New Insights into the Diagnosis and Age Determination of Retinal Hemorrhages from Abusive Head Trauma: A Systematic Review
Abstract
1. Introduction
1.1. Epidemiology and Social Burden
1.2. Referral Difficulties and Valuative Frameworks
- any clinically significant respiratory compromise at the scene of injury, during transport, in the emergency department (ED), or prior to admission;
- any bruising involving the child’s torso, ear(s), or neck;
- any subdural hemorrhages or fluid collections that are bilateral or interhemispheric;
- any skull fracture(s) other than an isolated, unilateral, nondiastatic, linear, parietal skull fracture that shows high values of sensitivity and specificity [29].
1.3. Triad of Clinical Presentation
- SDH;
- Encephalopathy;
- RH.
- The most ancient theory, undoubtedly, is the mechanism proposed as “Terson’s Syndrome” [44,45], according to which an increase in ICP causes a hydraulic transmission of the CSF pressure to the eye through the laminae of the optic nerve. In this way, an increase in intraocular pressure (IOP) above the intravascular pressure of the capillary and venous vessels of the ocular coats produce a reduction in blood flow or a complete interruption of circulation; the resulting ischemic insult, affecting both the eye, the dura mater, and the brain, produces a rupture of the thin monolayered endothelial layer of the capillaries and, therefore, a hemorrhage [46]. Additionally, the central optical vein is directly affected by the increased pressure inside the optic nerve, primarily causing congestion of the entire retinal vascular bed [47]. The extent and degree of the leak also directly depends on the extent of the pressure and ischemic insult [48]. However, the low incidence of papilledema in relation to the RH finding would seem to discredit this theory [28];
- Along with this theory, an additional hydraulic mechanism has been proposed, whereby the venous and capillary pressures of the retinal vessels undergo a sudden increase due to the transmission of force from the vitreous to the retina during shaking, causing its rupture [49];
- The theory of vitreoretinal traction is currently the most well known [50], supported by the fact that most hemorrhages are located where retina and vitreous are deeply attached. In theory, due to the intense production of rotational and linear movements with sudden acceleration and deceleration, the vitreous exerts a shearing force on the retina, causing a mechanical rupture of the vessels. Although according to numerous authors, this theory is undermined by the lack of supporting evidence [51]; the scientific plausibility of the proposed mechanism is reflected in the frequent finding of another highly suggestive ocular findings in AHT, i.e., retinoschisis and macular folds [52]. Circular ruptures of the retinal epithelium are in fact highly sensitive findings for AHT, but not specific; they can also be found in cases of direct ocular trauma or SDH. From a morphological point of view, this finding can assume varied aspects: it can in fact present itself as continuous or discontinuous; partial or complete; and peri-macular, trans-macular, or peripheral and often it appears surrounded by a hyperpigmented or frankly hemorrhagic linear ring [43]. In addition to such lesions, it is possible to observe dispersion of blood inside the vitreous (vitreous hemorrhage), a critical element as it often masks underlying lesions under ophthalmoscopic observations.
1.4. Other Ocular Findings Compatible with AHT
1.5. Differential Diagnosis of RH
2. Materials and Methods
2.1. Inclusion Criteria
- Patients: infant victims of AHT presenting RH independent of fatality of outcome;
- Intervention: clinical and radiological evaluation and/or judicial autopsy completed by histopathological investigations;
- Comparison: evidence prior to the investigated period;
- Outcome: accuracy of methods and indicators used, correspondence with histopathological results, concordance of different studies on RH dating, and enforceability in forensic practice.
2.2. Exclusion Criteria
- Date of publication prior to 2016;
- Field of interest unrelated to a forensic setting;
- Insufficient innovation in results;
- Unavailability of abstract in the English language;
- Any kind of publication other than articles published in impacted journals.
