Image-Guided Intraoperative Assessment of Surgical Margins in Oral Cavity Squamous Cell Cancer: A Diagnostic Test Accuracy Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Data Selection
- -
- Outcomes evaluated were not in line with the main question of the review;
- -
- The analysis involved anatomical regions other than the oral cavity (e.g., the oropharynx);
- -
- The cutoff set for close margins was not 5 mm;
- -
- Papers were short communications, oral presentations, letters to editor, posters, or analysis in the setting of a systematic review;
- -
- Studies were performed before 1 January 2016;
- -
- Frequency of intraoral ultrasound probes was below 15 MHz;
- -
- Studies were not performed on humans;
- -
- Articles were not in English language.
2.2. Quality Assessment and Data Extraction
3. Results
3.1. Study Characteristics
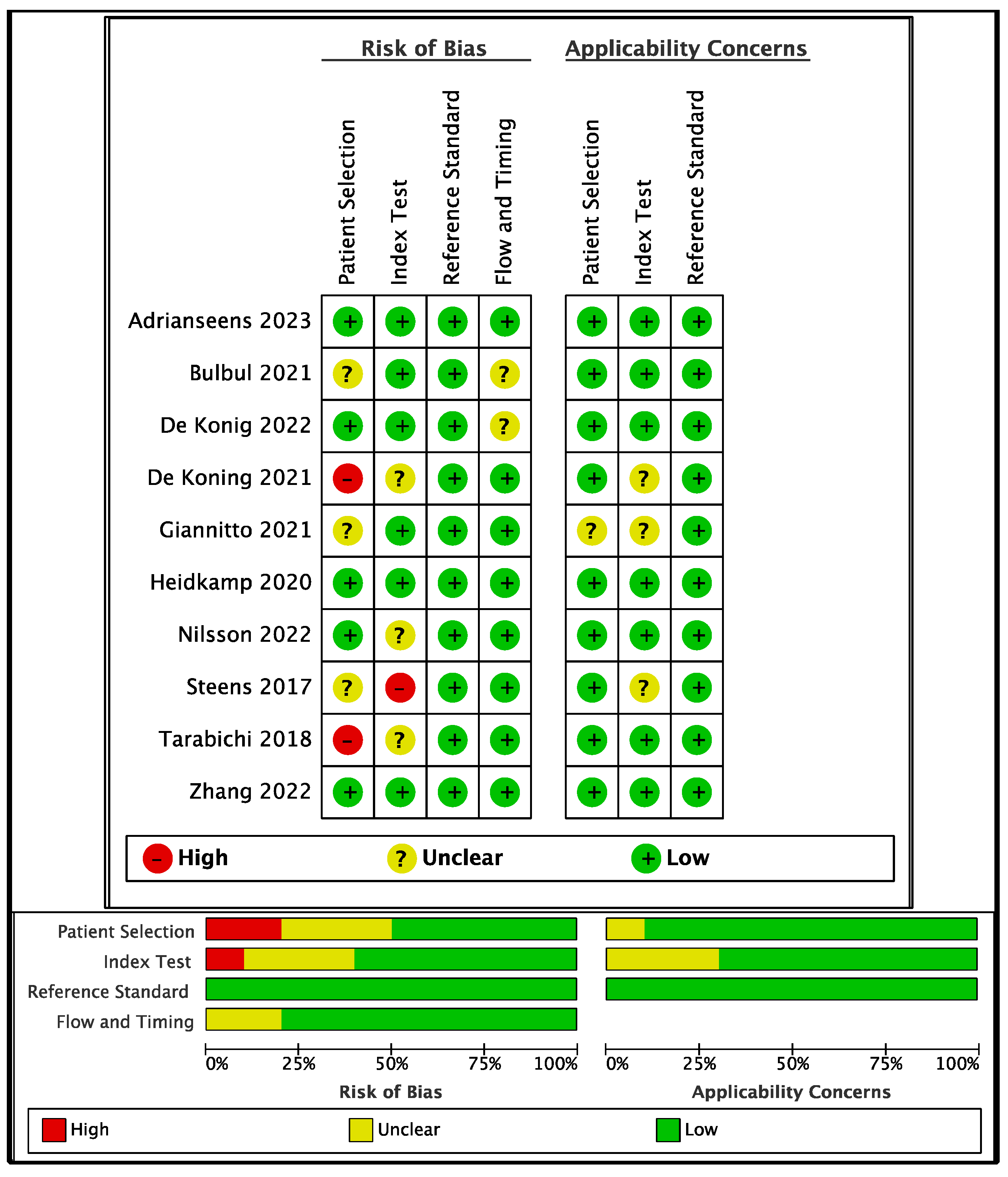
3.2. Bias and Applicability
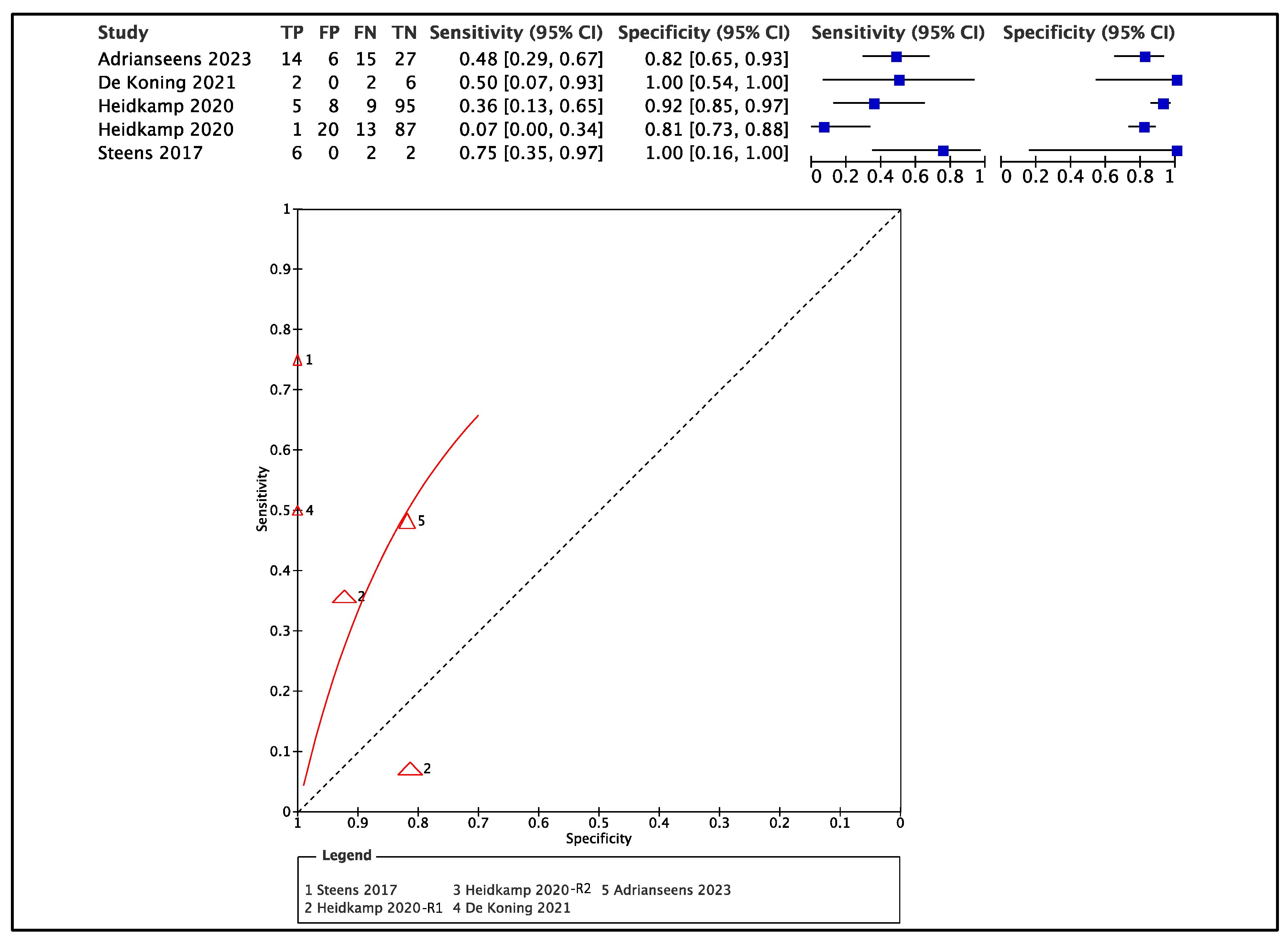
3.3. Data Extraction
4. Discussion
4.1. Accuracy of Ex Vivo Ultrasound
4.2. Accuracy of Ex Vivo MRI
4.3. Potential Sources of Bias and Heterogeneity
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanoni, D.K.; Migliacci, J.C.; Xu, B.; Katabi, N.; Montero, P.H.; Ganly, I.; Shah, J.P.; Wong, R.J.; Ghossein, R.A.; Patel, S.G. A Proposal to Redefine Close Surgical Margins in Squamous Cell Carcinoma of the Oral Tongue. JAMA Otolaryngol.–Head Neck Surg. 2017, 143, 555. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Sisson, K.; Moncrieff, M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015, 51, 464–469. [Google Scholar] [CrossRef]
- Thomas Robbins, K.; Triantafyllou, A.; Suárez, C.; López, F.; Hunt, J.L.; Strojan, P.; Williams, M.D.; Braakhuis, B.J.M.; de Bree, R.; Hinni, M.L.; et al. Surgical margins in head and neck cancer: Intra- and postoperative considerations. Auris Nasus Larynx 2019, 46, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers Version 1. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Buglione, M.; Cavagnini, R.; Di Rosario, F.; Sottocornola, L.; Maddalo, M.; Vassalli, L.; Grisanti, S.; Salgarello, S.; Orlandi, E.; Paganelli, C.; et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Dental pathologies and osteoradionecrosis (Part 1) literature review and consensus statement. Crit. Rev. Oncol./Hematol. 