Vascular Changes in the Macula of Patients after Previous Episodes of Vision Loss Due to Leber Hereditary Optic Neuropathy and Non-Arteritic Ischemic Optic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type
2.2. Patient Characterisctics
2.3. OCT-A Examination
2.4. Statistical Analysis
3. Results
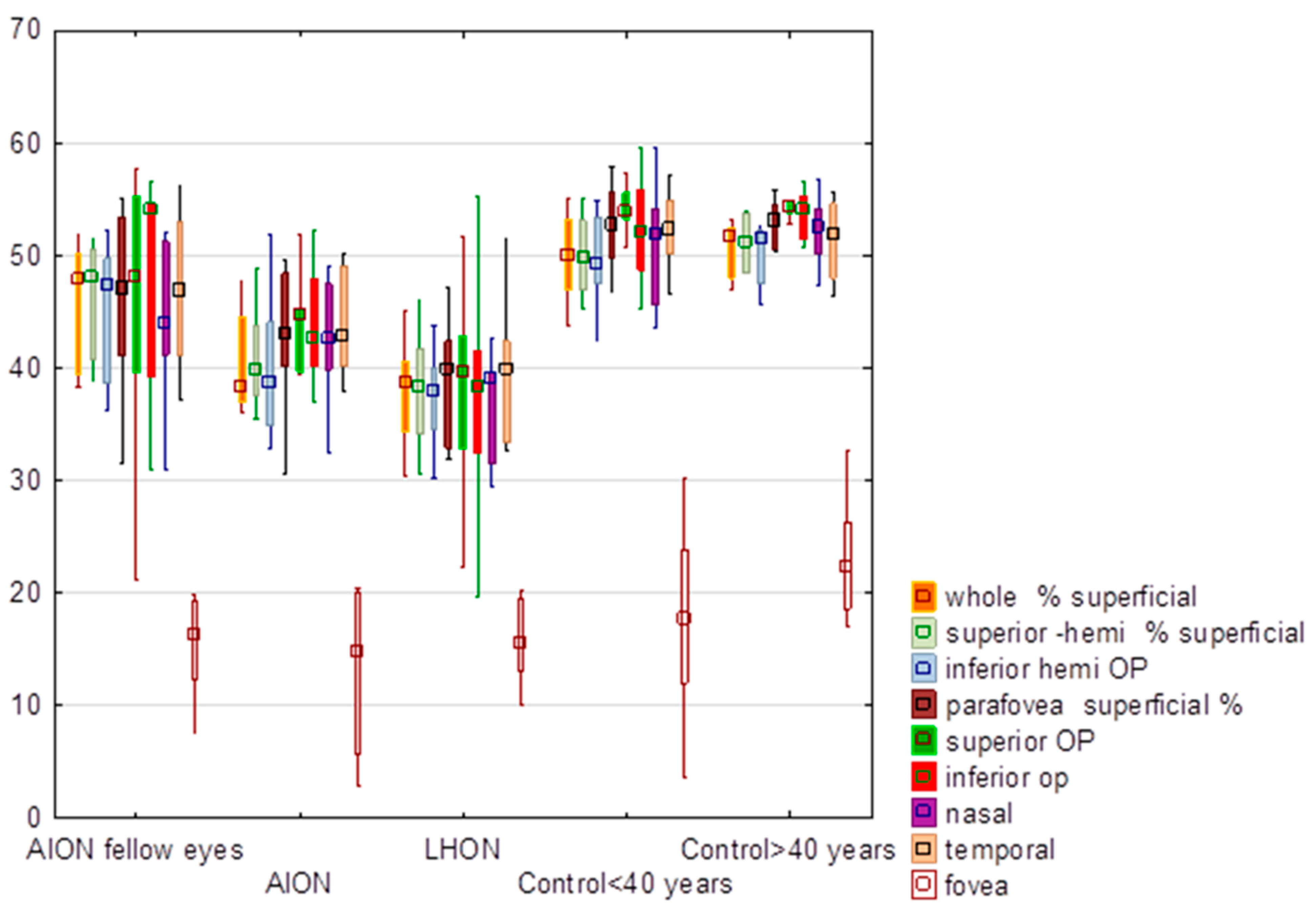
3.1. Superficial Vessel Density
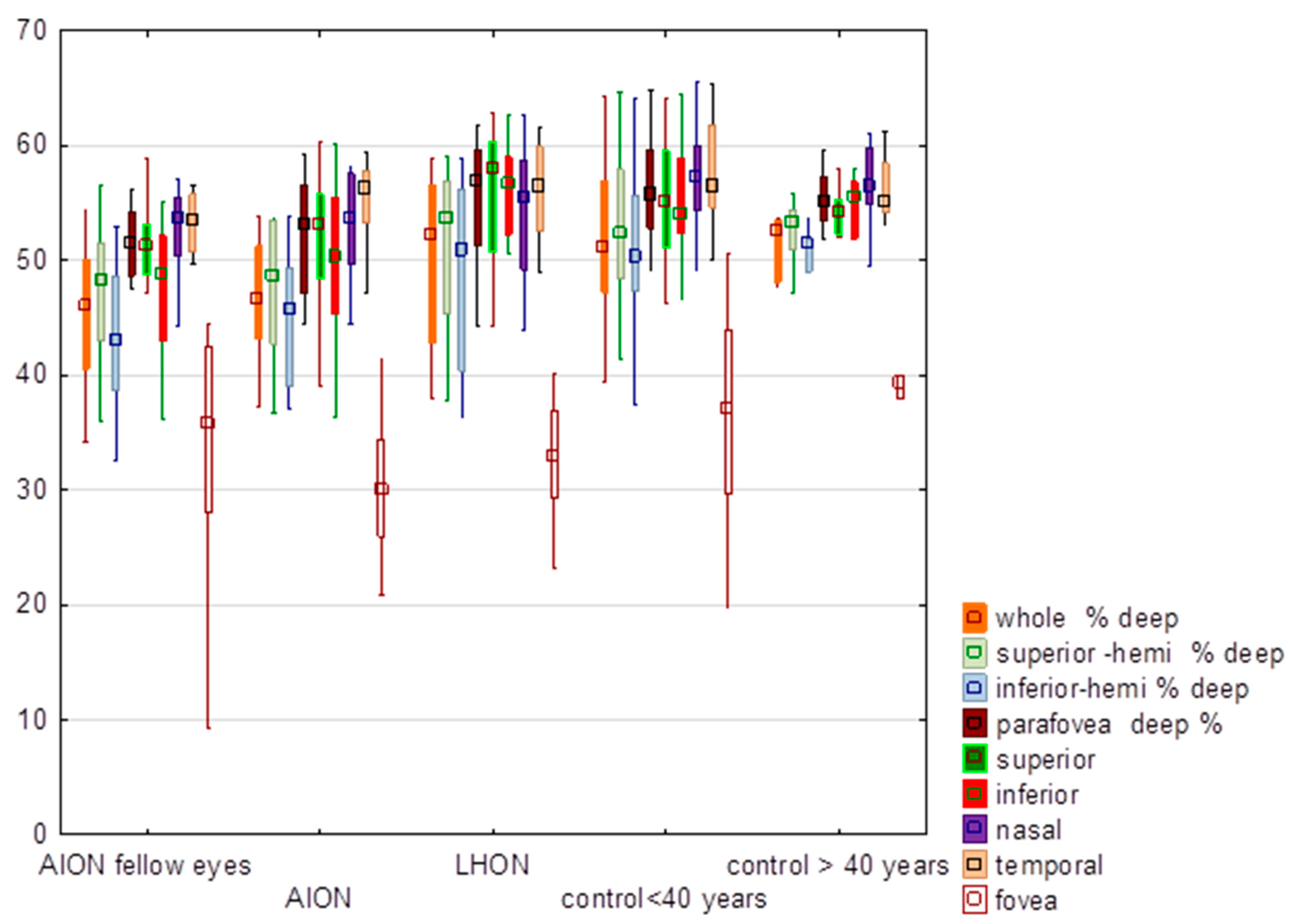
3.2. Deep Vessel Density
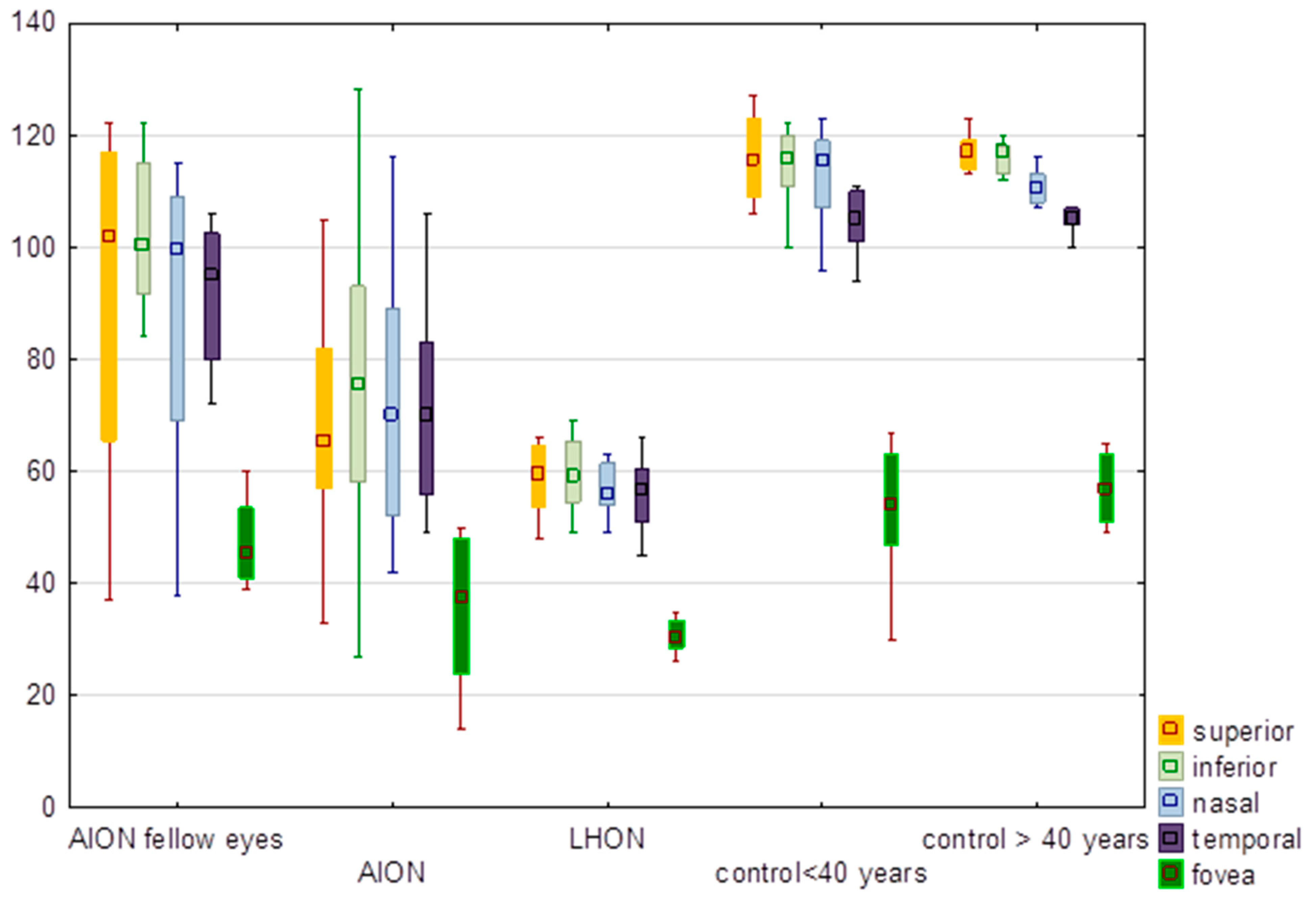
3.3. Inner Thickness of the Retina
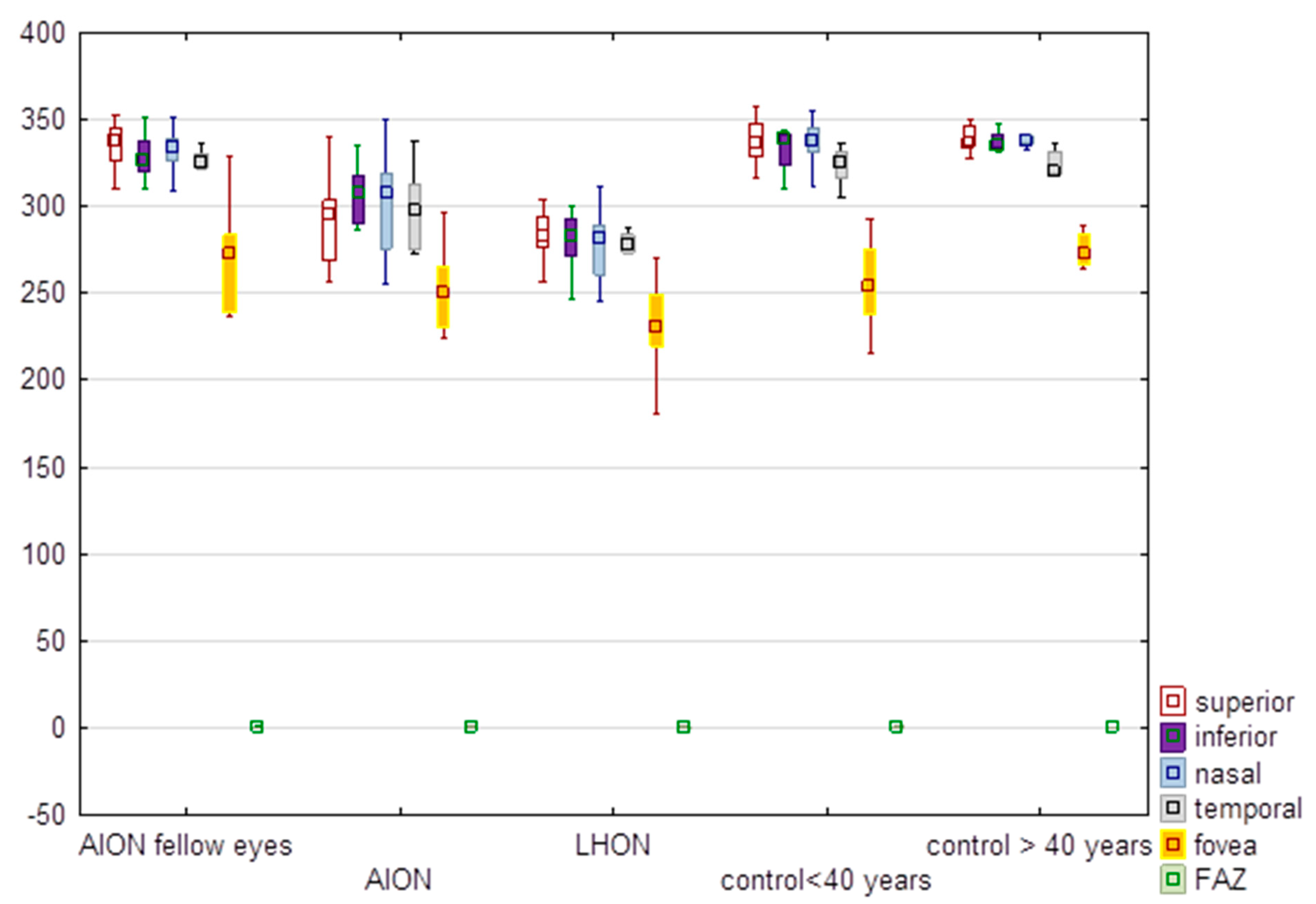
3.4. Full Thickness of the Retina and FAZ
3.5. Comparison of Superior and Inferior Hemifields of the Macula
3.6. Visual Function Results
3.7. Examples of Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiggs, J.L. Mitochondrial genetics and optic neuropathy. Annu. Rev. Vis. Sci. 2015, 1, 97–124. [Google Scholar] [CrossRef]
- Mackey, D.A.; Oostra, R.J.; Rosenberg, T.; Nikoskelainen, E.; Bronte-Stewart, J.; Poulton, J.; Harding, E.A.; Govan, G.; Bolhuis, A.P.; Norby, S. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am. J. Hum. Genet. 1996, 59, 481–485. [Google Scholar] [PubMed]
- Sundaramurthy, S.; SelvaKumar, A.; Ching, J.; Dharani, V.; Sarangapani, S.; Yu-Wai-Man, P. Leber hereditary optic neuropathy-new insights and old challenges. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2461–2472. [Google Scholar]
- Sadun, F.; De Negri, A.M.; Carelli, V.; Salomao, S.R.; Berezovsky, A.; Andrade, R.; Moraes, M.; Passos, A.; Belfort, R.; Da Rosa, A.B.; et al. Ophthalmologic findings in a large pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am. J. Ophthalmol. 2004, 137, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.d.V.; Bellusci, C.; Savini, G.; Carbonelli, M.; Berezovsky, A.; Tamaki, C.; Cinoto, R.; Sacai, P.Y.; Moraes-Filho, M.N.; Miura, H.M.; et al. Association of optic disc size with development and prognosis of Leber’s hereditary optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1666–1674. [Google Scholar] [CrossRef]
- Castillo, L.; Berrozpe-Villabona, C.; Miserachs-García, S.; Haulani, H.; Gómez-Gutiérrez, C.; Díaz-García, R.S.; González-Martínez, A.; Fernández-Sanz, G.; Morilla-Grasa, A.; García, V.; et al. Quantitative assessment of macular and circumpapillary retinal vessel density across all stages of Leber hereditary optic neuropathy using swept source optical coherence tomography angiography. Acta Ophthalmol. 2022, 6, e1646–e1656. [Google Scholar] [CrossRef]
