Artificial-Intelligence-Driven Algorithms for Predicting Response to Corticosteroid Treatment in Patients with Post-Acute COVID-19
Abstract
:1. Introduction
2. Related Work
2.1. Acute-Phase and Corticotherapy
2.1.1. Administration Timing and Dosing
2.1.2. Mortality
2.1.3. Machine Learning Approaches
2.2. Post-Acute Phase and Corticotherapy
2.3. Summary and Research Gap
3. Methodology
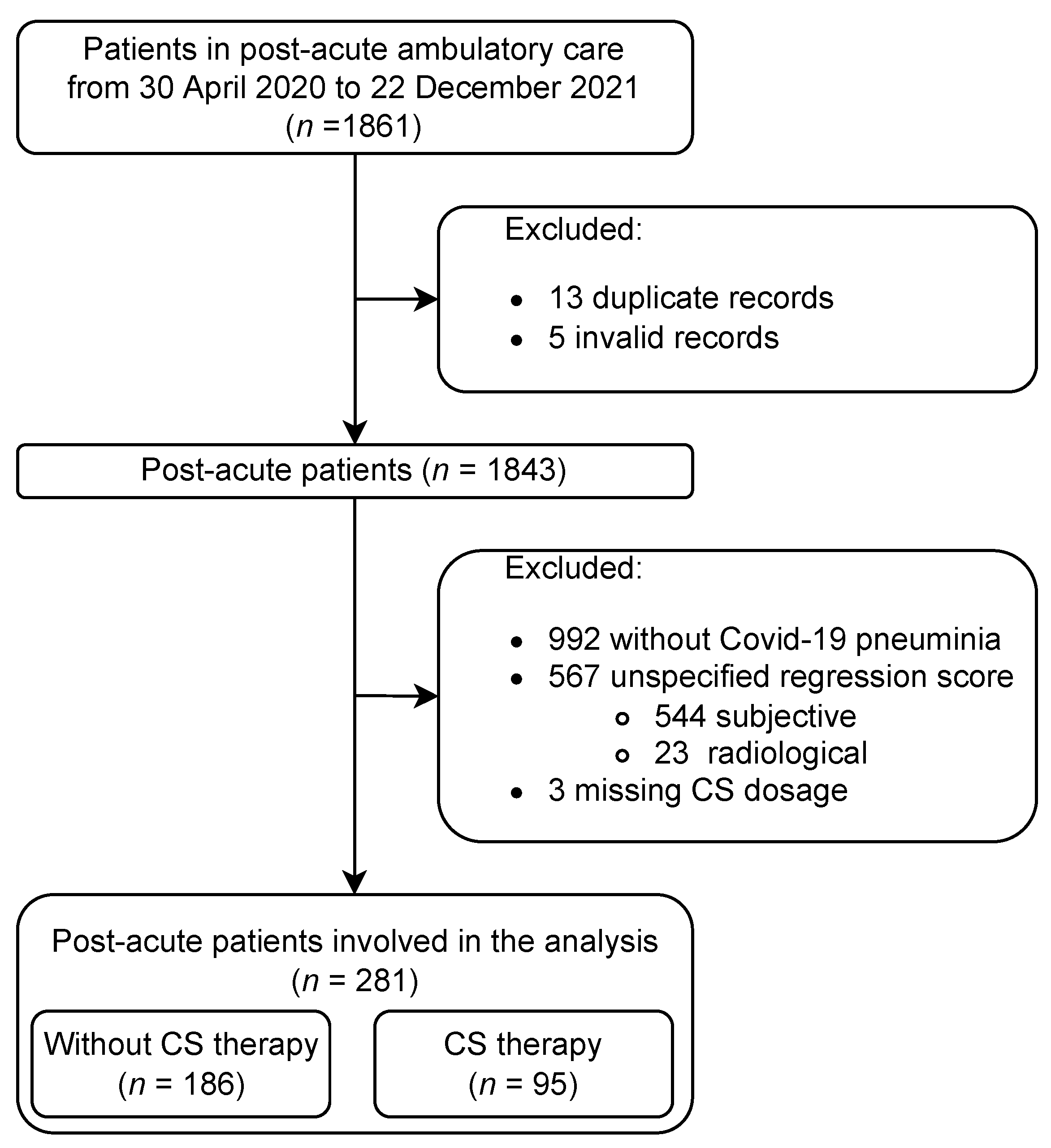
3.1. Dataset
3.1.1. Patient Selection Process