2015, 97, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Salz, T.; Ostroff, J.S.; Nightingale, C.L.; Atkinson, T.M.; Davidson, E.C.; Jinna, S.R.; Kriplani, A.; Lesser, G.J.; Lynch, K.A.; Mayer, D.K.; et al. The Head and Neck Survivorship Tool (HN-STAR) Trial (WF-1805CD): A protocol for a cluster-randomized hybrid effectiveness-implementation pragmatic trial to improve the follow-up care of head and neck cancer survivors. Contemp. Clin. Trials 2021, 107, 106448. [Google Scholar] [CrossRef]
- Rathod, S.; Livergant, J.; Klein, J.; Witterick, I.; Ringash, J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015, 51, 888–900. [Google Scholar] [CrossRef]
- Leeuw, I.V.-D.; Dawson, C.; Licitra, L.; Eriksen, J.G.; Hosal, S.; Singer, S.; Laverty, D.P.; Golusinski, W.; Machczynski, P.; Gomes, A.V.; et al. European Head and Neck Society recommendations for head and neck cancer survivorship care. Oral Oncol. 2022, 133, 106047. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Tarabichi, O.; Sethi, R.K.; Parikh, A.S.; Varvares, M.A. Does Clearance of Positive Margins Improve Local Control in Oral Cavity Cancer? A Meta-analysis. Otolaryngol. Neck Surg. 2019, 161, 235–244. [Google Scholar] [CrossRef]
- Hakim, S.G.; Bialy, R.; Falougy, M.; Steller, D.; Tharun, L.; Rades, D.; Sieg, P.; Alsharif, U. Impact of stratified resection margin classification on local tumor control and survival in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2021, 124, 1284–1295. [Google Scholar] [CrossRef]
- Smits, R.W.H.; Koljenović, S.; Hardillo, J.A.; Ten Hove, I.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.C.; Schut, T.C.B.; Langeveld, T.P.M.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement: Resection Margins in Oral Cancer Surgery. Head Neck 2016, 38, E2197–E2203. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.; Howe, C.L.; Borgstrom, M.; Baker, A.; Wang, S.J.; Bearelly, S. Impact of Close Margins in Head and Neck Mucosal Squamous Cell Carcinoma: A Systematic Review. Laryngoscope 2021, 132, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Warshavsky, A.; Rosen, R.; Nard-Carmel, N.; Abu-Ghanem, S.; Oestreicher-Kedem, Y.; Abergel, A.; Fliss, D.M.; Horowitz, G. Assessment of the Rate of Skip Metastasis to Neck Level IV in Patients with Clinically Node-Negative Neck Oral Cavity Squamous Cell Carcinoma: A Systematic Review and Meta-analysis. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 542. [Google Scholar] [CrossRef]
