[18F]FES PET Resolves the Diagnostic Dilemma of COVID-19-Vaccine-Associated Hypermetabolic Lymphadenopathy in ER-Positive Breast Cancer
Abstract
1. Introduction
2. Case Presentation
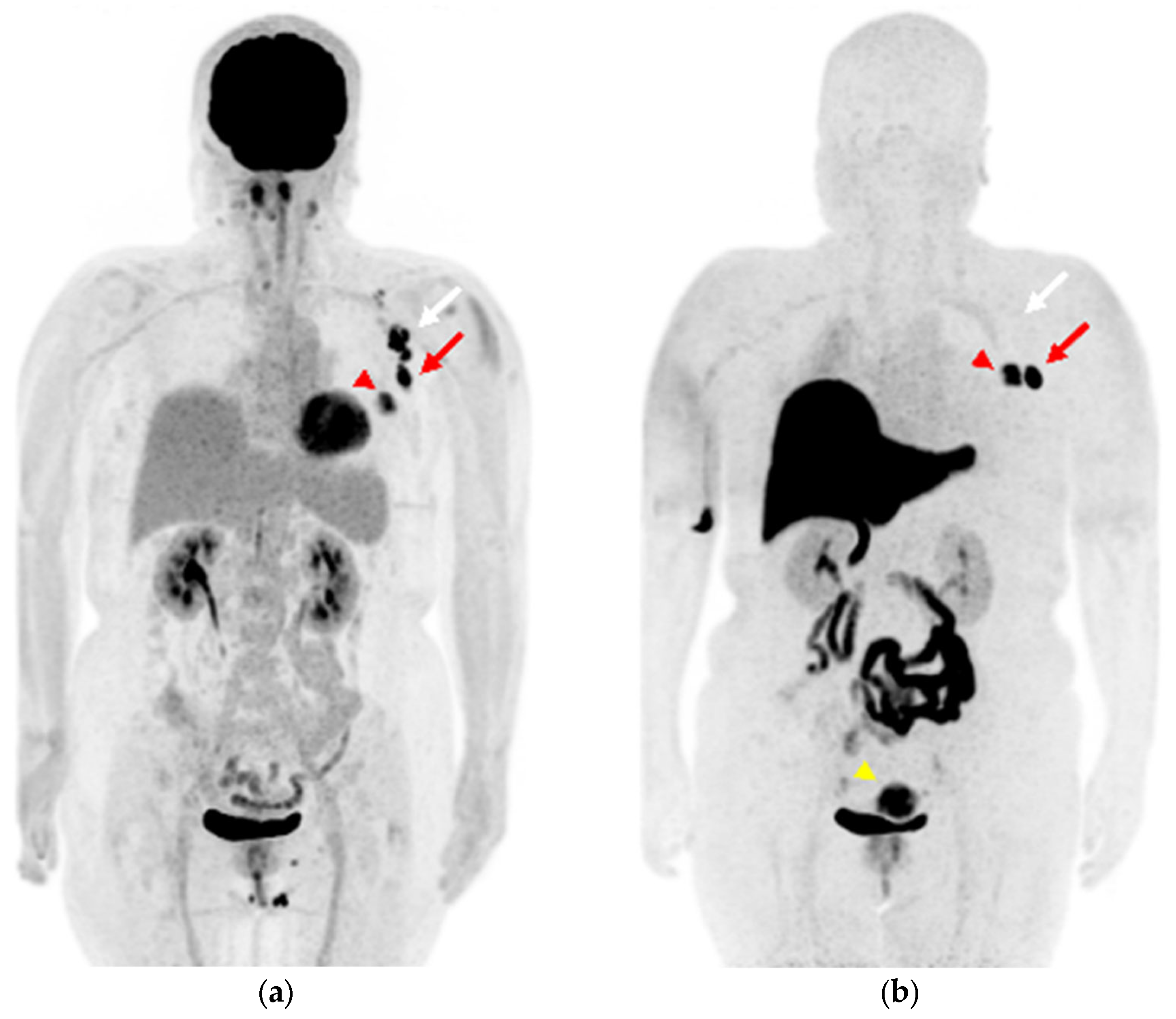
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA COVID-19 vaccine: Incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Eifer, M.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Shams, J.; Keret, N.; Gorfine, M.; Eshet, Y. COVID-19 mRNA Vaccination: Age and Immune Status and Its Association with Axillary Lymph Node PET/CT Uptake. J. Nucl. Med. 2022, 63, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Van Nijnatten, T.J.A.; Jochelson, M.S.; Lobbes, M.B.I. Axillary lymph node characteristics in breast cancer patients versus post-COVID-19 vaccination: Overview of current evidence per imaging modality. Eur. J. Radiol. 2022, 152, 110334. [Google Scholar] [CrossRef] [PubMed]
- Skawran, S.; Gennari, A.G.; Dittli, M.; Treyer, V.; Muehlematter, U.J.; Maurer, A.; Burger, I.A.; Mader, C.; Messerli, O.; Grunig, H.; et al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: Frequency, intensity, and potential clinical impact. Eur. Radiol. 2022, 32, 508–516. [Google Scholar] [CrossRef]
- Seban, R.D.; Richard, C.; Nascimento-Leite, C.; Ghidaglia, J.; Provost, C.; Gonin, J.; Tourneau, C.L.; Romano, E.; Deleval, N.; Champion, L. Absolute Lymphocyte Count After COVID-19 Vaccination Is Associated with Vaccine-Induced Hypermetabolic Lymph Nodes on 18F-FDG PET/CT: A Focus in Breast Cancer Care. J. Nucl. Med. 2022, 63, 1231–1238. [Google Scholar] [CrossRef]
- Linden, H.M.; Kurland, B.F.; Peterson, L.M.; Schubert, E.K.; Gralow, J.R.; Specht, J.M.; Ellis, G.K.; Lawton, T.J.; Livingston, R.B.; Petra, P.H.; et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin. Cancer Res. 2011, 17, 4799–4805. [Google Scholar] [CrossRef]
- Linden, H.M.; Stekhova, S.A.; Link, J.M.; Gralow, J.R.; Livingston, R.B.; Ellis, G.K.; Petra, P.H.; Peterson, L.M.; Schubert, E.K.; Dunnwald, L.K.; et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J. Clin. Oncol. 2006, 24, 2793–2799. [Google Scholar] [CrossRef]
- McGuire, A.H.; Dehdashti, F.; Siegel, B.A.; Lyss, A.P.; Brodack, J.W.; Mathias, C.J.; Mintun, M.A.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J. Nucl. Med. 1991, 32, 1526–1531. [Google Scholar]
- Nakamoto, Y.; Kitajima, K.; Toriihara, A.; Nakajo, M.; Hirata, K. Recent topics of the clinical utility of PET/MRI in oncology and neuroscience. Ann. Nucl. Med. 2022, 36, 798–803. [Google Scholar] [CrossRef]
- Antwi, K.; Caobelli, F.; Kudura, K.; Buchholz, H.G.; Hoffmann, M.; Schreckenberger, M. Hypermetabolic Ipsilateral Supraclavicular and Axillary Lymphadenopathy: Optimal Time Point for Performing an 18F-FDG PET/CT after COVID-19 Vaccination. Diagnostics 2022, 12, 3073. [Google Scholar] [CrossRef]
