.R.; Pacella, A.; Ortore, R.; Khasawneh, L. Recurring paroxysmal positional vertigo: Evaluation of the vascular factor. Acta Otorhinolaryngol. Ital. 2021, 41, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A. Comparative anatomy of cochlear blood vessels. Am. J. Otolaryngol. 1988, 9, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Adams, J.C.; Nadol, J.B., Jr. Pathophysiology of Meniere’s syndrome: Are symptoms caused by endolymphatic hydrops? Otol. Neurotol. 2005, 26, 74–81. [Google Scholar] [CrossRef]
- Friis, M.; Klauss, O. A potential portal flow in the inner ear. Laryngoscope 2007, 117, 194–198. [Google Scholar] [CrossRef]
- Friberg, U.; Rask-Andersen, H. Vascular occlusion in the endolymphatic sac in Meniere’s disease. Ann. Otol. Rhinol. Laryngol. 2002, 11, 237–245. [Google Scholar] [CrossRef]
- Del Rio, M.; Arriaga, M.A. Benign Positional Vertigo: Prognostic Factors. Otolaryngol. Head Neck Surg. 2004, 130, 426–429. [Google Scholar] [CrossRef]
- Luryi, A.L.; Lawrence, J.; Bojrab, D.I.; LaRouere, M.; Babu, S.; Zappia, J.; Sargent, E.W.; Chan, E.; Naumann, I.; Hong, R.S.; et al. Recurrence in Benign Paroxysmal Positional Vertigo: A Large, Single-Institution Study. Otol. Neurotol. 2018, 39, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.T.; Zhao, X.Q.; Ju, Y.; Wang, Y.; Chen, M.M.; Cui, Y. Clinical Characteristics and Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo. Front. Neurol. 