- Beunk, L.; Brown, K.; Nagtegaal, I.; Friedl, P.; Wolf, K. Cancer invasion into musculature: Mechanics molecules and implications. Semin. Cell Dev. Biol. 2019, 93, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bizzoca, M.E.; Grigolato, R.; Maffini, F.A.; Tagliabue, M.; Negro, R.; Leuci, S.; Mignogna, M.D.; Muzio, L.L. From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy Microanatomy Surgical Treatments and Basic Research. Life 2020, 10, 197. [Google Scholar] [CrossRef]
- Buchakjian, M.R.; Ginader, T.; Tasche, K.K.; Pagedar, N.A.; Smith, B.J.; Sperry, S.M. Independent Predictors of Prognosis Based on Oral Cavity Squamous Cell Carcinoma Surgical Margins. Otolaryngol. Neck Surg. 2018, 159, 675–682. [Google Scholar] [CrossRef]
- Almangush, A.; Pirinen, M.; Heikkinen, I.; Mäkitie, A.A.; Salo, T.; Leivo, I. Tumour budding in oral squamous cell carcinoma: A meta-analysis. Br. J. Cancer 2017, 118, 577–586. [Google Scholar] [CrossRef]
- Nentwig, K.; Unterhuber, T.; Wolff, K.-D.; Ritschl, L.M.; Nieberler, M. The impact of intraoperative frozen section analysis on final resection margin status recurrence and patient outcome with oral squamous cell carcinoma. Clin. Oral Investig. 2021, 25, 6769–6777. [Google Scholar] [CrossRef]
- Demir, B.; Incaz, S.; Uckuyulu, E.I.; Oysu, C. Accuracy of Frozen Section Examination in Oral Cavity Cancers. Ear Nose Throat J. 2020, 101, NP354–NP357. [Google Scholar] [CrossRef]
- Wasif, M.; Mughal, A.; Hussain, M.; Zaidi, M.; Awan, M.S.; Sidddique, S.; Awan, O.; Ghaloo, S.K.; Suahil, A. The Utility of Frozen Sections in the Evaluation of Clear Margins in Oral Squamous Cell Carcinomas: A Cross-Sectional Study from a Tertiary Care Center. Cureus [Internet]. 16 February 2022. Available online: https://www.cureus.com/articles/84814-the-utility-of-frozen-sections-in-the-evaluation-of-clear-margins-in-oral-squamous-cell-carcinomas-a-cross-sectional-study-from-a-tertiary-care-center (accessed on 18 March 2023).
