Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders
Abstract
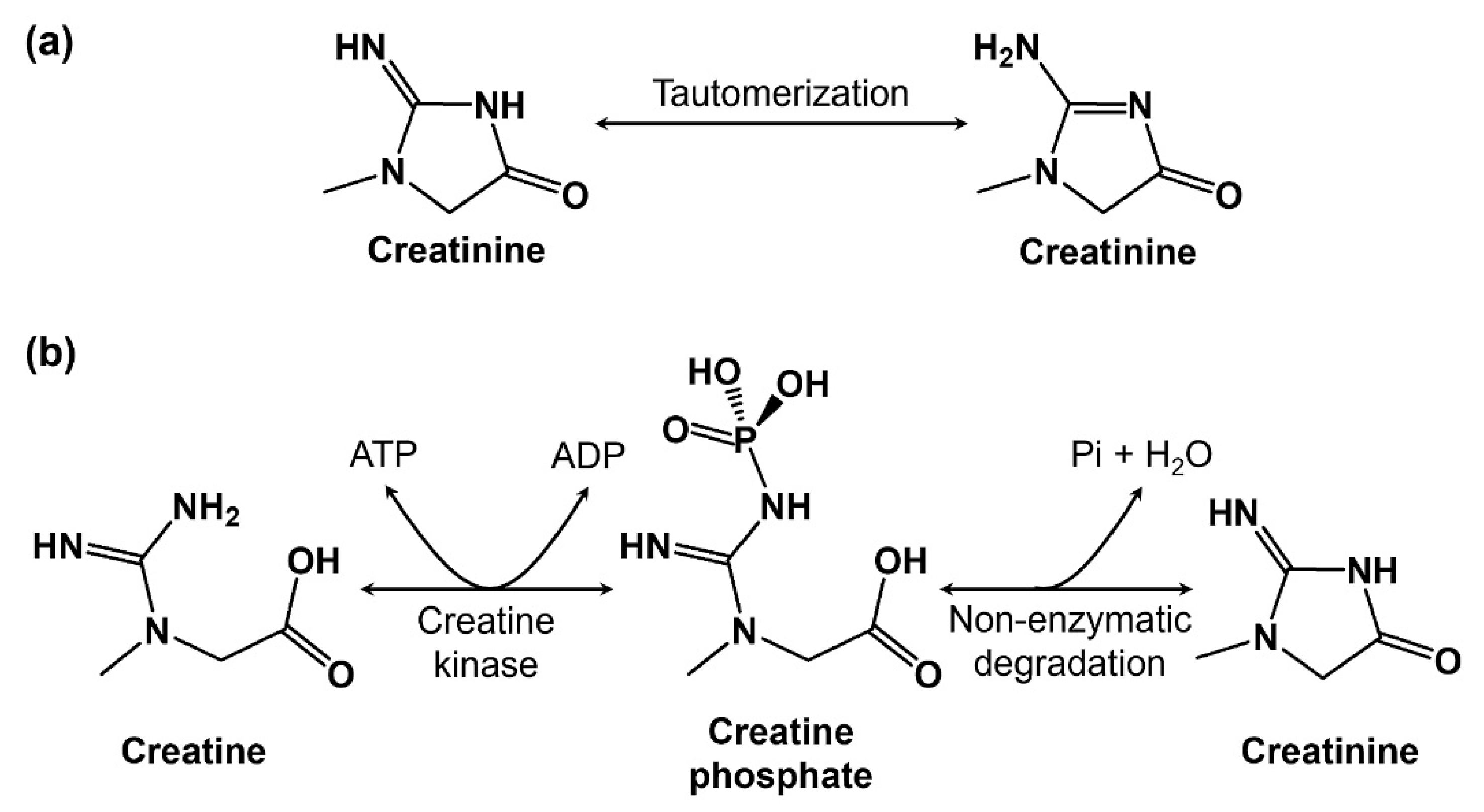
1. Introduction
| Age (Years) | Gender | Normal Range (µM) | Toxic Level (µM) |
|---|---|---|---|
| <14 | Male | 22.11–73.39 | <18, >80 |
| Female | 22.11–73.39 | <18, >80 | |
| 15–20 | Male | 44.21–80.46 | <35.37, >88 |
| Female | 37.14–68.97 | <31, >75.16 | |
| 20–70 | Male | 61.89–106.1 | <55, >113 |
| Female | 44.21–88.42 | <39, >95 | |
| >70 | Male | >61.89–106.1 | <45, >113 |
| Female | >44.21–88.42 | <39, >95 |
2. Creatinine Receptors
2.1. Enzymatic Receptors
2.2. Non-Enzymatic Receptors
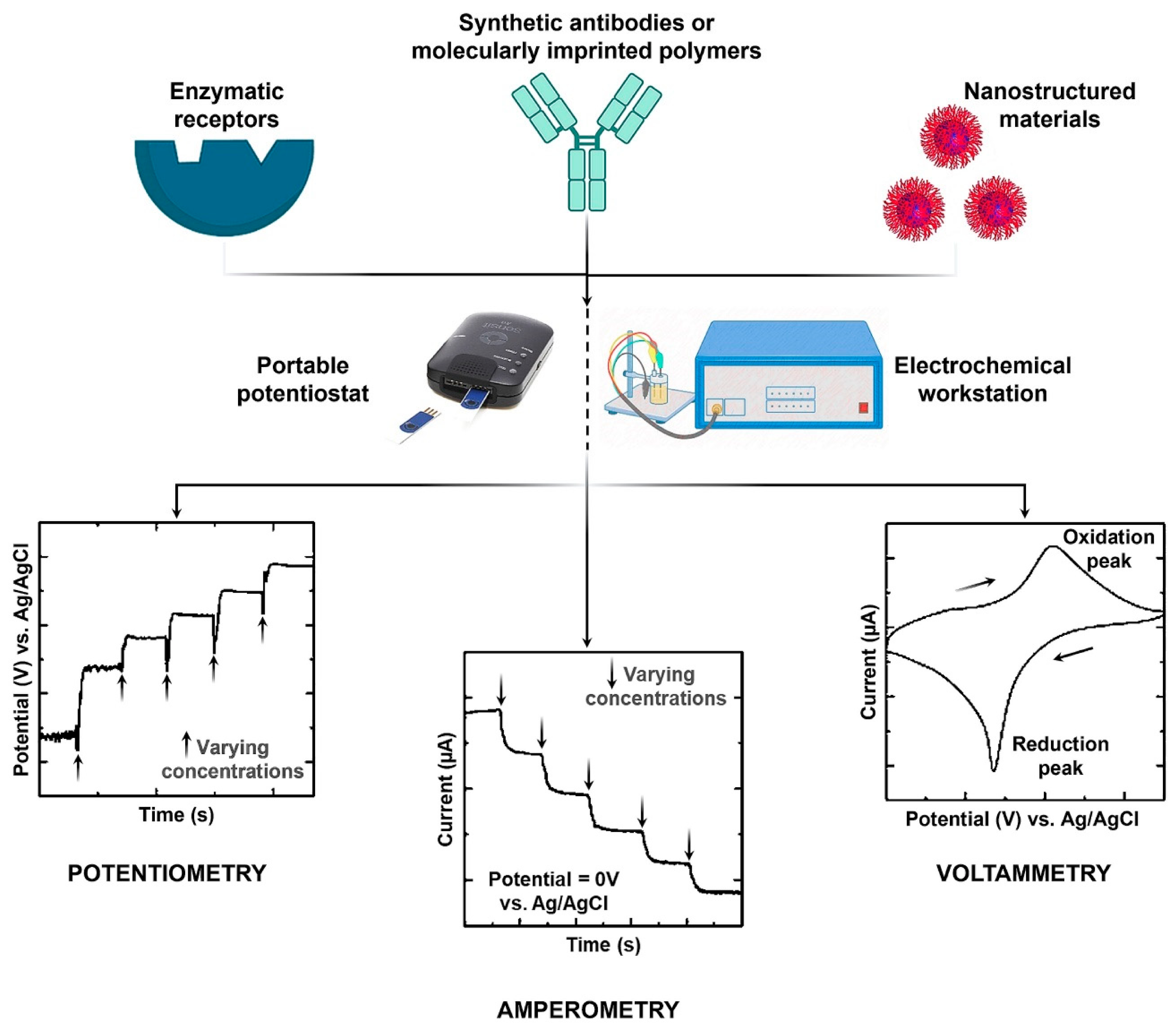
3. Electrochemical Creatinine (Bio)Sensors
3.1. Amperometric Creatinine Sensors
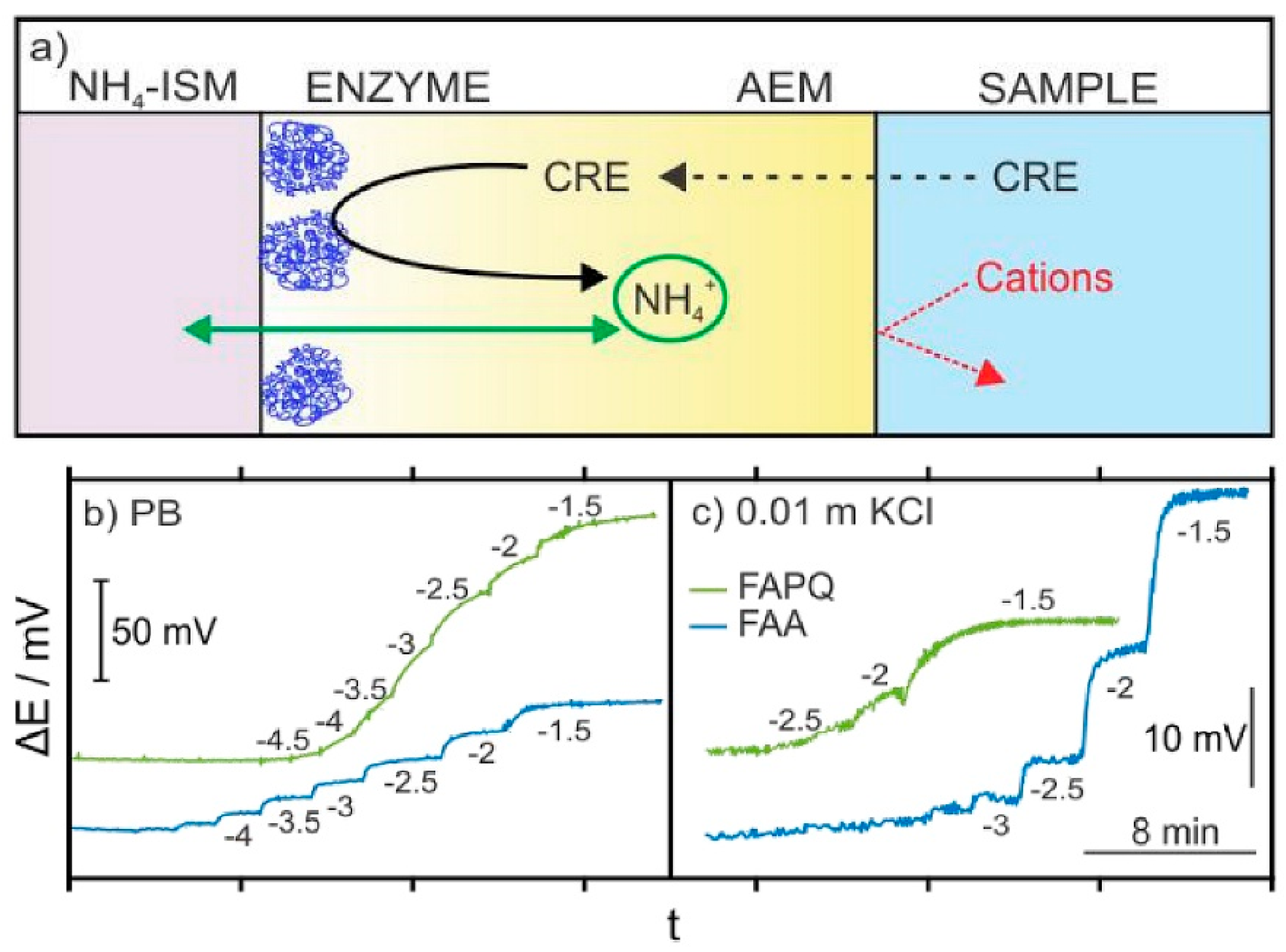
3.2. Potentiometric Creatinine Sensors
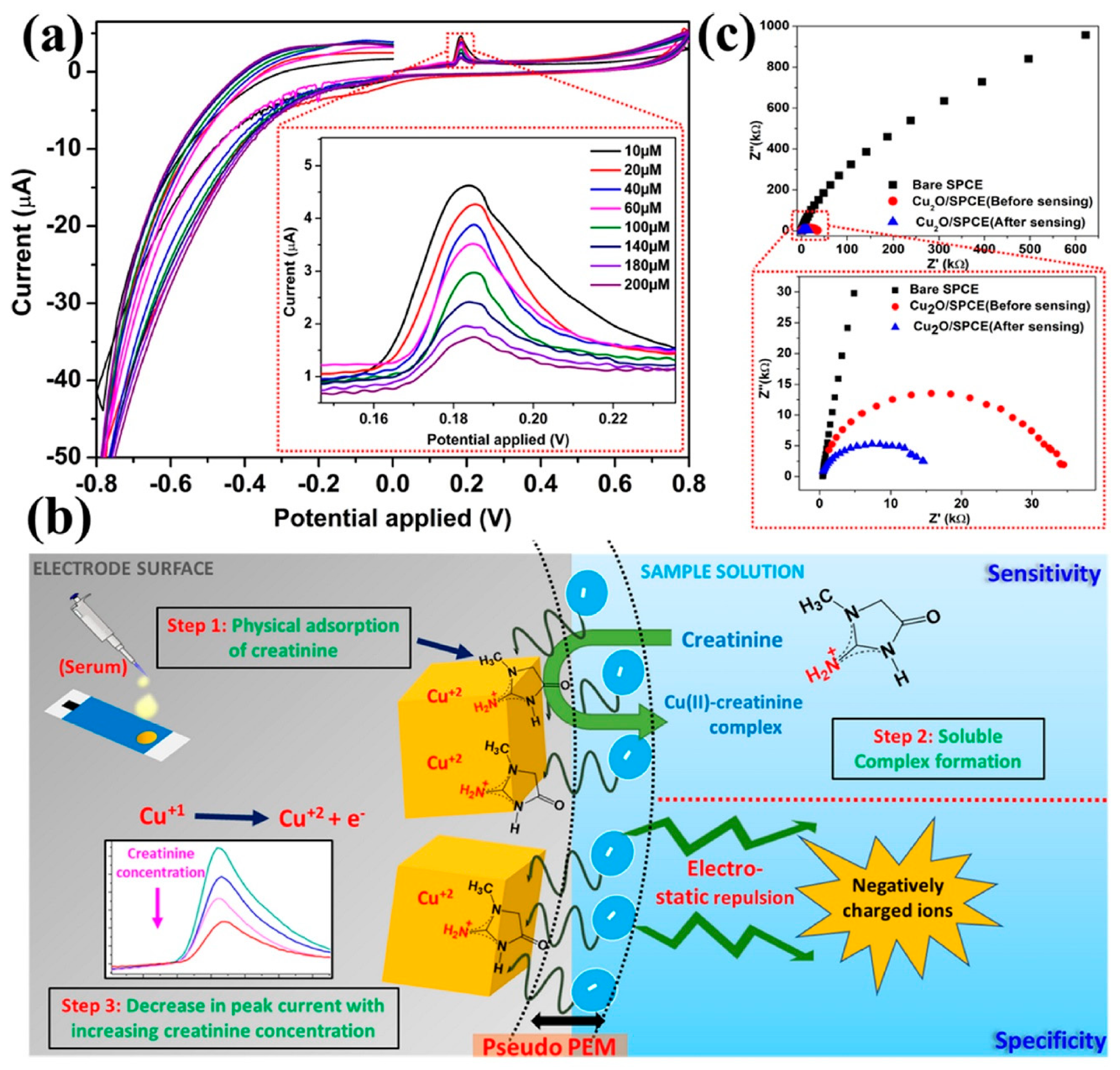
3.3. Voltammetric Creatinine Sensors
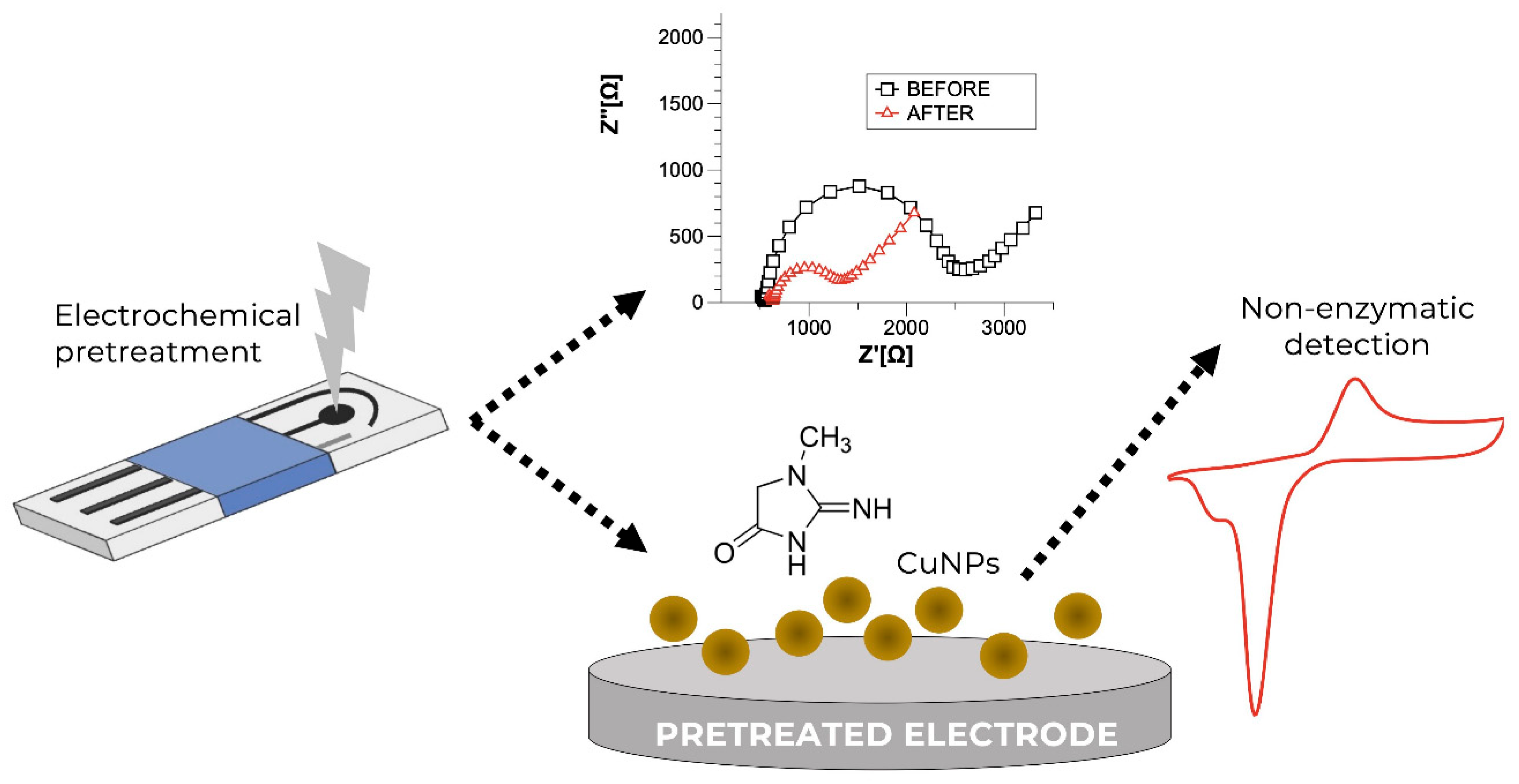
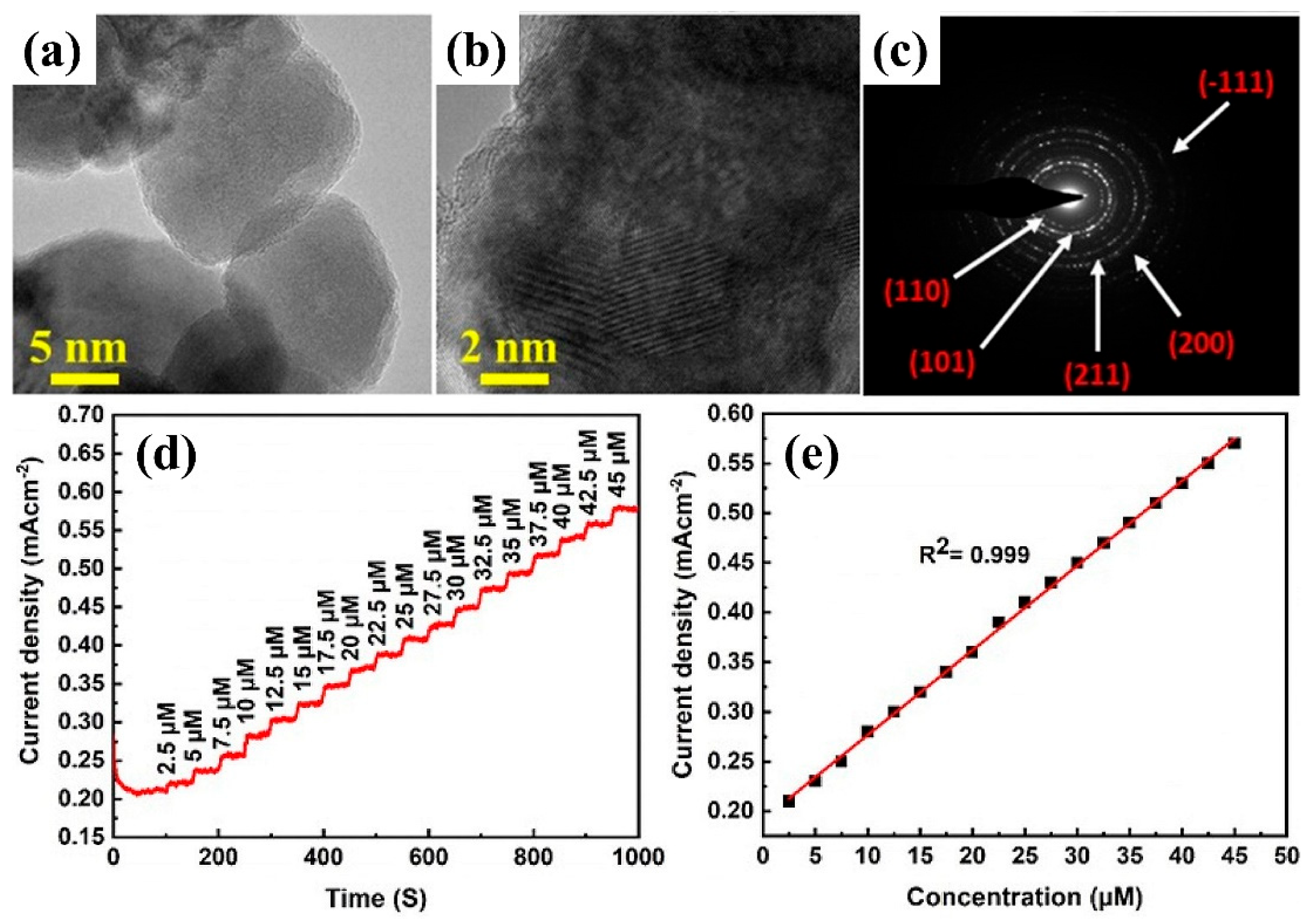
3.4. Other Electrochemical Sensors
4. Limitations and Challenges
| Material or Matrix | Enzymes 1 | Electrodes 2 | Range 3 (µM) | LOD 4 (µM) | τres 5 (s) | Sensitivity 6 (mA/unit.M) | Stability in Days 7 (Loss in Activity) | Recovery 8 (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| WE | RE | |||||||||
| ZnO/chitosan/carboxylated MWCNT/polyaniline | CA, CI, SO | Pt | Ag/AgCl | 10–650 | 0.5 | 10 | 0.030 μA/μM·cm2 | 120 (−15%) | 98.7 | [42] |
| Carboxylated MWCNT/polyaniline | CA, CI, SO | Pt | Ag/AgCl | 10–750 | 0.1 | 5 | 40 μA/μM·cm2 | 180 (−15%) | – | [42] |
| Polypyrrole | – | Au | Au | 0–1001.8 | 40.7 | <300 | – | – | – | [63] |
| Fe3O4/chitosan-graft-polyaniline | CRN, CR, SO | Pt | Ag/AgCl | 1–800 | 1 | 2 | 3.9 μA/μM·cm2 | 200 (−10%) | 99.93 | [101] |
| AuNPs, MWCNTs | CRN, CR, SO | Teflon cylinder | Ag/AgCl | 3–1000 | 0.1 | 9 | 1.32 µA/mM | – | 98 | [99] |
| Copper-polyaniline nanocomposite | CD | Carbon | Ag | 1–125 | 0.5 | 15 | 85 mA/M·cm2 | 3 | – | [58] |
| Polymethylene blue | – | Cu–doped carbon | Ag/AgCl | 2.2–132.6 | 2 × 10−4 | – | 0.133 μA/ng·mL | 180 (−4%) | 98.7 | [104] |
| Nafion-nsPANI | CD | Au | Ag/AgCl | 100–400 | – | – | 1300 μA/mM·cm2 | – | – | [22] |
| CuO@MIP | – | CPE | Ag/AgCl | 0.5–200 | 0.083 | – | 0.21 µA/µM | 14 (−20%) | – | [64] |
| ABTS+/CNT | – | Carbon | Ag/AgCl | 0–21300 | 11 | 60 | 27.3 µA/mM·cm2 | – | – | [56] |
| Nafion/Polyaniline | CD | Au | Au | 10–1000 | 2 | – | – | 30 (−20%) | – | [108] |
| Sb/NPC | – | GCE | Ag/AgCl | – | 0.744 | – | – | – | 90 | [52] |
| Prussian blue | CA, CI, SO | CGP | – | 50–1400 | – | 180 | – | 120 (−14%) | – | [57] |
| Conductive layer | CD | NH4+ ISE | – | 5–255 | 3 | 25 | – | – | – | [98] |
| β-cyclodextrin/poly(3,4-ethylene dioxythiophene) | – | GCE | SCE | 100–10,000 | 50 | 60 | – | 30 (−5%) | – | [81] |
| Fe3O4@polyaniline | – | GCE | – | 0.02–1 | 0.18 | – | – | 30 (−10%) | 104.9 | [82] |
| PVA-styryl pyridinium | CD | pH–FET | Ag/AgCl | 20–2000 | 20 | 120–180 | 40 | <1 | – | [103] |
| Silicalite | CD | pH–FET | Ag/AgCl | 0–2000 | 5 | – | 40 | 365 (−43.3%) | – | [105] |
| BEA gold (Zeolites) | CD | pH–FET | Ag/AgCl | 0–2000 | 10 | – | 40 | – | – | [80] |
| Calix [4]pyrrole | – | GCE | Ag/AgCl | 10–10,000 | ~1 | 54.1 | – | – | [83] | |
| Zeolite paraffin | – | CPE | Ag/AgCl | 0.1–100 | 0.079 | <50 | 52 | – | – | [84] |
| MWCNTs | CD | CPE | Ag/AgCl | 1000–50,000 | – | 900–3600 | 57.01 | – | – | [78] |
| Cu2O@MIP | – | SPCE | 0–0.075 | 0.022 | – | 2.16 µA/nM | 35 | 93 | [109] | |
| Polyethyleneimine/phosphotungstic acid | – | ITO | SCE | 0.125–62.5 | 0.06 | 200 | – | – | [110] | |
| Cu | – | Carbon | SCE | 6–378 | 0.0746 | – | – | – | 98 | [111] |