- Kousal, B.; Kolarova, H.; Meliska, M.; Bydzovsky, J.; Diblik, P.; Kulhanek, J.; Votruba, M.; Honzik, T.; Liskova, P. Peripapillary microcirculation in Leber hereditary optic neuropathy. Acta Ophthalmol. 2019, 97, e71–e76. [Google Scholar] [CrossRef]
- Takayama, K.; Ito, Y.; Kaneko, H.; Kataoka, K.; Ra, E.; Terasaki, H. Optical coherence tomography angiography in Leber hereditary optic neuropathy. Acta Ophthalmol. 2017, 95, e344–e345. [Google Scholar] [CrossRef]
- Borrelli, E.; Balasubramanian, S.; Triolo, G.; Barboni, P.; Sadda, S.R.; Sadun, A.A. Topographic Macular Microvascular Changes and Correlation with Visual Loss in Chronic Leber Hereditary Optic Neuropathy. Am. J. Ophthalmol. 2018, 192, 217–228. [Google Scholar] [CrossRef]
- Asanad, S.; Meer, E.; Fantini, M.; Borrelli, E.; Sadun, A.A. Leber’s hereditary optic neuropathy: Shifting our attention to the macula. Am. J. Ophthalmol. Case Rep. 2018, 13, 13–15. [Google Scholar] [CrossRef]
- Hayreh, S.S. Ischemic optic neuropathy. Prog. Retin. Eye Res. 2009, 28, 34–62. [Google Scholar] [CrossRef]
- Arnold, A.C. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J. Neuroophthalmol. 2003, 23, 157–163. [Google Scholar] [CrossRef]
- Tournaire-Marques, E. Ischemic optic neuropathies. J. Fr. Ophtalmol. 2020, 43, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tsokolas, G.; Tsaousis, K.T.; Diakonis, V.F.; Matsou, A.; Tyradellis, S. Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review. Eye Brain 2020, 12, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Koman-Wierdak, E.; Nowomiejska, K.; Brzozowska, A.; Nowakowska, D.; Toro, M.D.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Rejdak, R. Kinetic and static perimetry after 16 years and additional OCT-A analysis in eyes with long-lasting optic disc drusen. PLoS ONE 2021, 16, e0247399. [Google Scholar] [CrossRef]
- Koman-Wierdak, E.; Róg, J.; Brzozowska, A.; Toro, M.D.; Bonfiglio, V.; Załuska-Ogryzek, K.; Karakuła-Juchnowicz, H.; Rejdak, R.; Nowomiejska, K. Analysis of the Peripapillary and Macular Regions Using OCT Angiography in Patients with Schizophrenia and Bipolar Disorder. J. Clin. Med. 2021, 10, 4131. [Google Scholar] [CrossRef]
- Noll, C.; Hiltensperger, M.; Aly, L.; Wicklein, R.; Afzali, A.M.; Mardin, C.; Gasperi, C.; Berthele, A.; Hemmer, B.; Korn, T.; et al. Association of the retinal vasculature, intrathecal immunity, and disability in multiple sclerosis. Front. Immunol. 2022, 13, 997043. [Google Scholar] [CrossRef]