- Chaturvedi, P.; Datta, S.; Mishra, A.; Bal, M.; Nair, D.; More, Y.; Ingole, P.; Sawakare, S.; Agarwal, J.P.; Kane, S.V.; et al. Frozen section is not cost beneficial for the assessment of margins in oral cancer. Indian J. Cancer 2019, 56, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kamat, M.; Rai, B.; Puranik, R.; Datar, U. A comprehensive review of surgical margin in oral squamous cell carcinoma highlighting the significance of tumor-free surgical margins. J. Cancer Res. Ther. 2019, 15, 449–454. [Google Scholar] [CrossRef]
- Mannelli, G.; Comini, L.V.; Piazza, C. Surgical margins in oral squamous cell cancer: Intraoperative evaluation and prognostic impact. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 98–103. [Google Scholar] [CrossRef]
- Tasche, K.K.; Buchakjian, M.; Pagedar, N.; Sperry, S.M. Definition of “Close Margin” in Oral Cancer Surgery and Association of Margin Distance with Local Recurrence Rate. JAMA Otolaryngol. Neck Surg. 2017, 143, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.; Campanile, Y.; Warner, A.; Laxague, F.; Fnais, N.; Fung, K.; Mendez, A.; MacNeil, D.; Yoo, J.; Palma, D. Surgical margins of the oral cavity: Is 5 mm really necessary? J. Otolaryngol.-Head Neck Surg. 2022, 51, 38. [Google Scholar] [CrossRef] [PubMed]
- Brouwer de Koning, S.G.; Schaeffers, A.W.M.A.; Schats, W.; van den Brekel, M.W.M.; Ruers, T.J.M.; Karakullukcu, M.B. Assessment of the deep resection margin during oral cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2021, 47, 2220–2232. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. (Eds.) Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2; Cochrane: London, UK, 2022. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Adriaansens, C.M.E.M.; de Koning, K.J.; de Bree, R.; Dankbaar, J.W.; Breimer, G.E.; van Es, R.J.J.; Noorlag, R. Ultrasound-guided resection for squamous cell carcinoma of the buccal mucosa: A feasibility study. Head Neck 2023, 45, 647–657. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Tarabichi, O.; Parikh, A.S.; Yoon, B.C.; Juliano, A.; Sadow, P.M.; Faquin, W.; Gropler, M.; Walker, R.; Puram, S.V.; et al. The utility of intra-oral ultrasound in improving deep margin clearance of oral tongue cancer resections. Oral Oncol. 2021, 122, 105512. [Google Scholar] [CrossRef]
- de Koning, K.J.; van Es, R.J.; Klijn, R.J.; Breimer, G.E.; Dankbaar, J.W.; Braunius, W.W.; van Cann, E.M.; Dieleman, F.J.; Rijken, J.A.; Tijink, B.M.; et al. Application and accuracy of ultrasound-guided resections of tongue cancer. Oral Oncol. 2022, 133, 106023. [Google Scholar] [CrossRef] [PubMed]
- de Koning, K.J.; Koppes, S.A.; de Bree, R.; Dankbaar, J.W.; Willems, S.M.; van Es, R.J.; Noorlag, R. Feasibility study of ultrasound-guided resection of tongue cancer with immediate specimen examination to improve margin control—Comparison with conventional treatment. Oral Oncol. 2021, 116, 105249. [Google Scholar] [CrossRef]
- Giannitto, C.; Mercante, G.; Disconzi, L.; Boroni, R.; Casiraghi, E.; Canzano, F.; Cerasuolo, M.; Gaino, F.; De Virgilio, A.; Fiamengo, B.; et al. Frozen Section Analysis and Real-Time Magnetic Resonance Imaging of Surgical Specimen Oriented on 3D Printed Tongue Model to Assess Surgical Margins in Oral Tongue Carcinoma: Preliminary Results. Front. Oncol. 2021, 11, 5049. [Google Scholar] [CrossRef] [PubMed]
- Heidkamp, J.; Weijs, W.L.J.; Grunsven, A.C.H.V.E.; Vries, I.D.L.; Maas, M.C.; Rovers, M.M.; Fütterer, J.J.; Steens, S.C.A.; Takes, R.P. Assessment of surgical tumor-free resection margins in fresh squamous-cell carcinoma resection specimens of the tongue using a clinical, M.R.I.; system. Head Neck 2020, 42, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Knutsson, J.; Landström, F.J.; Magnuson, A.; von Beckerath, M. Ultrasound-assisted resection of oral tongue cancer. Acta Otolaryngol. 2022, 142, 743–748. [Google Scholar] [CrossRef]