| rGO-AgNPs | – | GCE | – | 5 × 10−5–1.5 × 10–3 | 1.51×10−5 | – | – | – | 100 | [102] |
| 2-hydroxymethacrylate/methyl methacrylate/graphene oxide | – | GCE | – | 44.2–26.2 | 16.6 | 120 | – | – | – | [112] |
| MWCNTs-inu-TiO2 | – | CPE | Ag/AgCl | 0.2–1000 | 0.06 | – | – | 240 (−10%) | – | [107] |
| Nafion/graphene QDs | – | Au | Ag/AgCl | 0.97–450 | – | – | – | – | – | [87] |
| Cobalt chloride | – | Carbon | AgCl | 44–354 | – | – | – | – | – | [28] |
| AgNPs/folic acid/MWCNTs | – | CPE | SCE | 0.01–200 | 0.008 | 1.5 | 50 | 14 (−5%) | 96 | [53] |
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pundir, C.S.; Yadav, S.; Kumar, A. Creatinine sensors. TrAC Trends Anal. Chem. 2013, 50, 42–52. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Panasyuk-Delaney, T.; Mirsky, V.M.; Wolfbeis, O.S. Capacitive sensors based on a photografted molecularly imprinted polymers. Biocybern. Biomed. Eng. 2001, 21, 43–54. [Google Scholar] [CrossRef]
- Pottel, H.; Vrydags, N.; Mahieu, B.; Vandewynckele, E.; Croes, K.; Martens, F. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin. Chim. Acta 2008, 396, 49–55. [Google Scholar] [CrossRef]
- Ceriotti, F.; Boyd, J.C.; Klein, G.; Henny, J.; Queraltó, J.; Kairisto, V.; Panteghini, M.; the IFCC Committee on Reference Intervals and Decision Limits (C-RIDL). Reference Intervals for Serum Creatinine Concentrations: Assessment of Available Data for Global Application. Clin. Chem. 2008, 54, 559–566. [Google Scholar] [CrossRef]
- Varshney, A.; Rehan, M.; Subbarao, N.; Rabbani, G.; Khan, R.H. Elimination of Endogenous Toxin, Creatinine from Blood Plasma Depends on Albumin Conformation: Site Specific Uremic Toxicity & Impaired Drug Binding. PLoS ONE 2011, 6, e17230. [Google Scholar] [CrossRef]
- Rowat, A.; Graham, C.; Dennis, M. Dehydration in hospital-admitted stroke patients: Detection, frequency, and association. Stroke 2012, 43, 857–859. [Google Scholar] [CrossRef]
- Prowle, J.R.; Kolic, I.; Purdell-Lewis, J.; Taylor, R.; Pearse, R.M.; Kirwan, C.J. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 1015–1023. [Google Scholar] [CrossRef]
- Mohabbati-Kalejahi, E.; Azimirad, V.; Bahrami, M.; Ganbari, A. A review on creatinine measurement techniques. Talanta 2012, 97, 1–8. [Google Scholar] [CrossRef]
- Jaffé, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Biol. Chem. 1886, 10, 391–400. [Google Scholar] [CrossRef]
- Lad, U.; Khokhar, S.; Kale, G.M. Electrochemical Creatinine Biosensors. Anal. Chem. 2008, 80, 7910–7917. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tung, S. Development and Applications of Portable Biosensors. J. Lab. Autom. 2015, 20, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Hasanzadeh, M.; Hassanpour, S.; Saadati, A.; Agazadeh, M.; Mokhtarzadeh, A. An innovative nucleic acid based biosensor toward detection of Legionella pneumophila using DNA immobilization and hybridization: A novel genosensor. Microchem. J. 2019, 148, 708–716. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Mobed, A.; Hasanzadeh, M.; Saadati, A.; Hassanpour, S.; Mokhtarzadeh, A. Bio-assay of Acintobacter baumannii using DNA conjugated with gold nano-star: A new platform for microorganism analysis. Enzym. Microb. Technol. 2020, 133, 109466. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Dong, Y.; Hui, M.; Xu, L.; Li, H.; Lv, J.; Yang, L.; Cui, Y. Uric acid and creatinine biosensors with enhanced room-temperature storage stability by a multilayer enzyme matrix. Anal. Chim. Acta 2022, 1227, 340264. [Google Scholar] [CrossRef]