3.1.2. Analysed Dataset
3.2. Experiment
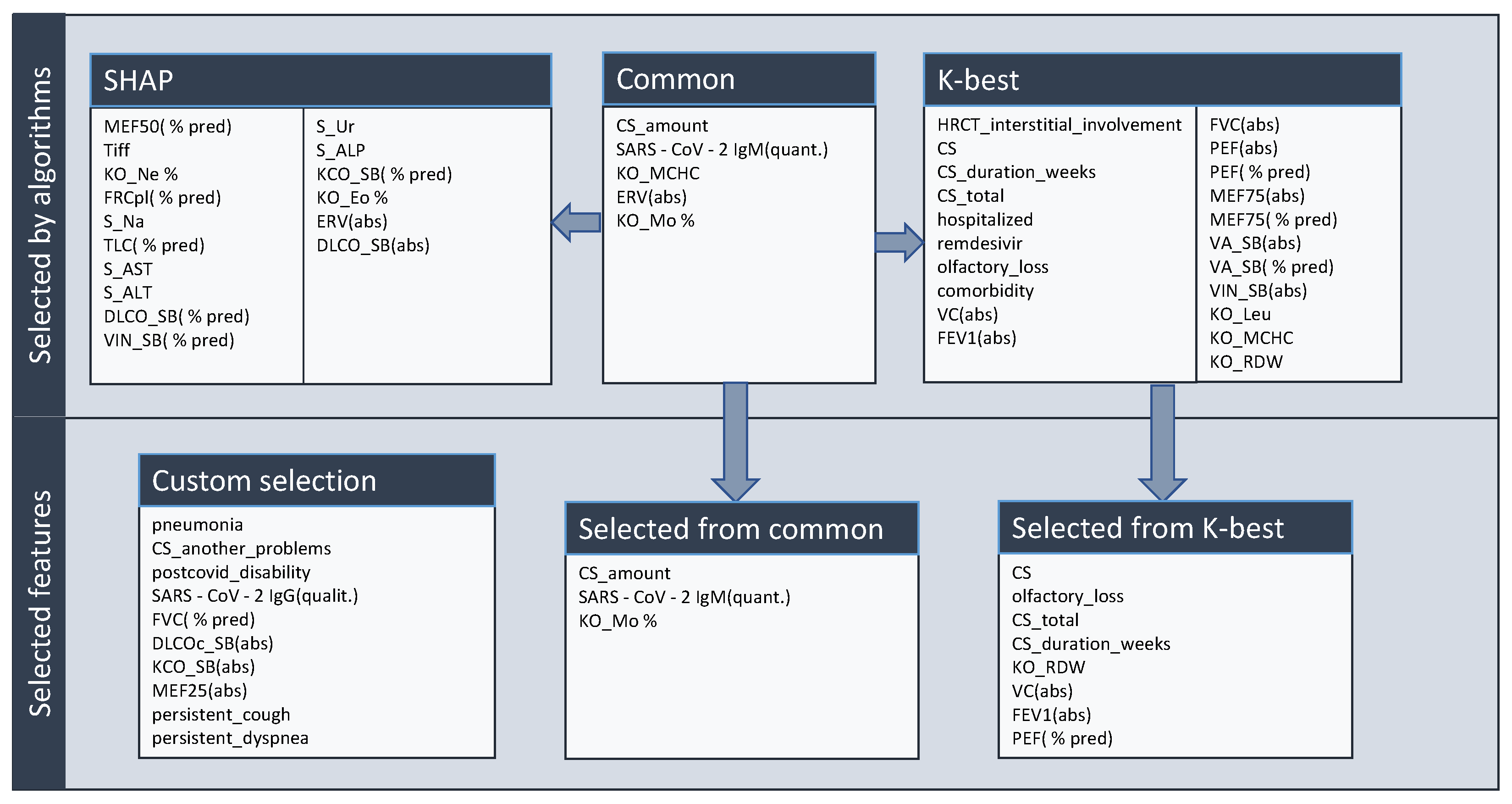
3.2.1. Feature Selection
3.2.2. Selected Methods
- Logistic Regression [29] (pp. 89–90),
- k-Nearest Neighbours [29] (pp. 56–59),
- Decision Tree [29] (pp. 167–169),
- XGBoost [29] (pp. 190–193),
- Random Forest [29] (pp. 194–195),
- Support Vector Machine [29] (pp. 145–146),
- Multi-layer Perceptron (MLP) [30],
- Adaboost classifier [29] (pp. 190–191),
- Light Gradient Boosting Machine (LGBM) [31].