- Steens, S.C.A.; Bekers, E.M.; Weijs, W.L.J.; Litjens, G.J.S.; Veltien, A.; Maat, A.; Broek, G.B.D.; van der Laak, J.A.W.M.; Fütterer, J.J.; van der Kaa, C.A.H.; et al. Evaluation of tongue squamous cell carcinoma resection margins using ex-vivo MR. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 821–828. [Google Scholar] [CrossRef]
- Tarabichi, O.; Kanumuri, V.; Juliano, A.F.; Faquin, W.C.; Cunnane, M.E.; Varvares, M.A. Intraoperative Ultrasound in Oral Tongue Cancer Resection: Feasibility Study and Early Outcomes. Otolaryngol. Neck Surg. 2017, 158, 645–648. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Chu, D.-G.; Mao, M.-H.; Feng, Z.-E.; Li, J.-Z.; Qin, L.-Z.; Han, Z.-X. The role of magnetic resonance imaging in assessing the extent of tongue squamous cell carcinoma: A prospective cohort study. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e822–e827. [Google Scholar] [CrossRef]
- Harvey, L.A.; Dijkers, M.P. Should trials that are highly vulnerable to bias be excluded from systematic reviews? Spinal Cord. 2019, 57, 715–716. [Google Scholar] [CrossRef]
- Berdugo, J.; Thompson, L.D.R.; Purgina, B.; Sturgis, C.D.; Tuluc, M.; Seethala, R.; Chiosea, S.I. Measuring Depth of Invasion in Early Squamous Cell Carcinoma of the Oral Tongue: Positive Deep Margin Extratumoral Perineural Invasion and Other Challenges. Head Neck Pathol. 2018, 13, 154–161. [Google Scholar] [CrossRef]
- Subramaniam, N.; Balasubramanian, D.; Murthy, S.; Kumar, N.; Vidhyadharan, S.; Vijayan, S.N.; Nambiar, A.; Thankappan, K.; Iyer, S. Predictors of locoregional control in stage, I/II oral squamous cell carcinoma classified by AJCC 8th edition. Eur. J. Surg. Oncol. EJSO 2019, 45, 2126–2130. [Google Scholar] [CrossRef]
- Noorlag, R.; de Bree, R.; Witjes, M.J. Image-guided surgery in oral cancer: Toward improved margin control. Curr. Opin. Oncol. 2022, 34, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Ow, T.J.; Lo, Y.T.; Gersten, A.; Schiff, B.A.; Tassler, A.B.; Smith, R.V. Refining the utility and role of Frozen section in head and neck squamous cell carcinoma resection: Frozen Section Analysis: Head and Neck. Laryngoscope 2016, 126, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.; Hashimoto, K.; Kayama, R.; Yamauchi, H.; Ikeda, K.; Ojiri, H. Radiological approach for the newly incorporated, T. staging factor depth of invasion (DOI), of the oral tongue cancer in the 8th edition of American Joint Committee on Cancer (AJCC) staging manual: Assessment of the necessity for elective neck dissection. Jpn. J. Radiol. 2020, 38, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Faisal, M.; Abu Bakar, M.; Sarwar, A.; Adeel, M.; Batool, F.; Malik, K.I.; Jamshed, A.; Hussain, R. Depth of invasion (DOI) as a predictor of cervical nodal metastasis and local recurrence in early stage squamous cell carcinoma of oral tongue (ESSCOT). PLoS ONE 2018, 13, e0202632. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, J.; Kang, L.; Zhang, X.; Wang, L.; Chen, X.; Yao, Q.; Li, H. Significance of depth of invasion determined by MRI in cT1N0 tongue squamous cell carcinoma. Sci. Rep. 2020, 10, 4695. [Google Scholar] [CrossRef]
- Marchi, F.; Filauro, M.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Santori, G.; Parrinello, G.; Canevari, F.R.; Piazza, C.; Peretti, G. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 9, 1571. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers Version 2.2020 NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, S.; Casaleggio, A.; Tagliafico, A.S.; Conforti, C.; Borda, F.; Fiannacca, M.; Filauro, M.; Iandelli, A.; Marchi, F.; Parrinello, G.; et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations. Int. J. Environ. Res. Public Health 2022, 19, 14900. [Google Scholar] [CrossRef]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review: Intraoral Ultrasound in Tongue Cancer. Laryngoscope 2019, 129, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Filauro, M.; Missale, F.; Marchi, F.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Parrinello, G.; Barabino, E.; Cittadini, G.; Farina, D.; et al. Intraoral ultrasonography in the assessment of DOI in oral cavity squamous cell carcinoma: A comparison with magnetic resonance and histopathology. Eur. Arch. Oto-Rhino-Laryngol. 2020, 278, 2943–2952. [Google Scholar] [CrossRef]