- Cesareo, M.; Giannini, C.; Di Marino, M.; Aloe, G.; Martucci, A.; Aiello, F.; Cusumano, A.; Mancino, R.; Ricci, F.; Sorge, R.P.; et al. Optical coherence tomography angiography in the multimodal assessment of the retinal posterior pole in autosomal dominant optic atrophy. Acta Ophthalmol. 2022, 100, e798–e806. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Shin, J.W.; Lee, J.Y.; Baek, M.S.; Kook, M.S. Association of Superficial and Deep Macular Microvasculature with Central Visual Field Sensitivity in Glaucomatous Eyes with High Myopia. J. Clin. Med. 2022, 11, 4430. [Google Scholar] [CrossRef]
- Carelli, V.; La Morgia, C.; Valentino, M.L.; Barboni, P.; Ross-Cisneros, F.N.; Sadun, A.A. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta 2009, 1787, 518–528. [Google Scholar] [CrossRef]
- Balducci, N.; Cascavilla, M.L.; Ciardella, A.; La Morgia, C.; Triolo, G.; Parisi, V.; Bandello, F.; A Sadun, A.; Carelli, V.; Barboni, P. Peripapillary vessel density changes in Leber’s hereditary optic neuropathy: A new biomarker. Clin. Exp. Ophthalmol. 2018, 46, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gaier, E.D.; Wang, M.; Gilbert, A.L.; Rizzo, J.F., 3rd; Cestari, D.M.; Miller, J.B. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PLoS ONE. 2018, 13, e0199793. [Google Scholar] [CrossRef]
- Yu, J.; Xu, H.; Huang, Y.; Gu, R.; Zong, Y.; Zhu, H.; Wang, M. Changes in Retinal Perfusion in Leber’s Hereditary Optic Neuropathy: An Optical Coherence Tomography-Angiography Study. Ophthalmic Res. 2021, 64, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Calzetti, G.; La Morgia, C.; Cattaneo, M.; Carta, A.; Bosello, F.; Amore, G.; Carbonelli, M.; Cascavilla, M.L.; Gandolfi, S.; Carelli, V.; et al. Longitudinal Study of Optic Disk Perfusion and Retinal Structure in Leber’s Hereditary Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2022, 63, 43. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Zhang, B.; Ma, J.; Zhong, Y. Microvascular alterations detected by optical coherence tomography angiography in non-arteritic anterior ischaemic optic neuropathy: A meta-analysis. Acta Ophthalmol. 2022, 100, e386–e395. [Google Scholar] [CrossRef]
- Rougier, M.B.; Gattoussi, S.; Le-Goff, M.; Korobelnik, J.F. OCT angiography analysis in acute non-arteritic anterior ischemic optic neuropathy: The importance of segmentation. Eur. J. Ophthalmol. 2021, 31, 3471–3475. [Google Scholar] [CrossRef]