2.3. Information Source and Search Process
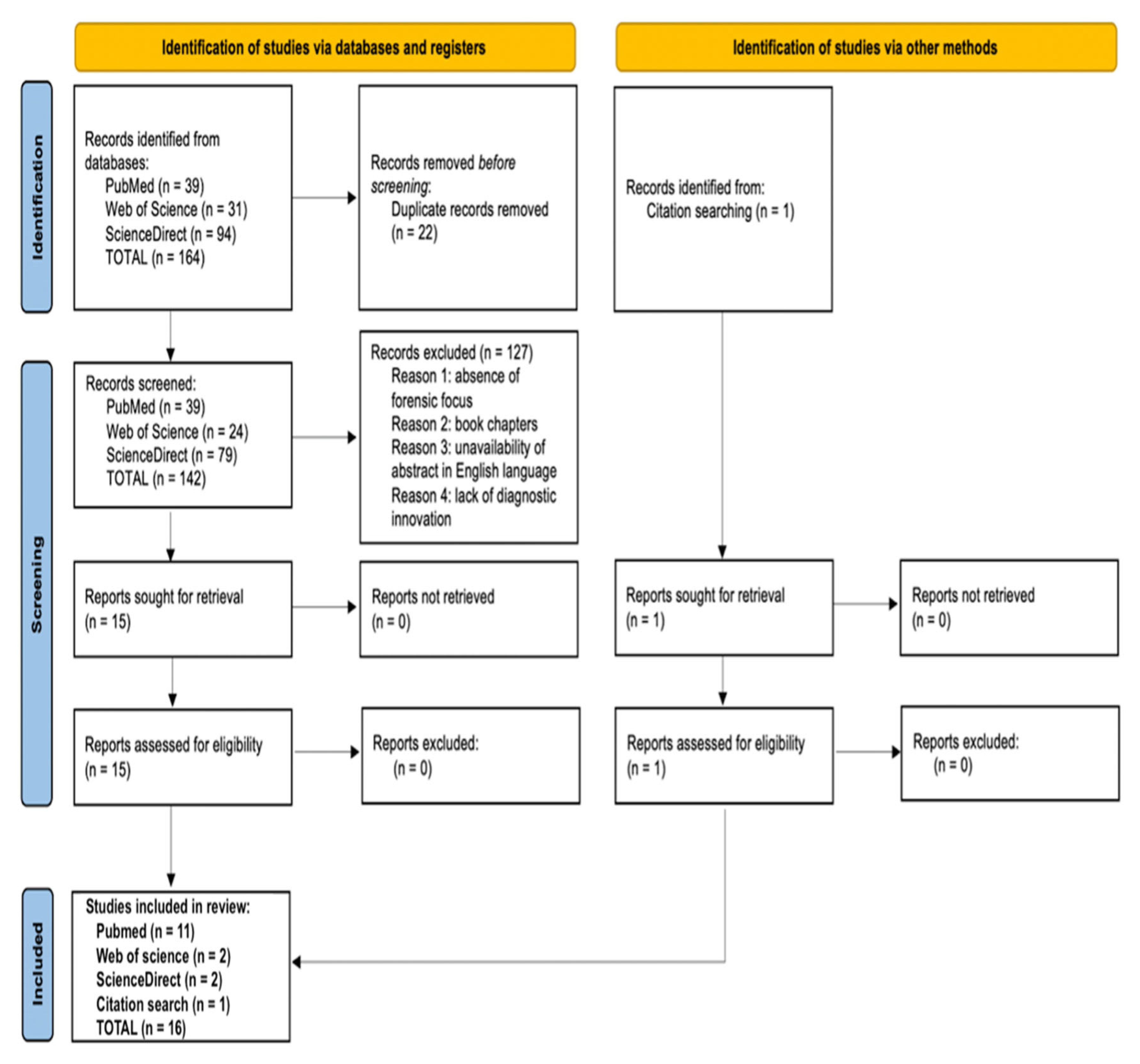
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
4. Discussion
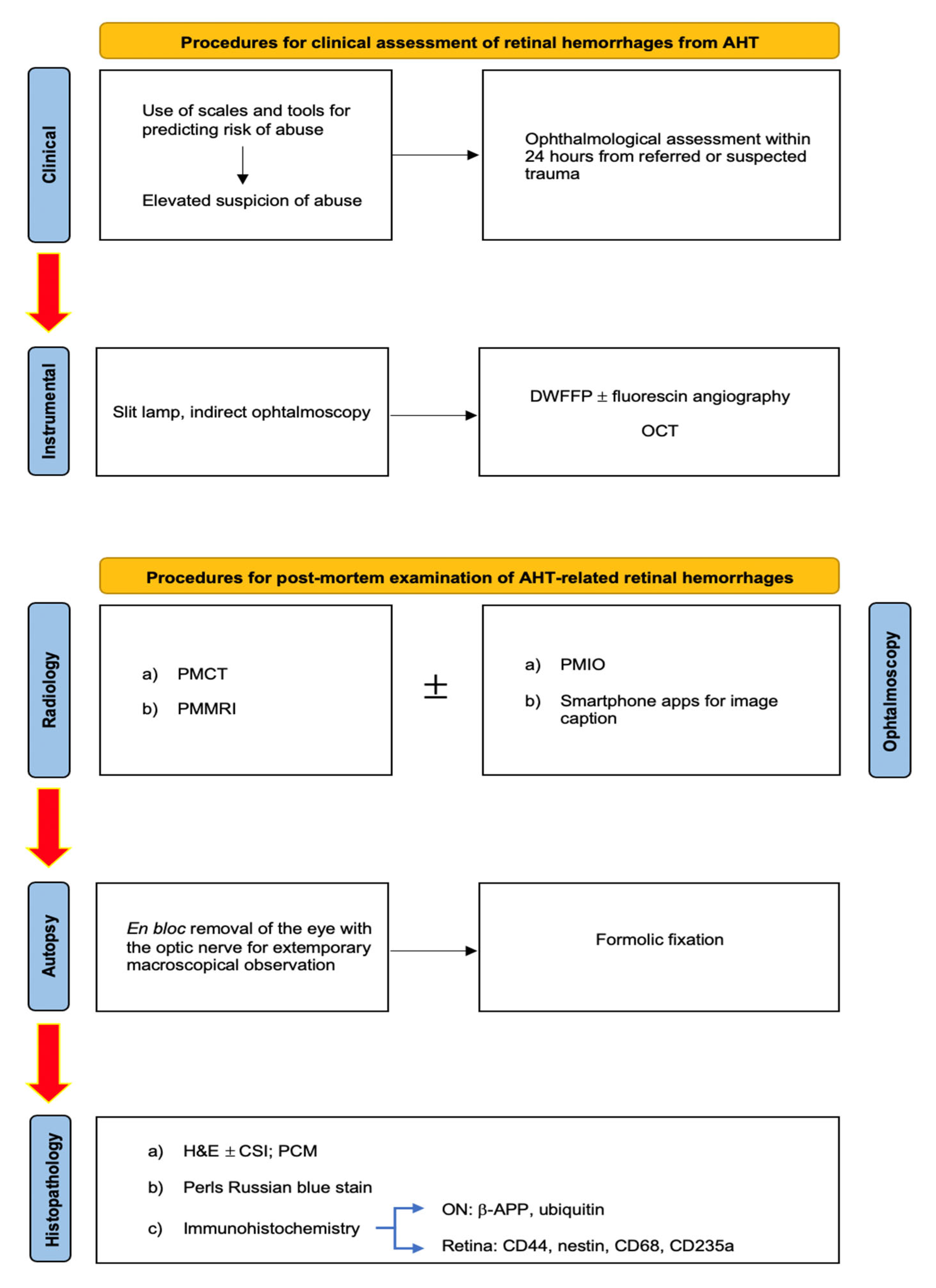
- Slit lamp, in order to ascertain the existence of damage to the anterior segment of the eye and to examine pupillary reflexes in subjects with acute neurological disorders;
- Indirect ophthalmoscopy, a relatively ancient tool to examine the posterior pole of the eye, with a limited focus on the retina but a very convenient cost-effectiveness to justify its wide application nowadays;
- Digital wide-field fundus photography (DWFFP), a technique capable of investigating the whole retina and to catch retinal images; it is considered to be highly economic with a good reliability (100% sensitivity, 85.7% specificity [102]), especially if combined with fluorescin angiography (FA);
- OCT, which is particularly useful to investigate the retinal posterior pole and optical nerve disc, including the different layers possibly showing hemorrhage. It allows the visualization of the vitreoretinal membrane, supporting a diagnosis based on a direct mechanical trauma mechanism.
- Grade 1—moderate deformation in the absence of an increase in nerve diameter;
- Grade 2—focal deformity and a moderate increase in diameter;
- Grade 3—loss of anatomical demarcation of the ONS with a severe increase in diameter.