- MLP: hidden layer sizes: (70, 8), optimizer: Adam, max iteration: 80, activation: ReLU;
- Decision tree: min samples leaf: 13, max depth: 8, criterion: entropy;
- Random forest: max depth: 6, criterion: entropy;
- k-Nearest Neighbours: weights: distance, neighbors number: 5;
- SVM: kernel: sigmoid;
- AdaBoost: number of estimators: 12, learning rate: 0.8;
- LGBM: learning rate: 0.5, max depth: 3.
3.3. Evaluation Metrics
4. Results and Discussion
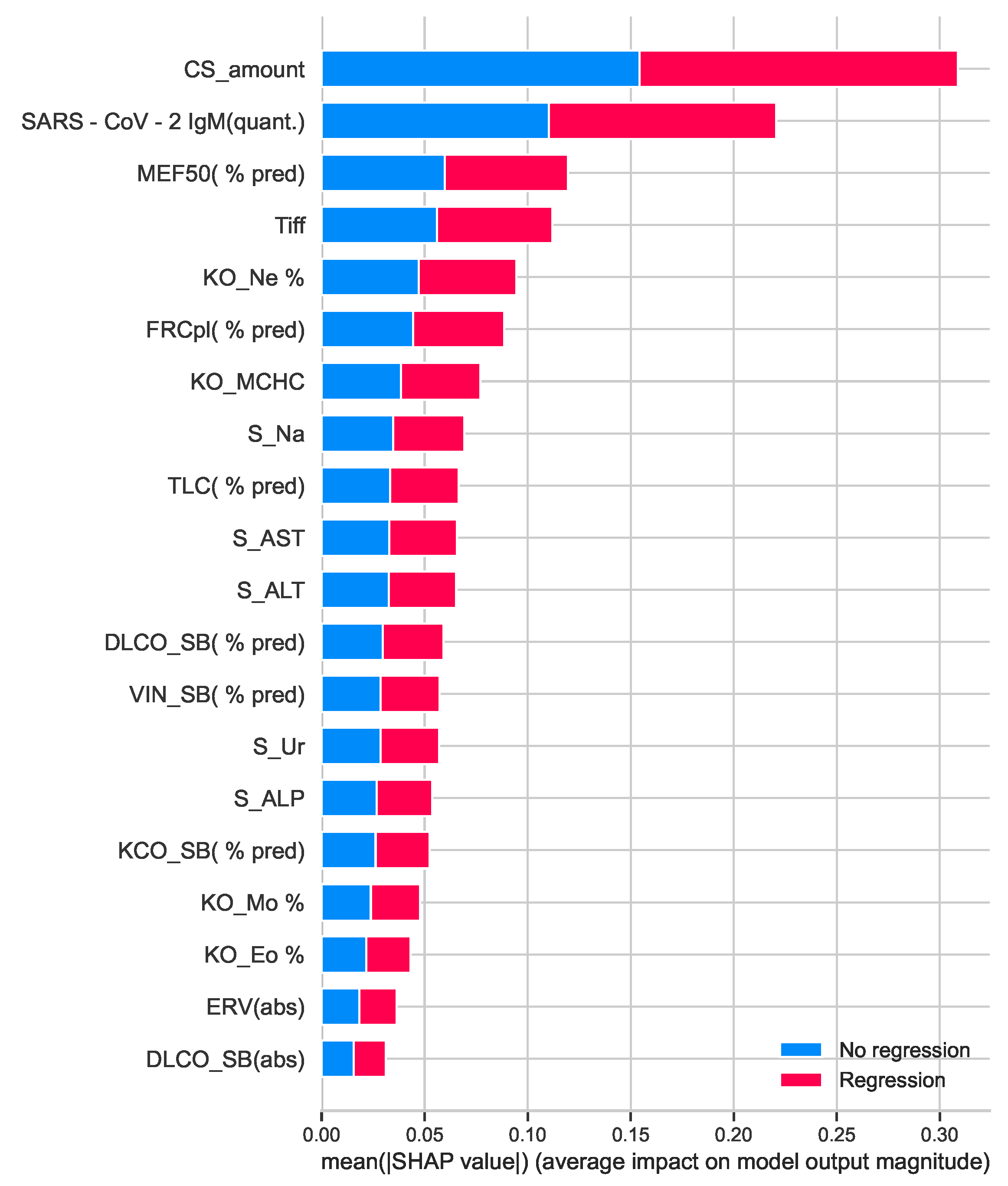
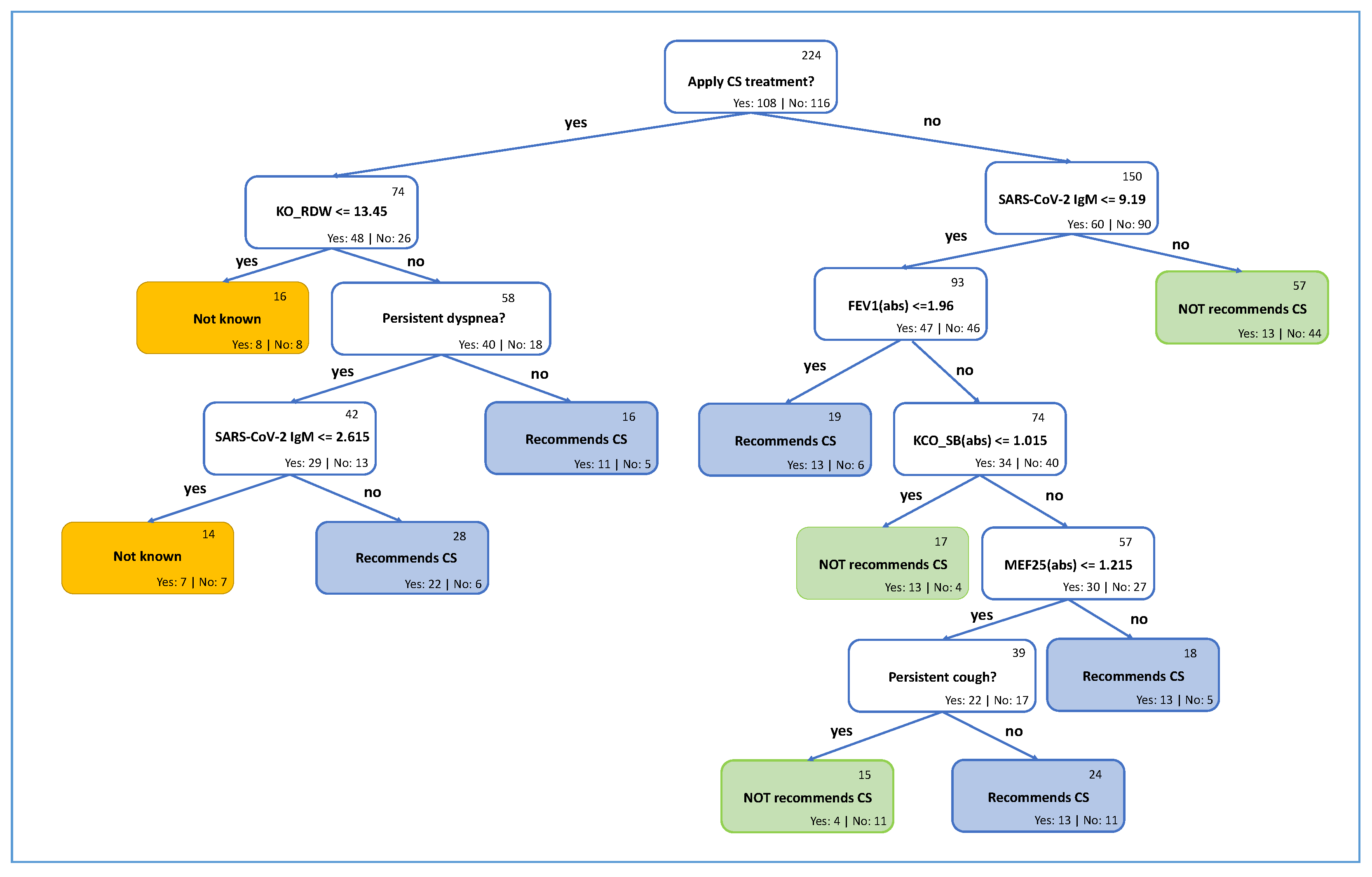
4.1. Explainable Recommendation Approach
4.2. Limitations
5. Conclusions
- The artificial intelligence-based (or also called evidence-based) model predicts with 73.52% accuracy whether treating a patient with CS therapy is recommended. Pulmonary fibrosis is one of the most severe long-term consequences of COVID-19. We also identified the most valuable attributes used for the classification.