- Akbari, M.; Abdi, P.; Fard, M.A.; Afzali, M.; Ameri, A.; Yazdani-Abyaneh, A.; Mohammadi, M.; Moghimi, S. Retinal ganglion cell loss precedes retinal nerve fiber thinning in nonarteritic anterior ischemic optic neuropathy. J. Neuroophthalmol. 2016, 36, 141–146. [Google Scholar] [CrossRef]
- Fard, M.A.; Ghahvechian, H.; Sahrayan, A.; Subramanian, P.S. Early Macular Vessel Density Loss in Acute Ischemic Optic Neuropathy Compared to Papilledema: Implications for Pathogenesis. Transl. Vis. Sci. Technol. 2018, 7, 10. [Google Scholar] [CrossRef]
- Petrovic Pajic, S.; Lapajne, L.; Vratanar, B.; Fakin, A.; Jarc-Vidmar, M.; Habjan, M.S.; Volk, M.; Maver, A.; Peterlin, B.; Hawlina, M. The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy. J. Clin. Med. 2022, 11, 6045. [Google Scholar] [CrossRef]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.K.; Durbin, M.; Kardon, R. Retinal Ganglion Cell Layer Thinning Within One Month of Presentation for Non-Arteritic Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3588–3593. [Google Scholar]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.K.; Durbin, M.; Kardon, R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult. Scler. 2016, 22, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Pierro, L.; Arrigo, A.; Aragona, E.; Cavalleri, M.; Bandello, F. Vessel Density and Vessel Tortuosity Quantitative Analysis of Arteritic and Non-arteritic Anterior Ischemic Optic Neuropathies: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Primiano, G.; Abed, E.; Corbo, G.; Minnella, A.M.; Servidei, S.; Vollono, C.; Savastano, M.C.; Falsini, B. Macular impairment in mitochondrial diseases: A potential biomarker of disease severity. Sci. Rep. 2020, 10, 8554. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.A.; Yadegari, S.; Ghahvechian, H.; Moghimi, S.; Soltani-Moghaddam, R.; Subramanian, P.S. Optical Coherence Tomography Angiography of a Pale Optic Disc in Demyelinating Optic Neuritis and Ischemic Optic Neuropathy. J. Neuro-Ophthalmol. 2019, 39, 339–344. [Google Scholar] [CrossRef]
- Borrelli, E.; Triolo, G.; Cascavilla, M.L.; La Morgia, C.; Rizzo, G.; Savini, G.; Balducci, N.; Nucci, P.; Giglio, R.; Darvizeh, F.; et al. Changes in Choroidal Thickness follow the RNFL Changes in Leber’s Hereditary Optic Neuropathy. Sci. Rep. 2016, 6, 37332. [Google Scholar] [CrossRef]