2019, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Kutlubaev, M.A.; Xu, Y.; Hornibrook, J. Benign Paroxysmal Positional Vertigo in Meniere’s Disease: Systematic Review and Meta-Analysis of Frequency and Clinical Characteristics. J. Neurol. 2021, 268, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M.M. Endaural labyrinthectomy. Ear Nose Throat J. 2008, 8, 204–205. [Google Scholar] [CrossRef]
- Casani, A.P.; Dallan, I.; Marchetti, M. Ruolo dei potenziali evocati vestibolari miogenici nella diagnostica vestibolare. Acta Otorhinolaryngol. Ital. 2003, 23, 113–119. [Google Scholar]
- Nadol, J.B.; Adam, J.C.; Kim, J.R. Degenerative changes in the organ of Corti and lateral cochlear wall in experimental endolymphatic hydrops and human Meniere’s disease. Acta Otolaryngol. Suppl. 1995, 519, 47–59. [Google Scholar] [CrossRef]
- Sisini, F.; Toro, E.; Gambaccini, M.; Zamboni, P. The oscillating component of the internal jugular vein flow: The overlooked element of cerebral circulation. Behav. Neurol. 2015, 2015, 170756. [Google Scholar] [CrossRef]
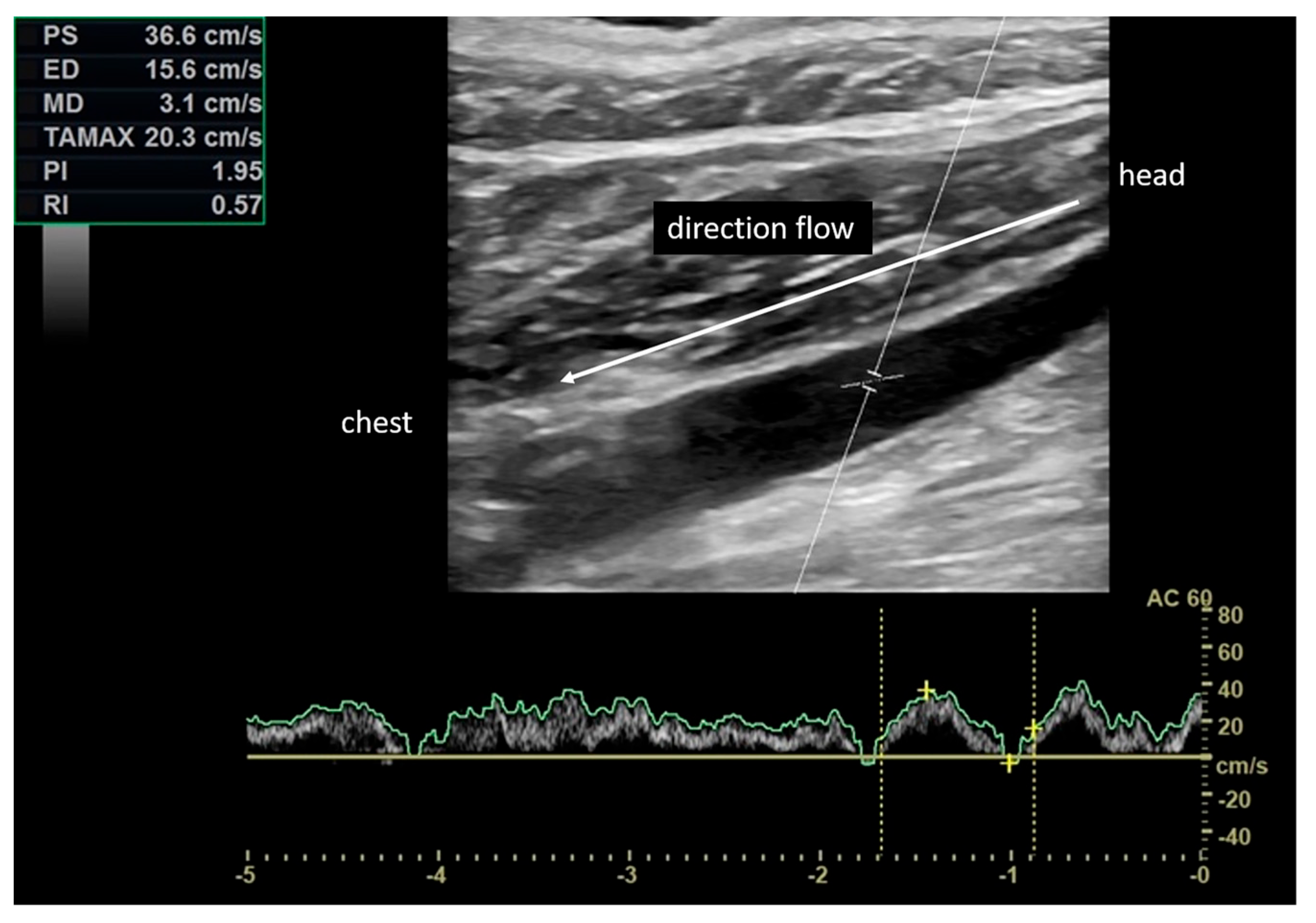
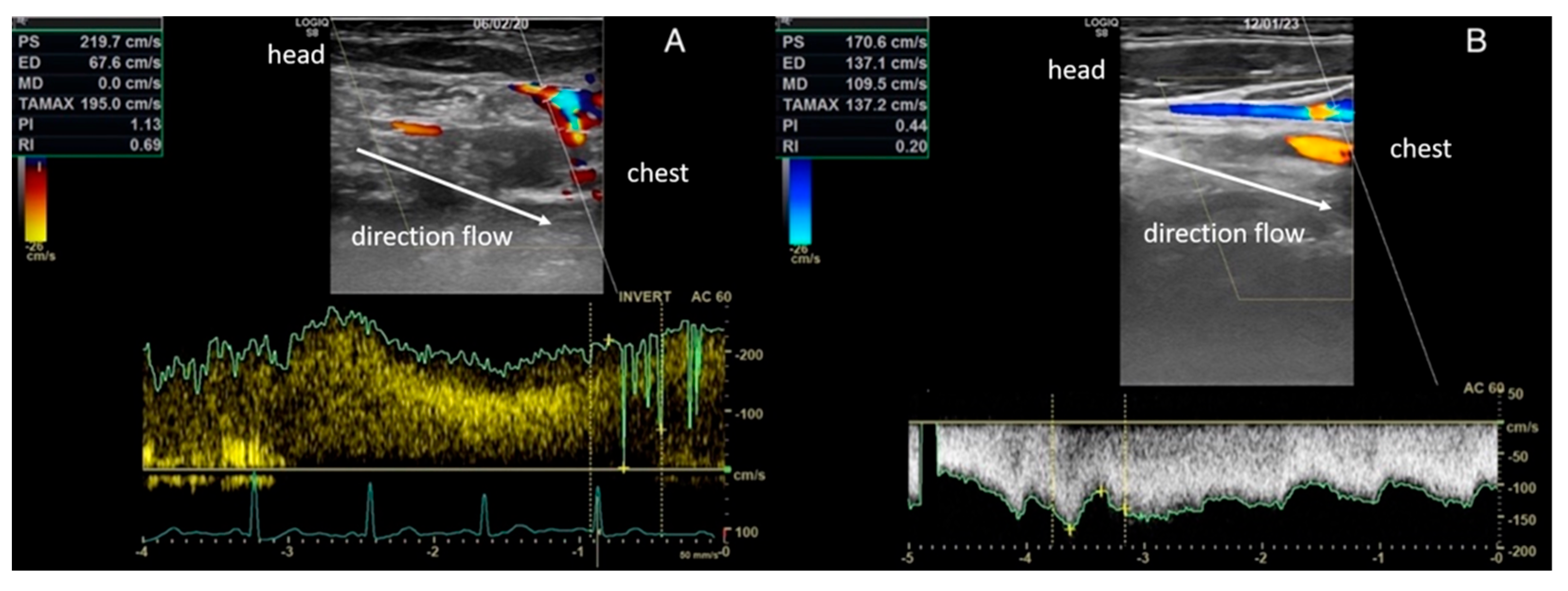
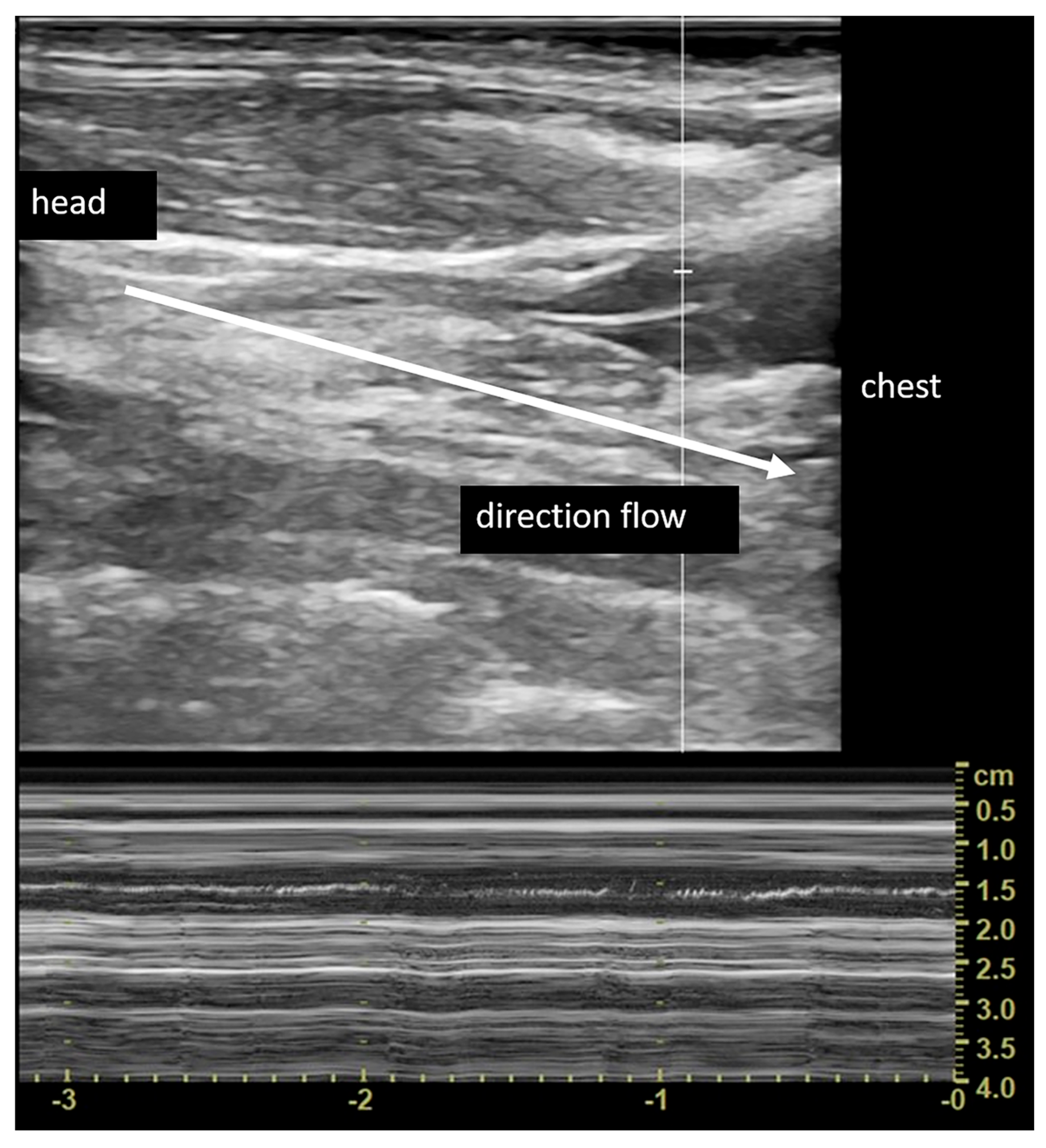
| Sex | Age (Years) | Δt | Side | SC Involved |
|---|---|---|---|---|
| F | 67 | 3 | Right | PSC |
| F | 51 | 48 | Left | PSC |