- Eshet, Y.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Eifer, M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology 2021, 300, E345–E347. [Google Scholar] [CrossRef]
- Plaza, M.J.; Wright, J.; Fernandez, S. COVID-19 vaccine-related unilateral axillary lymphadenopathy: Pattern on screening breast MRI allowing for a benign assessment. Clin. Imaging 2021, 80, 139–141. [Google Scholar] [CrossRef]
- Eifer, M.; Pinian, H.; Klang, E.; Alhoubani, Y.; Kanana, N.; Tau, N.; Davidson, T.; Konen, E.; Catalano, O.A.; Eshet, Y.; et al. FDG PET/CT radiomics as a tool to differentiate between reactive axillary lymphadenopathy following COVID-19 vaccination and metastatic breast cancer axillary lymphadenopathy: A pilot study. Eur. Radiol. 2022, 32, 5921–5929. [Google Scholar] [CrossRef]
- Yamane, T.; Ueda, S.; Seto, A.; Kuji, I. 18F-Fluoroestradiol PET/CT Correctly Diagnosed 18F-FDG-Avid Inflammatory Lymph Nodes in a Patient with Estrogen Receptor-Positive Breast Cancer. Clin. Nucl. Med. 2018, 43, 379–380. [Google Scholar] [CrossRef]
- Chae, S.Y.; Son, H.J.; Lee, D.Y.; Shin, E.; Oh, J.S.; Seo, S.Y.; Baek, S.; Kim, J.Y.; Na, S.J.; Moon, D.H. Comparison of diagnostic sensitivity of [18F]fluoroestradiol and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for breast cancer recurrence in patients with a history of estrogen receptor-positive primary breast cancer. EJNMMI Res. 2020, 10, 54. [Google Scholar] [CrossRef]
- Eifer, M.; Eshet, Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology 2021, 299, E248. [Google Scholar] [CrossRef]
- Nawwar, A.A.; Searle, J.; Hagan, I.; Lyburn, I.D. COVID-19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2655–2656. [Google Scholar] [CrossRef]
- Seban, R.D.; Champion, L.; Deleval, N.; Richard, C.; Provost, C. Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics 2021, 11, 676. [Google Scholar] [CrossRef]
- Schroeder, D.G.; Jang, S.; Johnson, D.R.; Takahashi, H.; Navin, P.J.; Broski, S.M.; Thorpe, M.P.; Johnson, G.B.; Young, J.R. Frequency and Characteristics of Nodal and Deltoid FDG and (11)C-Choline Uptake on PET Performed After COVID-19 Vaccination. AJR Am. J. Roentgenol. 2021, 217, 1206–1216. [Google Scholar] [CrossRef]
- Ah-Thiane, L.; Ferrer, L.; Maucherat, B.; Fleury, V.; Le Thiec, M.; Rusu, D.; Rousseau, C. Vaccine-Related Lymph Nodes: The Emerging Pitfalls of 18F-Fluorocholine and 68Ga-PSMA-11 PET/CT in the Era of COVID-19 Vaccination. Clin. Nucl. Med. 2022, 47, 575–582. [Google Scholar] [CrossRef]