- Dataset FNOL_PulFib2022 (https://github.com/VojtechMyska/AI_CS_response, accessed on 12 May 2022) of 281 patients from post-acute treatment. Each patient is subjected to rigorous testing (e.g., blood tests, spirometry, anamnesis, and comorbidities) at the start of the post-COVID treatment and the result three months after the treatment. In addition, information on whether the patient benefited from CS treatment is also included.
- A simplified interpretable decision tree-based model, which can be easily incorporated into the clinical practice, is also provided.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afshar, Z.M.; Ebrahimpour, S.; Javanian, M.; Koppolu, V.; Vasigala, V.K.R.; Hasanpour, A.H.; Babazadeh, A. Coronavirus disease 2019 (COVID-19), MERS and SARS: Similarity and difference. J. Acute Dis. 2020, 9, 194. [Google Scholar]
- Parums, D.V. Long COVID, or Post-COVID Syndrome, and the Global Impact on Health Care. Med. Sci. Monit. 2021, 27, e933446. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.M.; Churchill, A.; Nsair, A.; Hsu, J.J. Post-Acute COVID-19 Syndrome and the cardiovascular system: What is known? Am. Heart J. Plus Cardiol. Res. Pract. 2021, 5, 100025. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Cioboata, R.; Nicolosu, D.; Streba, C.T.; Vasile, C.M.; Olteanu, M.; Nemes, A.; Gheorghe, A.; Calarasu, C.; Turcu, A.A. Post-COVID-19 Syndrome Based on Disease Form and Associated Comorbidities. Diagnostics 2022, 12, 2502. [Google Scholar] [CrossRef]
- Sova, M.; Doubková, M.; Solichová, L.; Šterclová, M.; Genzor, S. Treatment of pulmonary involvement of patients after COVID-19 (Coronovirus disease 2019)—Position document of the Czech Pneumological and Phthisiological Society. Czech Pneumol. Phtiseol. Soc. 2020, 1–11. Available online: http://www.pneumologie.cz/upload/1612528705.1554.docx (accessed on 31 September 2021).
- Bieksiene, K.; Zaveckiene, J.; Malakauskas, K.; Vaguliene, N.; Zemaitis, M.; Miliauskas, S. Post COVID-19 Organizing Pneumonia: The Right Time to Interfere. Medicina 2021, 57, 283. [Google Scholar] [CrossRef]
- National Institutes of Health. Hospitalized Adults: Therapeutic Management. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/ (accessed on 19 April 2022).