- Álvarez Menéndez, G.; Amor-Gutiérrez, O.; Costa García, A.; Funes-Menéndez, M.; Prado, C.; Miguel, D.; Rodríguez-González, P.; González-Gago, A.; García Alonso, J.I. Development and evaluation of an electrochemical biosensor for creatinine quantification in a drop of whole human blood. Clin. Chim. Acta 2023, 543, 117300. [Google Scholar] [CrossRef]
- Do, J.-S.; Chang, Y.-H. Optimizing the sensing performance of amperometric creatinine detection based on creatinine deiminase/Nafion®-nanostructured polyaniline composite film by mixture design method. Sens. Actuators Rep. 2023, 5, 100135. [Google Scholar] [CrossRef]
- Koshland, D.E., Jr. The Key–Lock Theory and the Induced Fit Theory. Angew. Chem. Int. Ed. Engl. 1995, 33, 2375–2378. [Google Scholar] [CrossRef]
- Do, J.-S.; Chang, Y.-H.; Tsai, M.-L. Highly sensitive amperometric creatinine biosensor based on creatinine deiminase/Nafion®-nanostructured polyaniline composite sensing film prepared with cyclic voltammetry. Mater. Chem. Phys. 2018, 219, 1–12. [Google Scholar] [CrossRef]
- Schneider, J.; Gründig, B.; Renneberg, R.; Cammann, K.; Madaras, M.B.; Buck, R.P.; Vorlop, K.-D. Hydrogel matrix for three enzyme entrapment in creatine/creatinine amperometric biosensing. Anal. Chim. Acta 1996, 325, 161–167. [Google Scholar] [CrossRef]
- Khan, G.F.; Wernet, W. A highly sensitive amperometric creatinine sensor. Anal. Chim. Acta 1997, 351, 151–158. [Google Scholar] [CrossRef]
- Osaka, T.; Komaba, S.; Amano, A. Highly Sensitive Microbiosensor for Creatinine Based on the Combination of Inactive Polypyrrole with Polyion Complexes. J. Electrochem. Soc. 1998, 145, 406. [Google Scholar] [CrossRef]
- Kim, E.J.; Haruyama, T.; Yanagida, Y.; Kobatake, E.; Aizawa, M. Disposable creatinine sensor based on thick-film hydrogen peroxide electrode system. Anal. Chim. Acta 1999, 394, 225–231. [Google Scholar] [CrossRef]
- Killard, A.J.; Smyth, M.R. Creatinine biosensors: Principles and designs. Trends Biotechnol. 2000, 18, 433–437. [Google Scholar] [CrossRef]
- Dasgupta, P.; Kumar, V.; Krishnaswamy, P.R.; Bhat, N. Serum Creatinine Electrochemical Biosensor on Printed Electrodes Using Monoenzymatic Pathway to 1-Methylhydantoin Detection. ACS Omega 2020, 5, 22459–22464. [Google Scholar] [CrossRef]
- Premanode, B.; Toumazou, C. A novel, low power biosensor for real time monitoring of creatinine and urea in peritoneal dialysis. Sens. Actuators B Chem. 2007, 120, 732–735. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Mosbach, K. Molecular imprinting. Trends Biochem. Sci. 1994, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar] [CrossRef]
- Kandimalla, V.B.; Ju, H. Molecular imprinting: A dynamic technique for diverse applications in analytical chemistry. Anal. Bioanal. Chem. 2004, 380, 587–605. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Delaney, T.L.; Zimin, D.; Rahm, M.; Weiss, D.; Wolfbeis, O.S.; Mirsky, V.M. Capacitive Detection in Ultrathin Chemosensors Prepared by Molecularly Imprinted Grafting Photopolymerization. Anal. Chem. 2007, 79, 3220–3225. [Google Scholar] [CrossRef]
- Nehl, C.L.; Liao, H.; Hafner, J.H. Optical Properties of Star-Shaped Gold Nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Erden, P.E.; Kılıç, E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 2013, 107, 312–323. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Abedi, B.; Daneshvar, S.; Sarbaz, Y.; Shakeri Bavil, A. Future of the Renal Biopsy: Time to Change the Conventional Modality Using Nanotechnology. Int. J. Biomed. Imaging 2017, 2017, e6141734. [Google Scholar] [CrossRef]
- Domínguez-Aragón, A.; Conejo-Dávila, A.S.; Zaragoza-Contreras, E.A.; Dominguez, R.B. Pretreated Screen-Printed Carbon Electrode and Cu Nanoparticles for Creatinine Detection in Artificial Saliva. Chemosensors 2023, 11, 102. [Google Scholar] [CrossRef]