- Brophy, J.; Henkle, G.; Rohren, E.M. DOTATATE Uptake in an Axillary Lymph Node After COVID-19 Vaccination. Clin. Nucl. Med. 2022, 47, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Kolade, O.U.; Ayeni, A.O.; Brink, A.; Steyn, R.; More, S.; Prasad, V. SARS-CoV-2 vaccination site as possible pitfall on somatostatin receptor imaging. Clin. Transl. Imaging 2022, 10, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Demmert, T.T.; Maric, I.; Pomykala, K.L.; Lueckerath, K.; Siveke, J.; Schaarschmidt, B.M.; Hamacher, R.; Herrmann, K.; Fendler, W.P. Novel 68Ga-FAPI PET/CT Offers Oncologic Staging Without COVID-19 Vaccine-Related Pitfalls. J. Nucl. Med. 2023, 64, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, J.; Kapenhas, E.; Morgani, J.; Heimann, A. Contralateral axillary lymph node metastasis in second primary Breast cancer: Case report and review of the literature. Int. J. Surg. Case Rep. 2017, 40, 47–49. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Magnoni, F.; Colleoni, M.; Mattar, D.; Corso, G.; Bagnardi, V.; Frassoni, S.; Santomauro, G.; Jereczek-Fossa, B.A.; Veronesi, P.; Galimberti, V.; et al. Contralateral Axillary Lymph Node Metastases from Breast Carcinoma: Is it Time to Review TNM Cancer Staging? Ann. Surg. Oncol. 2020, 27, 4488–4499. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogami, M.; Tsujikawa, T.; Maeda, H.; Kosaka, N.; Takahashi, M.; Kinoshita, N.; Mori, T.; Makino, A.; Kiyono, Y.; Murakami, T.; et al. [18F]FES PET Resolves the Diagnostic Dilemma of COVID-19-Vaccine-Associated Hypermetabolic Lymphadenopathy in ER-Positive Breast Cancer. Diagnostics 2023, 13, 1851. https://doi.org/10.3390/diagnostics13111851
Nogami M, Tsujikawa T, Maeda H, Kosaka N, Takahashi M, Kinoshita N, Mori T, Makino A, Kiyono Y, Murakami T, et al. [18F]FES PET Resolves the Diagnostic Dilemma of COVID-19-Vaccine-Associated Hypermetabolic Lymphadenopathy in ER-Positive Breast Cancer. Diagnostics. 2023; 13(11):1851. https://doi.org/10.3390/diagnostics13111851
Chicago/Turabian StyleNogami, Munenobu, Tetsuya Tsujikawa, Hiroyuki Maeda, Nobuyuki Kosaka, Mizuho Takahashi, Naoki Kinoshita, Tetsuya Mori, Akira Makino, Yasushi Kiyono, Takamichi Murakami, and et al. 2023. "[18F]FES PET Resolves the Diagnostic Dilemma of COVID-19-Vaccine-Associated Hypermetabolic Lymphadenopathy in ER-Positive Breast Cancer" Diagnostics 13, no. 11: 1851. https://doi.org/10.3390/diagnostics13111851
APA StyleNogami, M., Tsujikawa, T., Maeda, H., Kosaka, N., Takahashi, M., Kinoshita, N., Mori, T., Makino, A., Kiyono, Y., Murakami, T., Goi, T., & Okazawa, H. (2023). [18F]FES PET Resolves the Diagnostic Dilemma of COVID-19-Vaccine-Associated Hypermetabolic Lymphadenopathy in ER-Positive Breast Cancer. Diagnostics, 13(11), 1851. https://doi.org/10.3390/diagnostics13111851