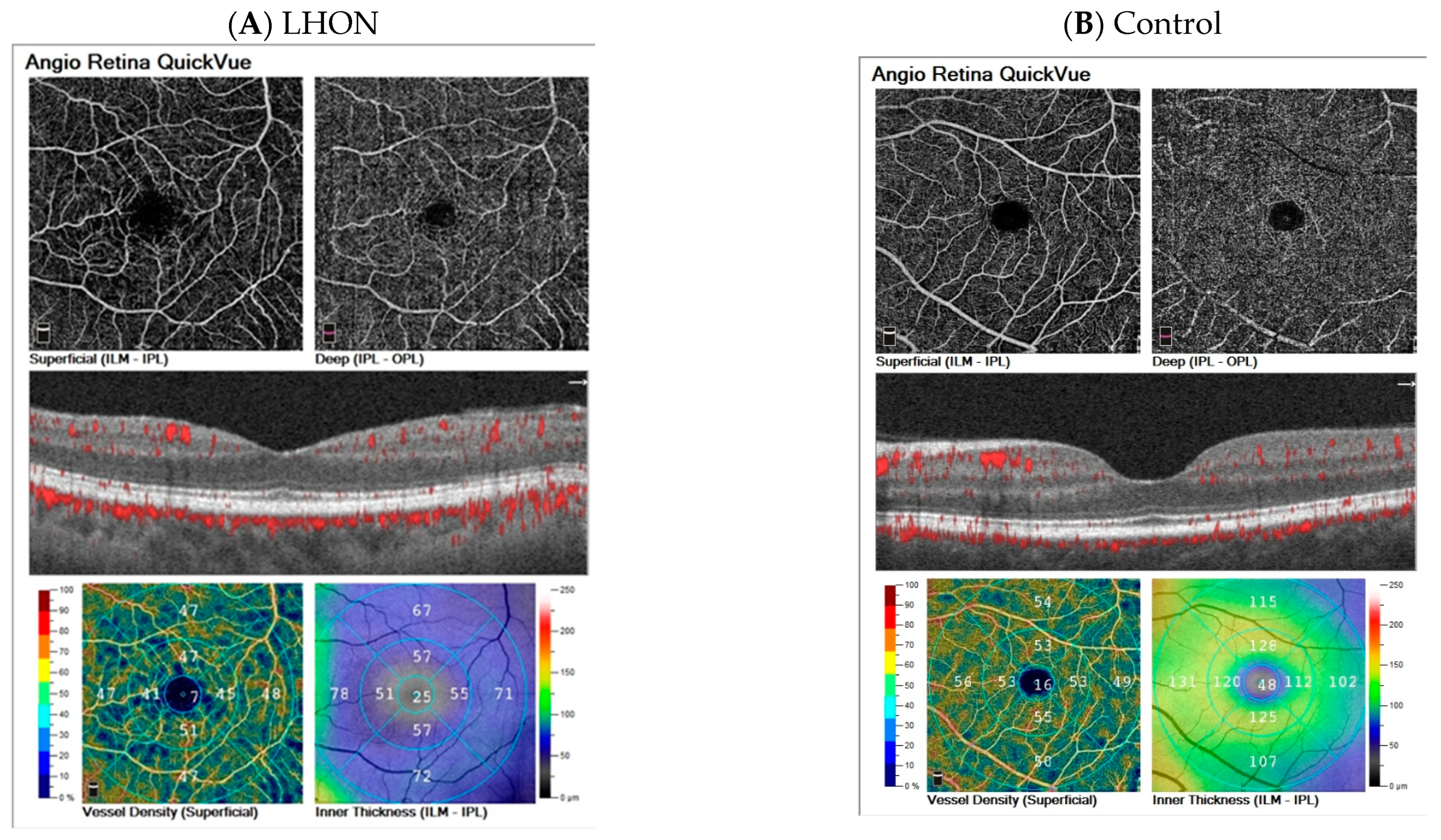
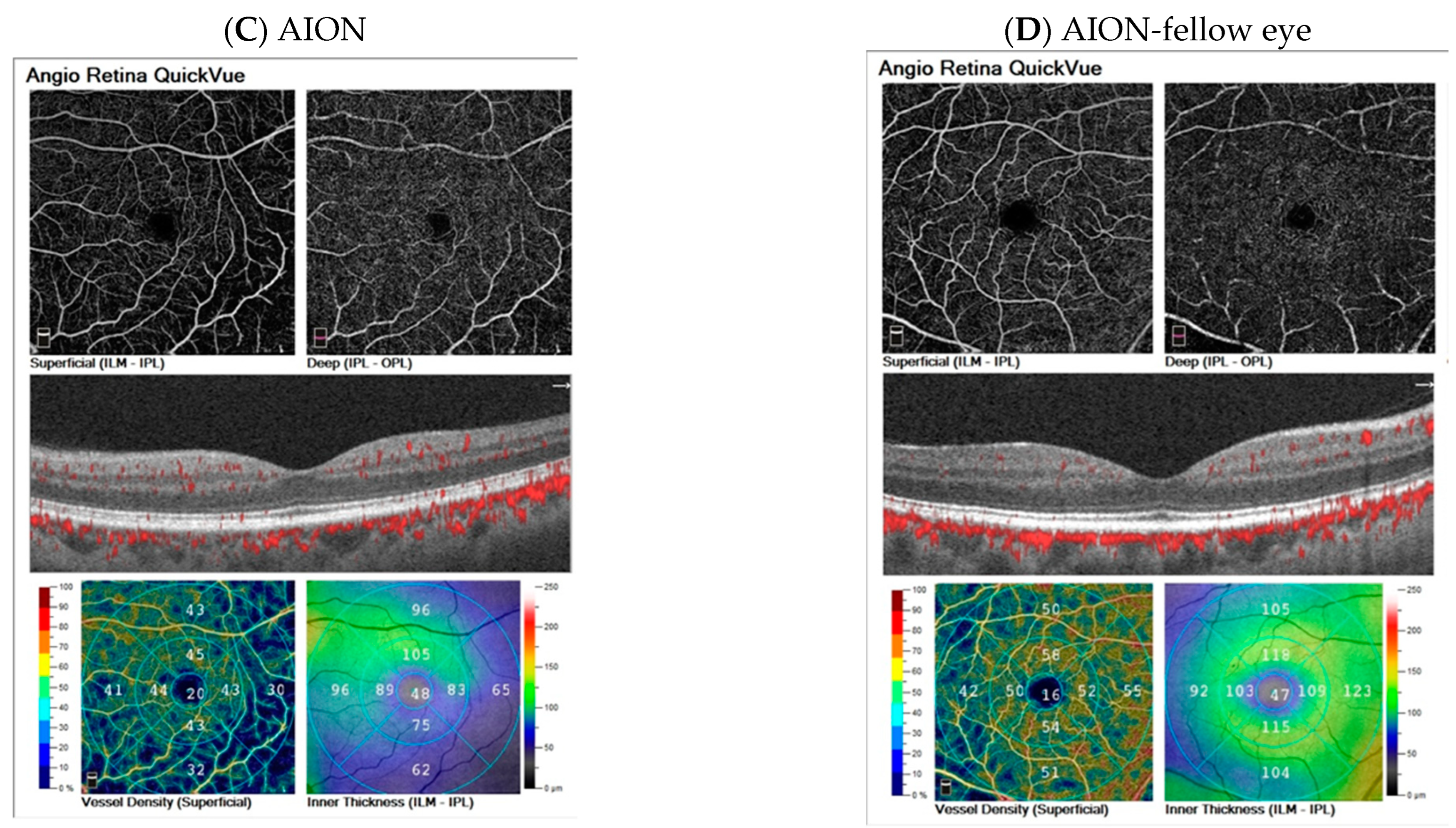
| Parameter | AION | AION Fellow Eye | LHON | Control < 40 Years | Control > 40 Years | Statistical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Kruskal–Wallis Test | Mann–Whitney Test for LHON and NA-AION Z | p | |
| Whole superficial | 40.35 | 38.30 | 4.48 | 45.60 | 47.75 | 5.63 | 36.41 | 38.65 | 8.39 | 50.05 | 50.05 | 3.48 | 50.65 | 51.65 | 2.55 | H = 29.63, p < 0.0001 * | −0.79 | 0.43 |
| Superior hemi superficial | 40.92 | 39.85 | 4.20 | 46.13 | 48.10 | 5.22 | 36.83 | 38.40 | 8.67 | 50.18 | 49.75 | 3.49 | 51.15 | 51.15 | 2.52 | H = 29.96, p < 0.0001 * | −0.92 | 0.36 |
| Inferior hemi | 39.74 | 38.65 | 5.98 | 45.00 | 47.35 | 6.23 | 35.91 | 37.85 | 8.16 | 49.90 | 49.15 | 3.67 | 50.15 | 51.45 | 2.92 | H = 27.75, p < 0.0001 * | −0.69 | 0.49 |
| Parafovea superficial | 42.67 | 42.90 | 5.82 | 46.23 | 47.10 | 8.24 | 37.19 | 39.80 | 8.71 | 52.42 | 52.75 | 3.69 | 52.92 | 53.10 | 2.14 | H = 28.46, p < 0.0001 * | −1.62 | 0.11 |
| Superior | 42.13 | 44.75 | 9.55 | 45.55 | 47.95 | 12.26 | 38.35 | 39.65 | 7.59 | 53.83 | 53.95 | 3.01 | 54.37 | 54.35 | 1.17 | H = 27.36, p < 0.0001 * | −1.68 | 0.09 |
| Inferior | 42.00 | 42.60 | 9.43 | 47.86 | 54.00 | 10.01 | 37.98 | 38.35 | 8.97 | 52.36 | 52.20 | 4.52 | 53.73 | 54.15 | 2.26 | H = 20.98, p = 0.0001 * | −1.52 | 0.13 |
| Nasal | 42.63 | 42.65 | 4.90 | 44.48 | 44.00 | 7.13 | 33.98 | 39.10 | 11.37 | 51.15 | 51.90 | 5.33 | 52.22 | 52.50 | 3.39 | H = 31.68, p < 0.0001 * | −2.80 | 0.01 |
| Temporal | 43.89 | 42.80 | 4.32 | 46.91 | 46.95 | 6.97 | 38.28 | 39.95 | 9.40 | 52.41 | 52.30 | 3.11 | 51.43 | 51.95 | 3.64 | H = 25.11, p < 0.0001 * | −1.58 | 0.11 |
| Fovea | 11.75 | 13.15 | 6.97 | 16.96 | 16.40 | 7.27 | 14.75 | 14.15 | 3.71 | 20.37 | 21.90 | 5.49 | 21.38 | 22.10 | 2.80 | H = 15.78 p = 0.003 * | 0.88 | 0.38 |
| Parameter | AION | AION Fellow Eye | LHON | Control < 40 Years | Control > 40 Years | Statistical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Kruskal–Wallis Test | Mann–Whitney Test for LHON and NA-AION Z | p | |
| Whole % deep | 46.15 | 46.50 | 5.87 | 45.24 | 46.05 | 6.84 | 47.63 | 52.10 | 13.54 | 51.57 | 51.10 | 6.36 | 51.33 | 52.60 | 2.74 | H = 8.06 p = 0.15 | 1.45 | 0.15 |
| Superior-hemi % deep | 47.48 | 48.65 | 6.37 | 47.23 | 48.20 | 6.57 | 48.94 | 53.65 | 13.11 | 52.46 | 52.30 | 6.40 | 52.48 | 53.30 | 3.05 | H = 7.37 p = 0.19 | 1.42 | 0.16 |