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S.; et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef]
- Estella, Á.; Garmendia, J.G.; de la Fuente, C.; Casas, J.M.; Yuste, M.E.; Villar, R.A.; Estecha, M.A.; Mateos, L.Y.; Bulnes, M.C.; Loza, A.; et al. Predictive factors of six-week mortality in critically ill patients with SARS-CoV-2: A multicenter prospective study. Med. Intensiv. 2021, 46, 179–191. [Google Scholar] [CrossRef]
- Mongardon, N.; Piagnerelli, M.; Grimaldi, D.; Perrot, B.; Lascarrou, J.B. Impact of late administration of corticosteroids in COVID-19 ARDS. Intensive Care Med. 2021, 47, 110–112. [Google Scholar] [CrossRef]
- Monreal, E.; de la Maza, S.S.; Natera-Villalba, E.; Beltrán-Corbellini, Á.; Rodríguez-Jorge, F.; Fernández-Velasco, J.I.; Walo-Delgado, P.; Muriel, A.; Zamora, J.; Alonso-Canovas, A.; et al. High versus standard doses of corticosteroids in severe COVID-19: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 761–769. [Google Scholar] [CrossRef]
- Kumar, G.; Patel, D.; Hererra, M.; Jefferies, D.; Sakhuja, A.; Meersman, M.; Dalton, D.; Nanchal, R.; Guddati, A.K. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J. Med. Virol. 2022, 94, 372–379. [Google Scholar] [CrossRef]
- Duarte-Millán, M.A.; Mesa-Plaza, N.; Guerrero-Santillán, M.; Morales-Ortega, A.; Bernal-Bello, D.; Farfán-Sedano, A.I.; García de Viedma-García, V.; Velázquez-Ríos, L.; Frutos-Pérez, B.; De Ancos-Aracil, C.L.; et al. Prognostic factors and combined use of tocilizumab and corticosteroids in a Spanish cohort of elderly COVID-19 patients. J. Med. Virol. 2022, 94, 1540–1549. [Google Scholar] [CrossRef]
- Lim, P.C.; Wong, K.L.; Rajah, R.; Chong, M.F.; Chow, T.S.; Subramaniam, S.; Lee, C.Y. Comparing the efficacy of tocilizumab with corticosteroid therapy in treating COVID-19 patients: A systematic review and meta-analysis. DARU J. Pharm. Sci. 2022, 30, 211–228. [Google Scholar] [CrossRef]
- Group, R.C. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Mishra, G.P.; Mulani, J. Corticosteroids for COVID-19: The search for an optimum duration of therapy. Lancet Respir. Med. 2021, 9, e8. [Google Scholar] [CrossRef]
- Kuno, T.; Sahashi, Y.; Kawahito, S.; Takahashi, M.; Iwagami, M.; Egorova, N.N. Prediction of in-hospital mortality with machine learning for COVID-19 patients treated with steroid and remdesivir. J. Med. Virol. 2022, 94, 958–964. [Google Scholar] [CrossRef]
- Lam, C.; Siefkas, A.; Zelin, N.S.; Barnes, G.; Dellinger, R.P.; Vincent, J.L.; Braden, G.; Burdick, H.; Hoffman, J.; Calvert, J.; et al. Machine Learning as a Precision-Medicine Approach to Prescribing COVID-19 Pharmacotherapy with Remdesivir or Corticosteroids. Clin. Ther. 2021, 43, 871–885. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, X.; Jiao, X.; Yu, Y.; Chi, J.; Chen, L.; Li, S.; Gao, Q. Development and Validation of a Machine Learning Model for Prediction of Response to Corticosteroid Therapy In COVID-19 Patients. SSRN 2021, 1–21. [Google Scholar] [CrossRef]
- Chen, H.; Xie, J.; Su, N.; Wang, J.; Sun, Q.; Li, S.; Jin, J.; Zhou, J.; Mo, M.; Wei, Y.; et al. Corticosteroid therapy is associated with improved outcome in critically ill coronavirus disease 2019 patients with hyperinflammatory phenotype. Chest 2021, 159, 1793–1802. [Google Scholar] [CrossRef]
- Ahmad, J.; Saudagar, A.K.J.; Malik, K.M.; Khan, M.B.; AlTameem, A.; Alkhathami, M.; Hasanat, M.H.A. Prognosis Prediction in COVID-19 Patients through Deep Feature Space Reasoning. Diagnostics 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. medRxiv 2021. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent Post–COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Nonhospitalized Adults: Therapeutic Management. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults–therapeutic-management/ (accessed on 12 May 2022).