| F | 61 | 22 | Left | PSC |
| F | 55 | 16 | Right | PSC |
| M | 74 | 1 | Right | PSC |
| F | 53 | 3 | Right | PSC |
| F | 45 | 6 | Left | PSC |
| F | 63 | 12 | Right | PSC |
| F | 51 | 24 | Right | PSC |
| M | 60 | 31 | Right | PSC |
| F | 48 | 60 | Right | PSC |
| F | 59 | 60 | Left | PSC |
| F | 67 | 1 | Right | LSC |
| M | 68 | 60 | Right | PSC |
| F | 57 | 27 | Right | PSC |
| F | 59 | 4 | Left | PSC |
| F | 71 | 7 | Right | LSC |
| F | 66 | 24 | Right | PSC |
| M | 52 | 4 | Right | LSC |
| F | 42 | 2 | Left | PSC |
| F | 61 | 6 | Left | PSC |
| F | 62 | 3 | Left | PSC |
| M | 51 | 5 | Right | LSC |
| F | 62 | 5 | Right | PSC |
| Vascular Segment | TAVm (cm/s) | Peak Velocity (cm/s) | ||||
|---|---|---|---|---|---|---|
| Ortho | Clino | Ortho | Clino | |||
| Mean ± sd | Mean ± sd | p Value | Mean ± sd | Mean ± sd | p Value | |
| CCA | - | 40.77 ± 8.97 | - | - | - | - |
| ICA | - | 32.86 ± 11.30 | - | - | - | - |
| ECA | - | 36.55 ± 7.82 | - | - | - | - |
| VA | - | 23.69 ± 10.35 | - | - | - | - |
| J3 | - | 22.12 ± 10.81 | - | - | - | - |
| J2 | - | 30.56 ± 15.97 | - | - | - | - |
| J1 | 59.70 ± 34.30 | 17.49 ± 10.90 | <0.0001 | 81.38 ± 44.37 | 46.27 ± 18.90 | <0.0001 |