- Nulent, T.J.K.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.; de Bree, R.; van Es, R.J. Intraoral ultrasonography to measure tumor thickness of oral cancer: A systematic review and meta-analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.; Liao, C.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Köhler, H.F.; Kreppel, M.; et al. Depth of invasion alone as an indication for postoperative radiotherapy in small oral squamous cell carcinomas: An International Collaborative Study. Head Neck 2019, 41, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Noorlag, R.; Nulent, T.J.K.; Delwel, V.E.; Pameijer, F.A.; Willems, S.M.; de Bree, R.; van Es, R.J. Assessment of tumour depth in early tongue cancer: Accuracy of, M.R.I.; and intraoral ultrasound. Oral Oncol. 2020, 110, 104895. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Kobayashi, T.; Nikkuni, Y.; Katsura, K.; Yamazaki, M.; Maruyama, S.; Tanuma, J.; Hayashi, T. A comparative study between, C.T.; MRI, and intraoral, U.S. for the evaluation of the depth of invasion in early stage (T1/T2) tongue squamous cell carcinoma. Oral Radiol. 2022, 38, 114–125. [Google Scholar] [CrossRef]
- Nilsson, O.; Knutsson, J.; Landström, F.J.; Magnuson, A.; von Beckerath, M. Ultrasound accurately assesses depth of invasion in, T1–T2 oral tongue cancer. Laryngoscope Investig. Otolaryngol. 2022, 7, 1448–1455. [Google Scholar] [CrossRef]
- Songra, A.; Ng, S.; Farthing, P.; Hutchison, I.; Bradley, P. Observation of tumour thickness and resection margin at surgical excision of primary oral squamous cell carcinoma—Assessment by ultrasound. Int. J. Oral Maxillofac. Surg. 2006, 35, 324–331. [Google Scholar] [CrossRef]
- Baek, C.; Son, Y.; Jeong, H.; Chung, M.K.; Park, K.; Ko, Y.; Kim, H.-J. Intraoral Sonography–Assisted Resection of, T.1–2 tongue Cancer for Adequate deep Resection. Otolaryngol.—Head Neck Surg. 2008, 139, 805–810. [Google Scholar] [CrossRef]
- Voizard, B.; Khoury, M.; Saydy, N.; Nelson, K.; Cardin, G.B.; Létourneau-Guillon, L.; Filali-Mouhim, A.; Christopoulos, A. Preoperative evaluation of depth of invasion in oral tongue squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2023, 136, 106273. [Google Scholar] [CrossRef]
- Casto, A.L.; Cannella, R.; Taravella, R.; Cordova, A.; Matta, D.; Campisi, G.; Attanasio, M.; Rinaldi, G.; Rodolico, V. Diagnostic and prognostic value of magnetic resonance imaging in the detection of tumor depth of invasion and bone invasion in patients with oral cavity cancer. La Radiol. Med. 2022, 127, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Cardenas, C.E.; Bahig, H.; Elgohari, B.; Wang, J.; Johnson, J.M.; Moreno, A.C.; Shah, S.J.; Garden, A.S.; Phan, J.; et al. Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study. Curr. Oncol. 2022, 29, 6303–6313. [Google Scholar] [CrossRef] [PubMed]
- Preda, L.; Conte, G.; Bonello, L.; Giannitto, C.; Travaini, L.L.; Raimondi, S.; Summers, P.E.; Mohssen, A.; Alterio, D.; Rocca, M.C.; et al. Combining standardized uptake value of FDG-PET and apparent diffusion coefficient of DW-MRI improves risk stratification in head and neck squamous cell carcinoma. Eur. Radiol. 2016, 26, 4432–4441. [Google Scholar] [CrossRef]
- Serifoglu, I.; Oz, I.I.; Damar, M.; Tokgoz, O.; Yazgan, O.; Erdem, Z. Diffusion-weighted imaging in the head and neck region: Usefulness of apparent diffusion coefficient values for characterization of lesions. Diagn. Interv. Radiol. 2015, 21, 208–214. [Google Scholar] [CrossRef] [PubMed]