- Yadav, S.; Devi, R.; Kumar, A.; Pundir, C.S. Tri-enzyme functionalized ZnO-NPs/CHIT/c-MWCNT/PANI composite film for amperometric determination of creatinine. Biosens. Bioelectron. 2011, 28, 64–70. [Google Scholar] [CrossRef]
- Jayasekhar Babu, P.; Tirkey, A.; Mohan Rao, T.J.; Chanu, N.B.; Lalchhandama, K.; Singh, Y.D. Conventional and nanotechnology based sensors for creatinine (A kidney biomarker) detection: A consolidated review. Anal. Biochem. 2022, 645, 114622. [Google Scholar] [CrossRef]
- Rao, H.; Lu, Z.; Ge, H.; Liu, X.; Chen, B.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. Electrochemical creatinine sensor based on a glassy carbon electrode modified with a molecularly imprinted polymer and a Ni@polyaniline nanocomposite. Microchim. Acta 2017, 184, 261–269. [Google Scholar] [CrossRef]
- Narimani, R.; Esmaeili, M.; Rasta, S.H.; Khosroshahi, H.T.; Mobed, A. Trend in creatinine determining methods: Conventional methods to molecular-based methods. Anal. Sci. Adv. 2021, 2, 308–325. [Google Scholar] [CrossRef]
- Kumar, R.R.; Shaikh, M.O.; Chuang, C.-H. A review of recent advances in non-enzymatic electrochemical creatinine biosensing. Anal. Chim. Acta 2021, 1183, 338748. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Nieh, C.-H.; Tsujimura, S.; Shirai, O.; Kano, K. Amperometric biosensor based on reductive H2O2 detection using pentacyanoferrate-bound polymer for creatinine determination. Anal. Chim. Acta 2013, 767, 128–133. [Google Scholar] [CrossRef]
- Erlenkötter, A.; Fobker, M.; Chemnitius, G.-C. Biosensors and flow-through system for the determination of creatinine in hemodialysate. Anal. Bioanal. Chem. 2002, 372, 284–292. [Google Scholar] [CrossRef]
- Stefan, R.-I.; Bokretsion, R.G.; van Staden, J.F.; Aboul-Enein, H.Y. Simultaneous determination of creatine and creatinine using amperometric biosensors. Talanta 2003, 60, 1223–1228. [Google Scholar] [CrossRef]
- Hsiue, G.-H.; Lu, P.-L.; Chen, J.-C. Multienzyme-immobilized modified polypropylene membrane for an amperometric creatinine biosensor. J. Appl. Polym. Sci. 2004, 92, 3126–3134. [Google Scholar] [CrossRef]
- Jamil, M.; Fatima, B.; Hussain, D.; Chohan, T.A.; Majeed, S.; Imran, M.; Khan, A.A.; Manzoor, S.; Nawaz, R.; Ashiq, M.N.; et al. Quantitative determination of creatinine from serum of prostate cancer patients by N-doped porous carbon antimony (Sb/NPC) nanoparticles. Bioelectrochemistry 2021, 140, 107815. [Google Scholar] [CrossRef] [PubMed]
- Fekry, A.M.; Abdel-Gawad, S.A.; Tammam, R.H.; Zayed, M.A. An electrochemical sensor for creatinine based on carbon nanotubes/folic acid /silver nanoparticles modified electrode. Measurement 2020, 163, 107958. [Google Scholar] [CrossRef]
- Stasyuk, N.Y.; Gayda, G.Z.; Zakalskiy, A.E.; Fayura, L.R.; Zakalska, O.M.; Sibirny, A.A.; Nisnevitch, M.; Gonchar, M.V. Amperometric biosensors for L-arginine and creatinine assay based on recombinant deiminases and ammonium-sensitive Cu/Zn(Hg)S nanoparticles. Talanta 2022, 238, 122996. [Google Scholar] [CrossRef]
- Shin, J.H.; Choi, Y.S.; Lee, H.J.; Choi, S.H.; Ha, J.; Yoon, I.J.; Nam, H.; Cha, G.S. A Planar Amperometric Creatinine Biosensor Employing an Insoluble Oxidizing Agent for Removing Redox-Active Interferences. Anal. Chem. 2001, 73, 5965–5971. [Google Scholar] [CrossRef] [PubMed]
- Ciou, D.-S.; Wu, P.-H.; Huang, Y.-C.; Yang, M.-C.; Lee, S.-Y.; Lin, C.-Y. Colorimetric and amperometric detection of urine creatinine based on the ABTS radical cation modified electrode. Sens. Actuators B Chem. 2020, 314, 128034. [Google Scholar] [CrossRef]