| Inferior-hemi % deep | 44.72 | 45.65 | 5.84 | 43.23 | 42.95 | 7.01 | 46.14 | 50.75 | 14.53 | 50.67 | 50.35 | 6.53 | 50.25 | 51.30 | 3.28 | H = 7.99 p = 0.16 | 1.42 | 0.16 |
| Parafovea deep % | 52.46 | 53.05 | 5.07 | 50.61 | 51.40 | 5.09 | 51.93 | 56.70 | 14.39 | 56.10 | 55.70 | 4.47 | 55.43 | 55.20 | 2.77 | H = 8.29 p = 0.14 | 0.96 | 0.34 |
| Superior | 52.17 | 53.15 | 5.90 | 50.66 | 51.15 | 5.33 | 53.53 | 57.90 | 11.62 | 55.39 | 55.05 | 5.16 | 54.32 | 54.20 | 2.21 | H = 8.09 p = 0.15 | 1.42 | 0.16 |
| Inferior | 50.05 | 50.35 | 7.64 | 47.38 | 48.75 | 6.39 | 51.72 | 56.60 | 14.64 | 54.73 | 54.05 | 5.40 | 54.90 | 55.45 | 2.67 | H = 9.71 p = 0.08 | 1.38 | 0.17 |
| Nasal | 53.05 | 53.55 | 4.47 | 52.50 | 53.65 | 4.20 | 50.11 | 55.40 | 16.48 | 57.05 | 57.25 | 4.02 | 56.33 | 56.40 | 4.09 | H = 7.44. p = 0.19 | 0.56 | 0.58 |
| Temporal | 54.57 | 56.20 | 4.88 | 51.95 | 53.40 | 5.51 | 52.48 | 56.50 | 15.09 | 57.29 | 56.40 | 4.07 | 56.17 | 55.15 | 3.10 | H = 7.67 p = 0.17 | 0.63 | 0.53 |
| Fovea | 30.20 | 29.90 | 6.26 | 33.23 | 35.70 | 11.73 | 31.28 | 32.90 | 8.26 | 36.44 | 37.00 | 9.15 | 39.33 | 39.30 | 6.18 | H = 7.46 p = 0.19 | 0.82 | 0.41 |
| Parameter | AION | AION Fellow Eye | LHON | Control < 40 Years | Control > 40 Years | Statistical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Kruskal–Wallis Test | Mann–Whitney Test for LHON and NA-AION Z | p | |
| Superior | 292.60 | 295.50 | 24.71 | 335.00 | 337.00 | 14.15 | 283.08 | 282.50 | 14.02 | 338.00 | 336.00 | 13.20 | 338.67 | 336.50 | 7.99 | H = 33.29. p < 0.0001 * | −1.12 | 0.26 |
| Inferior | 309.60 | 307.00 | 24.02 | 328.50 | 326.00 | 13.24 | 280.25 | 283.00 | 14.64 | 333.79 | 338.50 | 11.17 | 337.33 | 334.50 | 6.38 | H = 30.97. p < 0.0001 * | −2.90 | 0.004 * |
| Nasal | 302.70 | 307.50 | 28.86 | 332.38 | 333.50 | 12.84 | 277.67 | 281.50 | 20.79 | 336.43 | 338.00 | 13.21 | 339.50 | 337.50 | 6.66 | H = 30.59. p < 0.0001 * | −1.95 | 0.05 |
| Temporal | 298.60 | 297.00 | 23.43 | 323.13 | 324.50 | 12.52 | 276.25 | 278.00 | 12.61 | 323.79 | 325.50 | 10.34 | 324.50 | 320.50 | 7.97 | H = 27.76. p < 0.0001 * | −2.01 | 0.04 * |
| Fovea | 253.20 | 250.00 | 22.69 | 269.75 | 273.00 | 31.44 | 229.83 | 230.00 | 27.68 | 253.14 | 254.00 | 22.78 | 274.83 | 272.50 | 9.75 | H = 14.48. p = 0.006 * | −1.85 | 0.06 |
| Parameter | AION | AION Fellow Eye | LHON | Control < 40 Years | Control > 40 Years | Statistical Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Kruskall–Wallis Test | Mann–Whitney Test for LHON and NA-AION Z | p | |