| Literature Sources | Search in | Limits | Search Terms |
|---|---|---|---|
| MEDLINE | Advanced Search | Research articles Years (2016–2023) English language Humans | (margin* or specimen or ex-vivo or intraoperative or intra-operative) AND (oral* OR oral cavity or tongue) AND (CANCER OR TUMOUR OR TUMOR OR NEOPL*) AND (MRI Or MR OR magnetic resonance imaging OR nuclear magnetic resonance OR US OR ULTRASOUND OR ULTRASONOGRAPHY) ((‘oral cavity squamous cell carcinoma’ OR ‘oral cavity cancer’ OR ‘tongue cancer’) AND ‘magnetic resonance imaging’ OR ‘ultrasound’) AND ‘margin*’ AND [humans]/lim AND [english]/lim AND ([embase]/lim OR [medline]/lim OR [preprint]/lim OR [pubmed-not-medline]/lim) AND [2016–2023]/py |
| EMBASE | Advanced Search | Research articles Years (2016–2023) English language | ((margin* OR ‘specimen’/exp OR ‘ex vivo’/exp OR intraoperative OR ‘intra operative’) AND (oral* OR ‘mouth cavity’ OR tongue)) AND (cancer OR tumour OR tumor OR neopl*)) AND (mri OR mr OR ‘nuclear magnetic resonance’ OR ‘nuclear magnetic resonance imaging’ OR us OR ultrasound OR ultrasonography) |
| CENTRAL | Advanced Search | Research articles Years (2016–2023) English Language | ((margin* OR ‘specimen’/exp OR ‘ex vivo’/exp OR intraoperative OR ‘intra operative’) AND (oral* OR ‘mouth cavity’ OR tongue)) AND (cancer OR tumour OR tumor OR neopl*)) AND (mri OR mr OR ‘nuclear magnetic resonance’ OR ‘nuclear magnetic resonance imaging’ OR us OR ultrasound OR ultrasonography) |
| Study ID | Study Design | Anatomical Site | Index Test | Exclusion Criteria | T Stages Included | Control Cohort | Was Masking Applied | Sample Size | Excluded/ Dropouts |
|---|---|---|---|---|---|---|---|---|---|
| Ex-vivo ultrasound | |||||||||
| Adriaansens [30] 2023 | Prospective cohort study | Buccal mucosa | ioUS intraoperative and ex-vivo 16 MHz |
| T1-T2-T3-T4a | No | Unclear | 14 | 1 |
| Bulbul [31] 2021 | Retrospective case series study with Test and control groups | Oral tongue | ioUS intraoperative and ex-vivo 15 MHz |
| T1-T2-T3 | Yes | Yes | Test group: 23 Control group: 21 | 0 |
| De Konig [32] 2022 | Prospective-nonrandomized trial—consecutive enrolment Test-control cohorts (test prospective; cohort retrospective) | Oral tongue | ioUS intraoperative and ex-vivo 16 and 20 MHz |
| T1-T2-T3 | Yes | Unclear | Test group: 44 Control group: 96 | 4 |
| De Konig [33] 2021 | Prospective nonrandomized trial—consecutive enrolment Test-control arms (test prospective; cohort retrospective) | Oral tongue | ioUS intraoperative and ex-vivo 16 and 20 MHz |
| T1-T2-T3 | Yes | Unclear | Test group: 10 Control group: 98 | 0 |
| Nilsson [36] 2022 | Prospective-nonrandomized trial—consecutive enrollment Test-control cohorts (test prospective; cohort retrospective) | Oral tongue | ioUS intraoperative and ex-vivo 18 MHz |
| T1-T2-T3 | Yes | Unclear | Test group: 34 Control group: 76 | 0 |
| Tarabichi [38] 2018 | Retrospective cohort study | Oral tongue | ioUS intraoperative and ex-vivo 15 MHz | Not stated | T1-T2 | No | No | 12 | 0 |
| Ex-vivo MRI | |||||||||
| Giannitto [34] 2021 | Prospective-nonrandomized trial—consecutive enrolment | Oral tongue | ex vivo MRI (1.5T) | Not stated | T1-T2-T3 | No | Yes | 10 | 0 |
| Heidkamp [35] 2020 | Prospective nonrandomized trial—consecutive enrollment | Oral tongue | Ex-vivo MRI (3T) |
| T1-T2-T3-T4a | No | Yes | 10 | 0 |
| Steens [37] 2017 | Prospective-nonrandomized trial—consecutive enrolment | Oral tongue | Ex-vivo MRI (7T) | Not stated | T1-T2-T4a | No | No | 10 | 3 |
| Zhang [39] 2022 | Retrospective cohort study | Oral tongue | Ex-vivo MRI (1.5T) |
| T1-T2-T3 | No | Yes | 165 | 0 |
| Study ID | Mean TT or DOI | Mean TTΔ Imaging-Pathology | Free Margin Rate | Clear Margins (>5 mm) | Close Margins (1–4.9 mm) | Positive Margins (<1 mm) | NPV | Image-Guided Re-Resection | Reported * Sensitivity, Specificity, AUC | Potential Sources of Bias | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex-vivo ultrasound | |||||||||||