- Dong, Y.; Luo, X.; Liu, Y.; Yan, C.; Li, H.; Lv, J.; Yang, L.; Cui, Y. A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 2022, 248, 123592. [Google Scholar] [CrossRef]
- Zhybak, M.; Beni, V.; Vagin, M.Y.; Dempsey, E.; Turner, A.P.F.; Korpan, Y. Creatinine and urea biosensors based on a novel ammonium ion-selective copper-polyaniline nano-composite. Biosens. Bioelectron. 2016, 77, 505–511. [Google Scholar] [CrossRef]
- Tzianni, Ε.I.; Moutsios, I.; Moschovas, D.; Avgeropoulos, A.; Govaris, K.; Panagiotidis, L.; Prodromidis, M.I. Smartphone paired SIM card-type integrated creatinine biosensor. Biosens. Bioelectron. 2022, 207, 114204. [Google Scholar] [CrossRef]
- Caliskan, S.; Yildirim, E.; Anakok, D.A.; Cete, S. Design of a new biosensor platform for creatinine determination. J. Solid State Electrochem. 2022, 26, 549–557. [Google Scholar] [CrossRef]
- Collison, M.E.; Meyerhoff, M.E. Continuous-flow enzymatic determination of creatinine with improved on-line removal of endogenous ammonia. Anal. Chim. Acta 1987, 200, 61–72. [Google Scholar] [CrossRef]
- Kumar, P.; Kamboj, M.; Jaiwal, R.; Pundir, C.S. Fabrication of an improved amperometric creatinine biosensor based on enzymes nanoparticles bound to Au electrode. Biomarkers 2019, 24, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cheng, S.; Korin, Y.; Reed, E.F.; Gjertson, D.; Ho, C.; Gritsch, H.A.; Veale, J. Serum Creatinine Detection by a Conducting-Polymer-Based Electrochemical Sensor To Identify Allograft Dysfunction. Anal. Chem. 2012, 84, 7933–7937. [Google Scholar] [CrossRef] [PubMed]
- Nontawong, N.; Amatatongchai, M.; Thimoonnee, S.; Laosing, S.; Jarujamrus, P.; Karuwan, C.; Chairam, S. Novel amperometric flow-injection analysis of creatinine using a molecularly-imprinted polymer coated copper oxide nanoparticle-modified carbon-paste-electrode. J. Pharm. Biomed. Anal. 2019, 175, 112770. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Ahmad, R.; Khan, A.A.; Lee, N.E.; Lee, J.; Shah, A.U.; Khan, M.; Ali, T.; Ali, G.; Khan, Q.; et al. Anodic SnO2 Nanoporous Structure Decorated with Cu2O Nanoparticles for Sensitive Detection of Creatinine: Experimental and DFT Study. ACS Omega 2022, 7, 42377–42395. [Google Scholar] [CrossRef] [PubMed]
- Corba, A.; Sierra, A.F.; Blondeau, P.; Giussani, B.; Riu, J.; Ballester, P.; Andrade, F.J. Potentiometric detection of creatinine in the presence of nicotine: Molecular recognition, sensing and quantification through multivariate regression. Talanta 2022, 246, 123473. [Google Scholar] [CrossRef]
- Nakazato, K. Potentiometric, amperometric, and impedimetric CMOS biosensor array. In State of the Art in Biosensors: General Aspects; Intech: Rijeka, Croatia, 2013. [Google Scholar]
- Suzuki, H.; Matsugi, Y. Integrated microfluidic system for the simultaneous determination of ammonia, creatinine, and urea. Sens. Actuators B Chem. 2005, 108, 700–707. [Google Scholar] [CrossRef]
- Satoh, W.; Hosono, H.; Yokomaku, H.; Morimoto, K.; Upadhyay, S.; Suzuki, H. Integrated electrochemical analysis system with microfluidic and sensing functions. Sensors 2008, 8, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Komaba, S.; Amano, A.; Fujino, Y.; Mori, H. Electrochemical molecular sieving of the polyion complex film for designing highly sensitive biosensor for creatinine. Sens. Actuators B Chem. 2000, 65, 58–63. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsugi, Y. Microfabricated flow system for ammonia and creatinine with an air-gap structure. Sens. Actuators B Chem. 2004, 98, 101–111. [Google Scholar] [CrossRef]
- Pandey, P.C.; Mishra, A.P. Novel potentiometric sensing of creatinine. Sens. Actuators B Chem. 2004, 99, 230–235. [Google Scholar] [CrossRef]