| Superior | 292.60 | 295.50 | 24.71 | 335.00 | 337.00 | 14.15 | 283.08 | 282.50 | 14.02 | 338.00 | 336.00 | 13.20 | 338.67 | 336.50 | 7.99 | H = 33.29. p < 0.0001 * | −1.12 | 0.26 |
| Inferior | 309.60 | 307.00 | 24.02 | 328.50 | 326.00 | 13.24 | 280.25 | 283.00 | 14.64 | 333.79 | 338.50 | 11.17 | 337.33 | 334.50 | 6.38 | H = 30.97. p < 0.0001 * | −2.90 | 0.004 * |
| Nasal | 302.70 | 307.50 | 28.86 | 332.38 | 333.50 | 12.84 | 277.67 | 281.50 | 20.79 | 336.43 | 338.00 | 13.21 | 339.50 | 337.50 | 6.66 | H = 30.59. p < 0.0001 * | −1.95 | 0.05 |
| Temporal | 298.60 | 297.00 | 23.43 | 323.13 | 324.50 | 12.52 | 276.25 | 278.00 | 12.61 | 323.79 | 325.50 | 10.34 | 324.50 | 320.50 | 7.97 | H = 27.76. p < 0.0001 * | −2.01 | 0.04 * |
| Fovea | 253.20 | 250.00 | 22.69 | 269.75 | 273.00 | 31.44 | 229.83 | 230.00 | 27.68 | 253.14 | 254.00 | 22.78 | 274.83 | 272.50 | 9.75 | H = 14.48. p = 0.006 * | −1.85 | 0.06 |
| FAZ | 0.30 | 0.30 | 0.08 | 0.28 | 0.29 | 0.08 | 0.28 | 0.28 | 0.07 | 0.29 | 0.30 | 0.11 | 0.23 | 0.22 | 0.09 | H = 3.03. p = 0.55 | −0.69 | 0.49 |
| Parameter | NA-AION | AION Fellow Eyes | LHON | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | Mean | Median | Standard Deviation | |
| Vessel density Superficial | Z = 1.10; p = 0.92 | Z = 0.85; p = 0.40 | Z = 0.31; p = 0.75 | Z = 2.35; p = 0.02 * | ||||||||
| Superior | 42.13 | 44.75 | 9.55 | 45.55 | 42.13 | 44.75 | 9.55 | 45.55 | 42.13 | 44.75 | 9.55 | 45.55 |
| Inferior | 42.00 | 42.60 | 9.43 | 47.86 | 42.00 | 42.60 | 9.43 | 47.86 | 42.00 | 42.60 | 9.43 | 47.86 |
| Absolute value of the difference between hemifields | 3.35 | 2.70 | 2.43 | 5.56 | 3.35 | 2.70 | 2.43 | 5.56 | 3.35 | 2.70 | 2.43 | 5.56 |
| Statistical analysis | H = 5.42. p = 0.14 | |||||||||||
| Vessel density deep | Z = 1.48; p = 0.14 | Z = 2.10; p = 0.02 * | Z = 1.84; p = 0.07 | Z = 0.50; p = 0.61 | ||||||||
| Superior | 52.17 | 53.15 | 5.90 | 50.66 | 52.17 | 53.15 | 5.90 | 50.66 | 52.17 | 53.15 | 5.90 | 50.66 |
| Inferior | 50.05 | 50.35 | 7.64 | 47.38 | 50.05 | 50.35 | 7.64 | 47.38 | 50.05 | 50.35 | 7.64 | 47.38 |
| Absolute value of the difference between hemifields | 4.70 | 2.90 | 4.62 | 3.44 | 4.70 | 2.90 | 4.62 | 3.44 | 4.70 | 2.90 | 4.62 | 3.44 |
| Statistical analysis | H = 8.96. p = 0.03 *; no differences between groups | |||||||||||