- A rough temporal diagnosis has been proposed by distinguishing between recent hemorrhages, which are depicted as very dark areas inside the optic nerve parenchyma, and past lesions (hemosiderin deposits), which are described as scattered dark foci on the surface of the optic nerve.
- At the end of a thorough anamnestic and instrumental evaluation, autopsy remains a fundamental tool for dealing with fatal cases of abusive head trauma (AHT) [88]. Specifically, the search for ophthalmological findings requires the execution of a specific sectorial protocol at the cranial level [71]. By extracting the brain through a classic craniotomy, in fact, the exposure of the eye and its vasculo-nervous hilus requires the removal of the orbital roof by carefully cutting through the bone plane with tools of small dimensions, such as scissors or rongeurs. At this point, the exposed conjunctiva can be removed by cutting from the Tenon’s fascia to the sclera.
- At such a point, the eye and optic nerve (ON) become observable within their anatomical dwelling and should be removed en bloc (as for the intracanalicular portion of the ON) and fixed in formalin. Alternatively, an anterior approach is made possible by incising the conjunctiva and extracting the orbital content, which, however, does not allow for the preservation of posterior structures (e.g., the ON) and their observation in situ [98]. In cases of AHT, in fact, the importance of additional findings such as hemorrhagic infiltration involving periorbital adipose tissue, intrinsic musculature, peripapillary sclera, and especially the ON has already been asserted. Regarding peripapillary scleral hemorrhages (PSH), already known in a forensic context to be related to AHT [104,105], it has long been believed to derive from the typical acceleration/deceleration forces of shaking applied to the sclero-papillary