- Patel, A.K.; Sharma, P.S.; Prasad, B.B. Development of a Creatinine Sensor Based on a Molecularly Imprinted Polymer-Modified Sol-Gel Film on Graphite Electrode. Electroanalysis 2008, 20, 2102–2112. [Google Scholar] [CrossRef]
- Lakshmi, D.; Sharma, P.S.; Prasad, B.B. Imprinted polymer-modified hanging mercury drop electrode for differential pulse cathodic stripping voltammetric analysis of creatine. Biosens. Bioelectron. 2007, 22, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, M.; Rechnitz, G.A. An activated enzyme electrode for creatinine. Anal. Chim. Acta 1976, 85, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Radomska, A.; Bodenszac, E.; Głąb, S.; Koncki, R. Creatinine biosensor based on ammonium ion selective electrode and its application in flow-injection analysis. Talanta 2004, 64, 603–608. [Google Scholar] [CrossRef]
- Shih, Y.-T.; Huang, H.-J. A creatinine deiminase modified polyaniline electrode for creatinine analysis. Anal. Chim. Acta 1999, 392, 143–150. [Google Scholar] [CrossRef]
- Liu, Y.; Cánovas, R.; Crespo, G.A.; Cuartero, M. Thin-Layer Potentiometry for Creatinine Detection in Undiluted Human Urine Using Ion-Exchange Membranes as Barriers for Charged Interferences. Anal. Chem. 2020, 92, 3315–3323. [Google Scholar] [CrossRef]
- Marchenko, S.V.; Soldatkin, O.O.; Kasap, B.O.; Kurc, B.A.; Soldatkin, A.P.; Dzyadevych, S.V. Creatinine Deiminase Adsorption onto Silicalite-Modified pH-FET for Creation of New Creatinine-Sensitive Biosensor. Nanoscale Res. Lett. 2016, 11, 173. [Google Scholar] [CrossRef]
- Ozansoy Kasap, B.; Marchenko, S.V.; Soldatkin, O.O.; Dzyadevych, S.V.; Akata Kurc, B. Biosensors Based on Nano-Gold/Zeolite-Modified Ion Selective Field-Effect Transistors for Creatinine Detection. Nanoscale Res. Lett. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Naresh Kumar, T.; Ananthi, A.; Mathiyarasu, J.; Joseph, J.; Lakshminarasimha Phani, K.; Yegnaraman, V. Enzymeless creatinine estimation using poly(3,4-ethylenedioxythiophene)-β-cyclodextrin. J. Electroanal. Chem. 2011, 661, 303–308. [Google Scholar] [CrossRef]
- Wen, T.; Zhu, W.; Xue, C.; Wu, J.; Han, Q.; Wang, X.; Zhou, X.; Jiang, H. Novel electrochemical sensing platform based on magnetic field-induced self-assembly of Fe3O4@Polyaniline nanoparticles for clinical detection of creatinine. Biosens. Bioelectron. 2014, 56, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Hernández-Alonso, D.; Adriaenssens, L.; Blondeau, P.; Rius, F.X.; Ballester, P.; Andrade, F.J. Characterization of a new ionophore-based ion-selective electrode for the potentiometric determination of creatinine in urine. Biosens. Bioelectron. 2017, 87, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Khasanah, M.; Handajani, U.S.; Widati, A.A.; Abdulloh, A.; Rindarti, R.R. Construction and Performance of Creatinine Selective Electrode based on Carbon Paste-Imprinting Zeolite. Anal. Bioanal. Electrochem. 2018, 10, 429–438. [Google Scholar]
- Saidi, T.; Moufid, M.; Zaim, O.; El Bari, N.; Bouchikhi, B. Voltammetric electronic tongue combined with chemometric techniques for direct identification of creatinine level in human urine. Measurement 2018, 115, 178–184. [Google Scholar] [CrossRef]
- Gao, X.; Gui, R.; Guo, H.; Wang, Z.; Liu, Q. Creatinine-induced specific signal responses and enzymeless ratiometric electrochemical detection based on copper nanoparticles electrodeposited on reduced graphene oxide-based hybrids. Sens. Actuators B Chem. 2019, 285, 201–208. [Google Scholar] [CrossRef]