- Le Bon, S.D.; Konopnicki, D.; Pisarski, N.; Prunier, L.; Lechien, J.R.; Horoi, M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur. Arch.-Oto-Rhino-Laryngol. 2021, 278, 3113–3117. [Google Scholar] [CrossRef]
- Touisserkani, S.K.; Ayatollahi, A. Oral corticosteroid relieves post-COVID-19 anosmia in a 35-year-old patient. Case Rep. Otolaryngol. 2020, 2020, 5892047. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 2522–5839. [Google Scholar] [CrossRef]
- Rhys, H. Machine Learning with R, the Tidyverse, and Mlr; Simon and Schuster: New York, NY, USA, 2020. [Google Scholar]
- Taud, H.; Mas, J. Multilayer perceptron (MLP). In Geomatic Approaches for Modeling Land Change Scenarios; Springer: Berlin/Heidelberg, Germany, 2018; pp. 451–455. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. Lightgbm: A highly efficient gradient boosting decision tree. In Advances in Neural Information Processing Systems 30; NeurIPS: Long Beach, CA, USA, 2017. [Google Scholar]
- Hossin, M.; Sulaiman, M.N. A review on evaluation metrics for data classification evaluations. Int. J. Data Min. Knowl. Manag. Process. 2015, 5, 1–11. [Google Scholar]
- Tharwat, A. Classification assessment methods. Appl. Comput. Inform. 2021, 17, 168–192. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]

| DEMOGRAPHIC & HABITS | |||||
|---|---|---|---|---|---|
| Attributes | Values | ||||
| Number of patients | 281 | ||||
| Gender | Male | Female | |||
| Number of patients | 170 (60.50%) | 111 (39.50%) | |||
| Age | Mean (SD) | Median (Q1–Q3) | Minumum–maximum | ||
| Years | 64.33 (11.08) | 65 (58–72) | 30–90 | ||
| Body proportion | Mean (SD) | Median (Q1–Q3) | Minumum–maximum | ||
| Weight (kg) | 88.03 (15.71) | 86 (77–97) | 57–136 | ||
| Height (cm) | 169.97 (9.76) | 171 (163–176) | 145–198 | ||
| BMI | 30.49 (4.96) | 29.71 (26.88–32.89) | 20.75–47.37 | ||
| Smoking | Smoker | Ex-smoker | Non-smoker | N/A | |
| Number of patients | 11 (3.91%) | 55 (19.57%) | 201 (71.53%) | 14 (4.99%) | |
| THERAPY & LUNG DAMAGE | |||||
| Attributes | Number of patients | ||||
| Yes | No | ||||
| Hospitalized | 230 (81.85%) | 51 (18.15%) | |||
| Oxygen | 185 (65.83%) | 96 (34.17%) | |||
| Remdesivir | 22 (7.83%) | 259 (92.17%) | |||
| CS | |||||
| During hospitalization | 102 (36.30%) | 179 (63.70%) | |||
| Post-covid treatment | 95 (33.81%) | 186 (66.19%) | |||
| Another diagnostics | 4 (1.42%) | 277 (98.57%) | |||
| HRCT—Lung damage | |||||
| Interstitial involvement | 51 (18.15%) | 230 (81.85%) | |||
| Inflammatory changes | 125 (44.45%) | 156 (55.45%) | |||
| PERSISTENT HEALT ISSUES | |||||
| Attributes | Number of patients | ||||
| Yes | No | N/A | |||
| Dyspnea | 194 (69.04%) | 86 (30.60%) | 1 (0.36%) | ||
| Cough | 98 (34.86%) | 182 (64.78%) | 1 (0.36%) | ||
| Fatigue | 80 (28.47%) | 200 (71.17%) | 1 (0.36%) | ||
| Olfactory loss | 40 (14.23%) | 241 (85.77%) | 0 (0.00%) | ||
| Gastrointestinal problems | 70 (24.91%) | 211 (75.09%) | 0 (0.00%) | ||
| COVID-19 TESTING | |||||
| Attributes | Number of patients | ||||