| Inner thickness | Z = 0.95; p = 0.34 | Z = 0.42; p = 0.67 | Z = 0.67; p = 0.50 | Z = 1.41; p = 0.16 | ||||||||
| Superior | 71.90 | 65.50 | 26.09 | 91.00 | 71.90 | 65.50 | 26.09 | 91.00 | 71.90 | 65.50 | 26.09 | 91.00 |
| Inferior | 76.20 | 75.50 | 27.58 | 96.13 | 76.20 | 75.50 | 27.58 | 96.13 | 76.20 | 75.50 | 27.58 | 96.13 |
| Absolute value of the difference between hemifields | 11.70 | 6.50 | 11.51 | 9.88 | 11.70 | 6.50 | 11.51 | 9.88 | 11.70 | 6.50 | 11.51 | 9.88 |
| Statistical analysis | H = 10.13. p = 0.02 *; no differences between groups | |||||||||||
| Full thickness | Z = 1.17; p = 0.24 | Z = 1.86; p = 0.06 | Z = 1.83; p = 0.07 | Z = 2.24; p = 0.03 * | ||||||||
| Superior | 292.60 | 295.50 | 24.71 | 335.00 | 292.60 | 295.50 | 24.71 | 335.00 | 292.60 | 295.50 | 24.71 | 335.00 |
| Inferior | 309.60 | 307.00 | 24.02 | 328.50 | 309.60 | 307.00 | 24.02 | 328.50 | 309.60 | 307.00 | 24.02 | 328.50 |
| Absolute value of the difference between hemifields | 20.60 | 6.50 | 32.70 | 7.50 | 20.60 | 6.50 | 32.70 | 7.50 | 20.60 | 6.50 | 32.70 | 7.50 |
| Statistical analysis | H = 3.68. p = 0.30 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowomiejska, K.; Lesiuk, P.; Brzozowska, A.; Tońska, K.; Rejdak, R. Vascular Changes in the Macula of Patients after Previous Episodes of Vision Loss Due to Leber Hereditary Optic Neuropathy and Non-Arteritic Ischemic Optic Neuropathy. Diagnostics 2023, 13, 1726. https://doi.org/10.3390/diagnostics13101726
Nowomiejska K, Lesiuk P, Brzozowska A, Tońska K, Rejdak R. Vascular Changes in the Macula of Patients after Previous Episodes of Vision Loss Due to Leber Hereditary Optic Neuropathy and Non-Arteritic Ischemic Optic Neuropathy. Diagnostics. 2023; 13(10):1726. https://doi.org/10.3390/diagnostics13101726
Chicago/Turabian StyleNowomiejska, Katarzyna, Patrycja Lesiuk, Agnieszka Brzozowska, Katarzyna Tońska, and Robert Rejdak. 2023. "Vascular Changes in the Macula of Patients after Previous Episodes of Vision Loss Due to Leber Hereditary Optic Neuropathy and Non-Arteritic Ischemic Optic Neuropathy" Diagnostics 13, no. 10: 1726. https://doi.org/10.3390/diagnostics13101726
APA StyleNowomiejska, K., Lesiuk, P., Brzozowska, A., Tońska, K., & Rejdak, R. (2023). Vascular Changes in the Macula of Patients after Previous Episodes of Vision Loss Due to Leber Hereditary Optic Neuropathy and Non-Arteritic Ischemic Optic Neuropathy. Diagnostics, 13(10), 1726. https://doi.org/10.3390/diagnostics13101726