- Pedrozo-Peñafiel, M.J.; Lópes, T.; Gutiérrez-Beleño, L.M.; Da Costa, M.E.H.M.; Larrudé, D.G.; Aucelio, R.Q. Voltammetric determination of creatinine using a gold electrode modified with Nafion mixed with graphene quantum dots-copper. J. Electroanal. Chem. 2020, 878, 114561. [Google Scholar] [CrossRef]
- Sriramprabha, R.; Sekar, M.; Revathi, R.; Viswanathan, C.; Wilson, J. Fe2O2/polyaniline supramolecular nanocomposite: A receptor free sensor platform for the quantitative determination of serum creatinine. Anal. Chim. Acta 2020, 1137, 103–114. [Google Scholar] [CrossRef]
- Kumar, R.K.R.; Shaikh, M.O.; Kumar, A.; Liu, C.-H.; Chuang, C.-H. Zwitterion-Functionalized Cuprous Oxide Nanoparticles for Highly Specific and Enzymeless Electrochemical Creatinine Biosensing in Human Serum. ACS Appl. Nano Mater. 2023, 6, 2083–2094. [Google Scholar] [CrossRef]
- Reddy, K.K.; Gobi, K.V. Artificial molecular recognition material based biosensor for creatinine by electrochemical impedance analysis. Sens. Actuators B Chem. 2013, 183, 356–363. [Google Scholar] [CrossRef]
- Isildak, I.; Cubuk, O.; Altikatoglu, M.; Yolcu, M.; Erci, V.; Tinkilic, N. A novel conductometric creatinine biosensor based on solid-state contact ammonium sensitive PVC–NH2 membrane. Biochem. Eng. J. 2012, 62, 34–38. [Google Scholar] [CrossRef]
- Braiek, M.; Djebbi, M.A.; Chateaux, J.-F.; Bonhomme, A.; Vargiolu, R.; Bessueille, F.; Jaffrezic-Renault, N. A conductometric creatinine biosensor prepared through contact printing of polyvinyl alcohol/polyethyleneimine based enzymatic membrane. Microelectron. Eng. 2018, 187–188, 43–49. [Google Scholar] [CrossRef]
- Mohabbati-Kalejahi, E.; Azimirad, V.; Bahrami, M. Creatinine Diffusion Modeling in Capacitive Sensors. Sens. Imaging 2016, 17, 5. [Google Scholar] [CrossRef]
- Panasyuk-Delaney, T.; Mirsky, V.M.; Wolfbeis, O.S. Capacitive Creatinine Sensor Based on a Photografted Molecularly Imprinted Polymer. Electroanalysis 2002, 14, 221–224. [Google Scholar] [CrossRef]
- Guha, S.; Warsinke, A.; Tientcheu, C.M.; Schmalz, K.; Meliani, C.; Wenger, C. Label free sensing of creatinine using a 6 GHz CMOS near-field dielectric immunosensor. Analyst 2015, 140, 3019–3027. [Google Scholar] [CrossRef]
- Sawaira, T.; Jamil, A.; Aziz, S.; Mujahid, A.; Hussain, T.; Afzal, A. Low-cost, disposable sensors based on polystyrene waste and V–TiO2 microneedle composites for diagnosis of renal malfunction and muscle dystrophy. Compos. Commun. 2023, 37, 101398. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Andersen, J.E.T.; Zachau-Christiansen, B. Improved Performance of the Potentiometric Biosensor for the Determination of Creatinine. Anal. Lett. 2007, 40, 39–52. [Google Scholar] [CrossRef]
- Hsiung, S.-K.; Chou, J.-C.; Sun, T.-P.; Pan, C.-W.; Chou, N.-H. Dual Type Potentiometric Creatinine Biosensor. U.S. Patent 7,758,733, 20 July 2010. [Google Scholar]
- Serafín, V.; Hernández, P.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensor for creatinine based on the immobilization of creatininase, creatinase and sarcosine oxidase onto a ferrocene/horseradish peroxidase/gold nanoparticles/multi-walled carbon nanotubes/Teflon composite electrode. Electrochim. Acta 2013, 97, 175–183. [Google Scholar] [CrossRef]
- Elmosallamy, M.A.F. New potentiometric sensors for creatinine. Anal. Chim. Acta 2006, 564, 253–257. [Google Scholar] [CrossRef]
- Yadav, S.; Devi, R.; Bhar, P.; Singhla, S.; Pundir, C.S. Immobilization of creatininase, creatinase and sarcosine oxidase on iron oxide nanoparticles/chitosan-g-polyaniline modified Pt electrode for detection of creatinine. Enzym. Microb. Technol. 2012, 50, 247–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Liu, X.; Zhang, Y.; Wang, D. Molecular Imprinting Electrochemical Sensor for Sensitive Creatinine Determination. Int. J. Electrochem. Sci. 2018, 13, 2986–2995. [Google Scholar] [CrossRef]