junction with consequent traction of the intrascleral vessels of the Zinn’s arterial ring [106]. However, the experience of Oshima et al. [93] has allowed for a new hypothesis to be put forward based on the assumption that bleeding results from the Zinn–Haller arterial ring directly associated with traction of the ON. Consequently, the same author proposed the finding of PSH as an indicator of acceleration trauma stronger than RH.
- Furthermore, as reported by Puanglumyai’s team [71], the visualization of the ON may be obstructed by a local hemorrhagic infarction, which is why non-traumatic means (e.g., clamps) can be applied below the nerve to expose it more clearly. According to the same author, this method allows for rapid and effective in situ visualization of the hemorrhagic findings, sometimes better than after enucleation in the case of a minimal lesion size, which still requires histopathological confirmation. Ultimately, in confirmation of what has already been stated in the literature, the modest case series proposed by Puanglumyai et al. (11 victims of AHT) allowed for an appreciation of the specificity of the ONSH findings equal to 100%, with a topographic predilection for the retrobulbar portion considered to be more subjected to tension forces.
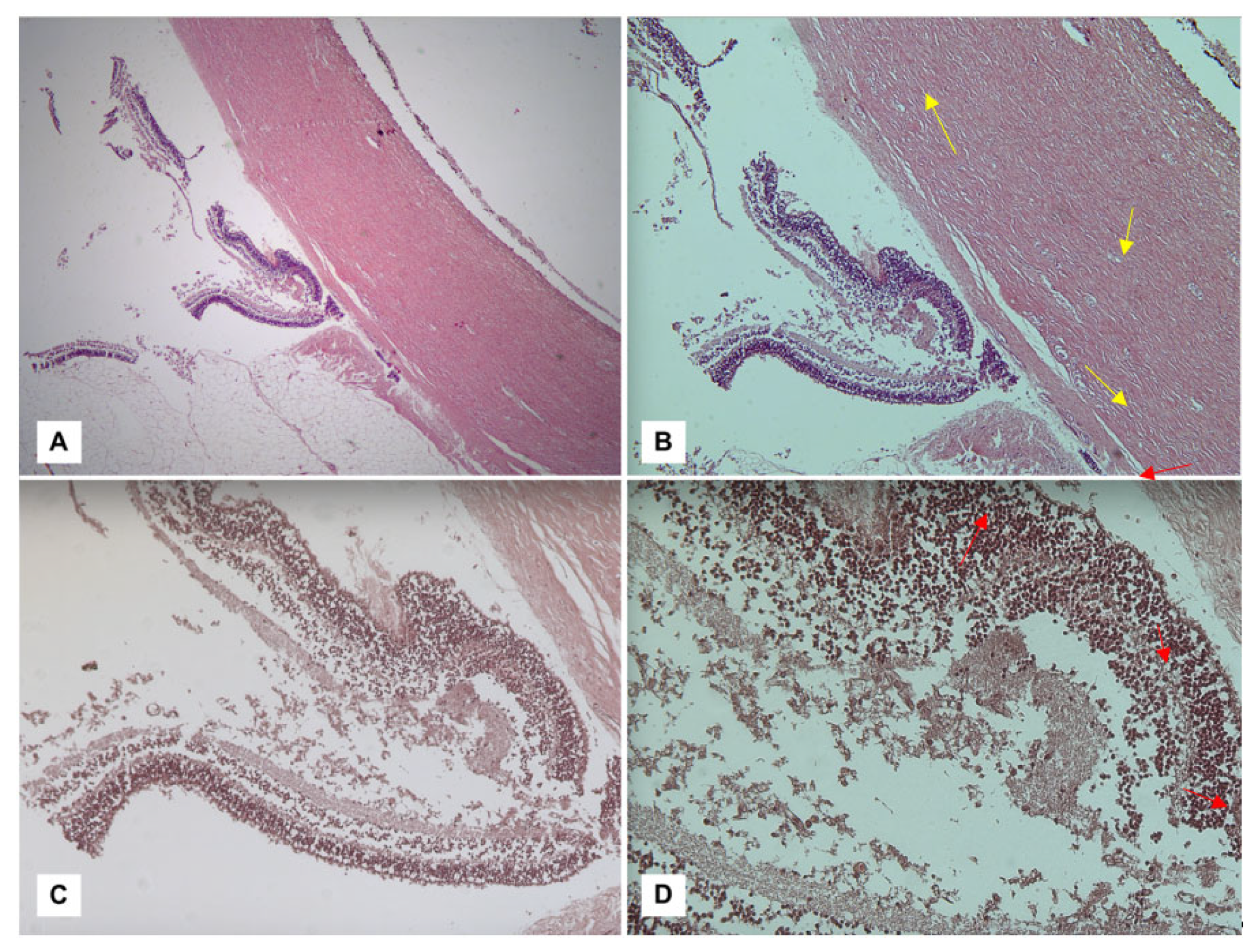
- The diagnostic culmination of an investigation into suspected AHT is currently constituted by histopathological investigations. Among the methods aimed at detecting ophthalmic hemorrhagic lesions, the classic hematoxylin–eosin (H&E) technique can be counted. As illustrated by Fieß et al. [88], in a discussion of their cases, in fact, their investigation conducted on acute retinal lesions allowed the visualization of extravascular red blood cells, distinguishing the different retinal layers affected by the hemorrhages as well as highlighting any folds or detachments (Figure 1).