| VV | 19.77 ± 25.23 | 12.82 ± 19.24 | 0.0193 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorba, A.; Tessari, M.; Natale, E.; Buzzi, F.; Baldazzi, G.; Cosacco, A.; Migliorelli, A.; Corazzi, V.; Bianchini, C.; Stomeo, F.; et al. Cerebral Outflow Discrepancies in Recurrent Benign Paroxysmal Positional Vertigo: Focus on Ultrasonographic Examination. Diagnostics 2023, 13, 1902. https://doi.org/10.3390/diagnostics13111902
Ciorba A, Tessari M, Natale E, Buzzi F, Baldazzi G, Cosacco A, Migliorelli A, Corazzi V, Bianchini C, Stomeo F, et al. Cerebral Outflow Discrepancies in Recurrent Benign Paroxysmal Positional Vertigo: Focus on Ultrasonographic Examination. Diagnostics. 2023; 13(11):1902. https://doi.org/10.3390/diagnostics13111902
Chicago/Turabian StyleCiorba, Andrea, Mirko Tessari, Erennio Natale, Fabio Buzzi, Giulia Baldazzi, Alessio Cosacco, Andrea Migliorelli, Virginia Corazzi, Chiara Bianchini, Francesco Stomeo, and et al. 2023. "Cerebral Outflow Discrepancies in Recurrent Benign Paroxysmal Positional Vertigo: Focus on Ultrasonographic Examination" Diagnostics 13, no. 11: 1902. https://doi.org/10.3390/diagnostics13111902
APA StyleCiorba, A., Tessari, M., Natale, E., Buzzi, F., Baldazzi, G., Cosacco, A., Migliorelli, A., Corazzi, V., Bianchini, C., Stomeo, F., Pelucchi, S., & Zamboni, P. (2023). Cerebral Outflow Discrepancies in Recurrent Benign Paroxysmal Positional Vertigo: Focus on Ultrasonographic Examination. Diagnostics, 13(11), 1902. https://doi.org/10.3390/diagnostics13111902