- Marchenko, S.V.; Kucherenko, I.S.; Soldatkin, O.O.; Soldatkin, A.P. Potentiometric Biosensor System Based on Recombinant Urease and Creatinine Deiminase for Urea and Creatinine Determination in Blood Dialysate and Serum. Electroanalysis 2015, 27, 1699–1706. [Google Scholar] [CrossRef]
- Pandey, I.; Bairagi, P.K.; Verma, N. Electrochemically grown polymethylene blue nanofilm on copper-carbon nanofiber nanocomposite: An electrochemical sensor for creatinine. Sens. Actuators B Chem. 2018, 277, 562–570. [Google Scholar] [CrossRef]
- Marchenko, S.V.; Piliponskiy, I.I.; Mamchur, O.O.; Soldatkin, O.O.; Kucherenko, I.S.; Kasap, B.O.; Kurç, B.A.; Dzyadevych, S.V.; Soldatkin, A.P. Development of a New Biosensor by Adsorption of Creatinine Deiminase on Monolayers of Micro- and Nanoscale Zeolites. In Nanophysics, Nanomaterials, Interface Studies, and Applications; Fesenko, O., Yatsenko, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 573–584. [Google Scholar]
- Rui, C.-S.; Kato, Y.; Sonomoto, K. Amperometric Flow-Injection Biosensor System for the Simultaneous Determination of Urea and Creatinine. Anal. Sci. 1992, 8, 845–850. [Google Scholar] [CrossRef]
- Kalaivani, G.J.; Suja, S.K. Enzyme-less sensing of the kidney dysfunction biomarker creatinine using an inulin based bio-nanocomposite. New J. Chem. 2019, 43, 5914–5924. [Google Scholar] [CrossRef]
- Ortiz, M.; Botero, M.L.; Fragoso, A.; O’Sullivan, C.K. Amperometric Detection of Creatinine in Clinical Samples Based on Gold Electrode Arrays Fabricated Using Printed Circuit Board Technology. Electroanalysis 2020, 32, 3054–3059. [Google Scholar] [CrossRef]
- Nur Ashakirin, S.; Zaid, M.H.M.; Haniff, M.A.S.M.; Masood, A.; Mohd Razip Wee, M.F. Sensitive electrochemical detection of creatinine based on electrodeposited molecular imprinting polymer modified screen printed carbon electrode. Measurement 2023, 210, 112502. [Google Scholar] [CrossRef]
- Han, P.; Xu, S.; Feng, S.; Hao, Y.; Wang, J. Direct determination of creatinine based on poly(ethyleneimine)/phosphotungstic acid multilayer modified electrode. Talanta 2016, 151, 114–118. [Google Scholar] [CrossRef]
- Raveendran, J.; Resmi, P.E.; Ramachandran, T.; Nair, B.G.; Babu, T.S. Fabrication of a disposable non-enzymatic electrochemical creatinine sensor. Sens. Actuators B Chem. 2017, 243, 589–595. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R.; Stanly, N. Fabrication of a molecularly imprinted silylated graphene oxide polymer for sensing and quantification of creatinine in blood and urine samples. Appl. Surf. Sci. 2019, 466, 28–39. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saddique, Z.; Faheem, M.; Habib, A.; UlHasan, I.; Mujahid, A.; Afzal, A. Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics 2023, 13, 1737. https://doi.org/10.3390/diagnostics13101737
Saddique Z, Faheem M, Habib A, UlHasan I, Mujahid A, Afzal A. Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics. 2023; 13(10):1737. https://doi.org/10.3390/diagnostics13101737
Chicago/Turabian StyleSaddique, Zohaib, Muhammad Faheem, Amir Habib, Iftikhar UlHasan, Adnan Mujahid, and Adeel Afzal. 2023. "Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders" Diagnostics 13, no. 10: 1737. https://doi.org/10.3390/diagnostics13101737
APA StyleSaddique, Z., Faheem, M., Habib, A., UlHasan, I., Mujahid, A., & Afzal, A. (2023). Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics, 13(10), 1737. https://doi.org/10.3390/diagnostics13101737