| Positive | Negative | Inconslusive or N/A | |||
| IgM (qualitatively) | 225 (80.07%) | 49 (17.44%) | 7 (2.49%) | ||
| IgG (qualitatively) | 272 (96.80%) | 2 (0.71%) | 7 (2.49%) | ||
| VACCINATION | |||||
| Attributes | Number of patients | ||||
| Before 1st examination | Yes | No | |||
| 1st dozen | 11 (3.91%) | 270 (96.09%) | |||
| 2nd dozen | 3 (1.07%) | 278 (1.07%) | |||
| 3rd dozen | 1 (0.36%) | 280 (99.64%) | |||
| Type | Corminaty | Spikevax | Vaxzevria | Janssen | N/A |
| 1st dozen | 177 (62.99%) | 19 (6.76%) | 16 (5.69%) | 16 (5.69%) | 53 (18.87%) |
| 2nd dozen | 181 (64.41%) | 17 (6.05%) | 16 (5.69%) | 0 (0.00%) | 67 (23.85%) |
| 3rd dozen | 78 (27.76%) | 0 (0.00%) | 1 (0.36%) | 12 (4.27%) | 190 (67.61%) |
| Method | Accuracy | Balanced Accuracy | ROC-AUC | F1 | Precision | Recall |
|---|---|---|---|---|---|---|
| Logistic Regression | 61.40% | 61.30% | 63.33% | 59.26% | 59.26% | 59.26% |
| Multilayer Perceptron | 70.18% | 69.81% | 72.10% | 66.67% | 70.83% | 62.96% |
| Decision Tree | 73.68% | 73.52% | 74.69% | 71.70% | 73.08% | 70.37% |
| Random Forest | 70.18% | 70.00% | 70.62% | 67.92% | 69.23% | 66.67% |
| k-Nearest Neighbors | 68.42% | 68.15% | 66.30% | 65.38% | 68.00% | 62.96% |
| Support Vector Machine | 63.16% | 61.85% | 70.37% | 48.78% | 71.43% | 37.04% |
| AdaBoost | 70.18% | 70.37% | 63.58% | 70.18% | 66.67% | 74.07% |
| XGBoost | 61.40% | 61.11% | 66.30% | 57.69% | 60.00% | 55.56% |
| Light Gradient Boosting Machine | 63.16% | 62.59% | 69.14% | 57.14% | 63.64% | 51.85% |
| Actual label | Not recommended | 23 | 7 |
|---|---|---|---|
| Recommended | 8 | 19 | |
| Not recommended | Recommended | ||
| Predicted label | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myska, V.; Genzor, S.; Mezina, A.; Burget, R.; Mizera, J.; Stybnar, M.; Kolarik, M.; Sova, M.; Dutta, M.K. Artificial-Intelligence-Driven Algorithms for Predicting Response to Corticosteroid Treatment in Patients with Post-Acute COVID-19. Diagnostics 2023, 13, 1755. https://doi.org/10.3390/diagnostics13101755
Myska V, Genzor S, Mezina A, Burget R, Mizera J, Stybnar M, Kolarik M, Sova M, Dutta MK. Artificial-Intelligence-Driven Algorithms for Predicting Response to Corticosteroid Treatment in Patients with Post-Acute COVID-19. Diagnostics. 2023; 13(10):1755. https://doi.org/10.3390/diagnostics13101755
Chicago/Turabian StyleMyska, Vojtech, Samuel Genzor, Anzhelika Mezina, Radim Burget, Jan Mizera, Michal Stybnar, Martin Kolarik, Milan Sova, and Malay Kishore Dutta. 2023. "Artificial-Intelligence-Driven Algorithms for Predicting Response to Corticosteroid Treatment in Patients with Post-Acute COVID-19" Diagnostics 13, no. 10: 1755. https://doi.org/10.3390/diagnostics13101755
APA StyleMyska, V., Genzor, S., Mezina, A., Burget, R., Mizera, J., Stybnar, M., Kolarik, M., Sova, M., & Dutta, M. K. (2023). Artificial-Intelligence-Driven Algorithms for Predicting Response to Corticosteroid Treatment in Patients with Post-Acute COVID-19. Diagnostics, 13(10), 1755. https://doi.org/10.3390/diagnostics13101755