| Adriaansens [30] 2023 | 8.6 mm (TT) | 1.7 mm in vivo; 1.6 mm ex vivo | 8% | 1/13 (8%) | 9/13 (69%) | 3/13 (23%) | 0.64 * | Yes (7/13 patients; 20/62 sections) |
|
| Authors suggest surgical margins >7.5 mm The positive margin rate increases with DOI |
| Bulbul [31] 2021 | 6.4 mm test vs. 10.8 mm control (DOI) | 0.4 mm ex vivo | 70% (T) vs. 48%(C) | Test: 16/23 (70%) Control: 10/21 (48%) | Test: 7/23 (30%) Control: 11/21 (52%) | Test: 0/23 (0) Control: 1/21 (4%) | 0.69 | No | Not reported |
| The positive margin rate increases with DOI |
| De Konig [33] 2021 | 6.2 mm (DOI) vs. 6.1 mm (DOI) | 1.9 mm in vivo; 1.4 mm ex vivo | 70% (T) vs. 17%(C) | Test 7/10 (70%) Control: 15 (17%) | Test: 2/10 (20%) Control: 67 (74%) | Test: 1/10 (10%) Control: 9 (10%) | 0.75 | Yes (20%) | Not reported * Extracted: Figure 3 |
| Authors suggest surgical margins >10 mm The positive margin rate increases with DOI ioUS lowered the rate of close margins; no impact on positive margins |
| De Konig [32] 2022 | 7.1 mm (DOI) vs. 7.8 mm (DOI) | 0.4 mm in vivo; 0.9 mm ex vivo | 55% (T) vs. 16% (C) | Test: 22/40 (55%) Control: 15/96 (16%) | Test: 16 /40 (40%) Control: 67/96 (70%) | Test: 2/40 (5%) Control: 15/96 (16%) | 0.55 | Yes (33%) |
|
|
|
| Nilsson [36] 2022 | NA | 1.4 mm | 55% (T) vs. 28% (C) | Test: 19/34 (55%) Control: 22/76 (28%) | Test: 14/34 (41%) Control: 45/76 (59%) | Test: 1/34 (2%) Control: 9/76 (11%) | 0.55 | Yes | Not reported |
| Authors suggest surgical margins >10 mm (accuracy profile too low for closer margins) |
| Tarabichi [38] 2018 | 5.45 mm (TT) | 1 mm | 92% | 11/12 (92%) | 1/12 (8%) | 0 | 0.91 | No | Not reported |
|
|
| Ex-vivo MRI | |||||||||||
| Giannitto [34] 2021 | NA | NA | 90% | 9/10 (90%) | 1/10 (10%) | 0 | 0.90 | No |
|
|
|
| Heidkamp [35] 2020 | Median 8 mm (DOI) | NA | 70% | 7/10 (70%) | 3/10 (30%) | NA | 0.75* | No |
|
|
|
| Steens [37] 2017 | 4 mm (DOI) | 0.45 mm | 20% | 2/10 (20%) | 7/10 (70%) | 1/10 (10%) | 0.50 | No | Not reported * Extracted: Figure 3 |
|
|
| Zhang [39] 2022 | NA | NA | \ | Not applicable | Not applicable | Not applicable | NA | No | Not reported |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnicelli, G.; Disconzi, L.; Cerasuolo, M.; Casiraghi, E.; Costa, G.; De Virgilio, A.; Esposito, A.A.; Ferreli, F.; Fici, F.; Lo Casto, A.; et al. Image-Guided Intraoperative Assessment of Surgical Margins in Oral Cavity Squamous Cell Cancer: A Diagnostic Test Accuracy Review. Diagnostics 2023, 13, 1846. https://doi.org/10.3390/diagnostics13111846
Carnicelli G, Disconzi L, Cerasuolo M, Casiraghi E, Costa G, De Virgilio A, Esposito AA, Ferreli F, Fici F, Lo Casto A, et al. Image-Guided Intraoperative Assessment of Surgical Margins in Oral Cavity Squamous Cell Cancer: A Diagnostic Test Accuracy Review. Diagnostics. 2023; 13(11):1846. https://doi.org/10.3390/diagnostics13111846
Chicago/Turabian StyleCarnicelli, Giorgia, Luca Disconzi, Michele Cerasuolo, Elena Casiraghi, Guido Costa, Armando De Virgilio, Andrea Alessandro Esposito, Fabio Ferreli, Federica Fici, Antonio Lo Casto, and et al. 2023. "Image-Guided Intraoperative Assessment of Surgical Margins in Oral Cavity Squamous Cell Cancer: A Diagnostic Test Accuracy Review" Diagnostics 13, no. 11: 1846. https://doi.org/10.3390/diagnostics13111846
APA StyleCarnicelli, G., Disconzi, L., Cerasuolo, M., Casiraghi, E., Costa, G., De Virgilio, A., Esposito, A. A., Ferreli, F., Fici, F., Lo Casto, A., Marra, S., Malvezzi, L., Mercante, G., Spriano, G., Torzilli, G., Francone, M., Balzarini, L., & Giannitto, C. (2023). Image-Guided Intraoperative Assessment of Surgical Margins in Oral Cavity Squamous Cell Cancer: A Diagnostic Test Accuracy Review. Diagnostics, 13(11), 1846. https://doi.org/10.3390/diagnostics13111846