- An uncomplicated vaginal birth and cesarian section are frequently related to microscopic hemorrhages in retina, orbital fat, and extra-ocular soft tissues for reasons unrelated to resuscitation attempts, but ONSH are exceptional findings in such cases;
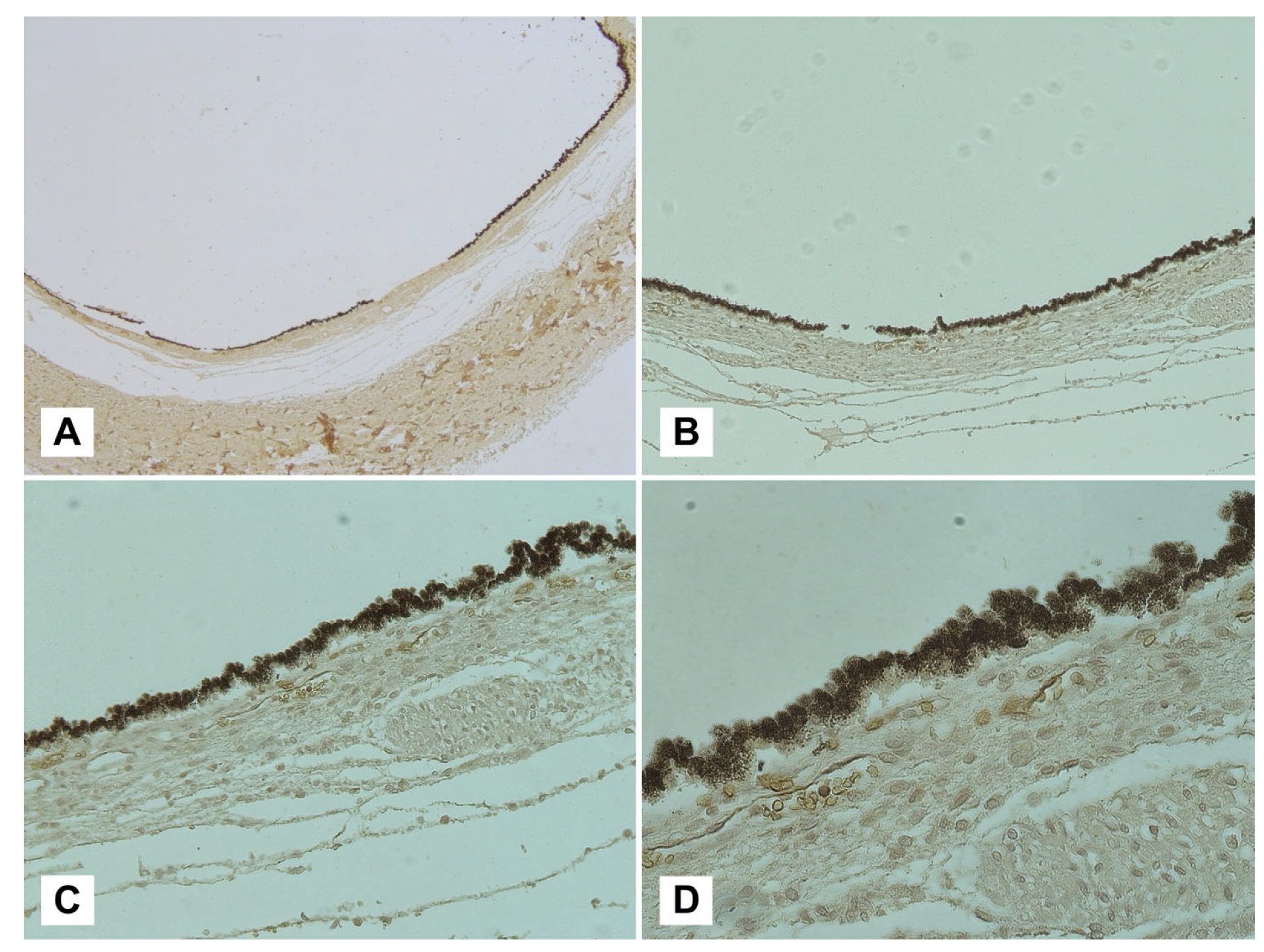
- Water-soluble ferritin may be lost during routine histological processing techniques, leaving hemosiderin as the most represented compound.
- β-APP and ubiquitin are valid markers for axonal and retinal damage and NO in cases of AHT, especially when used together;
- GFAP, on the other hand, did not show a statistically significant correlation with AHT;
- An RH extended to 20%–30% of the retinal area should be considered a strong indicator of AHT, as well as vitreal and orbital fat hemorrhage and macular folds.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosén, M.; Lynøe, N.; Elinder, G.; Hallberg, B.; Sundgren, P.; Eriksson, A. Shaken baby syndrome and the risk of losing scientific scrutiny. Acta Paediatr. 2017, 106, 1905–1908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardieu, A. Étude Médico-légale sur les sévices et mauvais traitements exercés sur des enfants. Ann. Hyg. Publique Med. Leg. 1860, 13, 361–398. (In French) [Google Scholar]
- Caffey, J. On the theory and practice of shaking infants. Its potential residual effects of permanent brain damage and mental retardation. Am. J. Dis. Child. 1972, 124, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Duhaime, A.C.; Gennarelli, T.A.; Thibault, L.E.; Bruce, D.A.; Margulies, S.S.; Wiser, R. The shaken baby syndrome. A clinical, pathological, and biomechanical study. J. Neurosurg. 1987, 66, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Minns, R.A.; Jones, P.A.; Tandon, A.; Fleck, B.W.; Mulvihill, A.O.; Elton, R.A. Prediction of inflicted brain injury in infants and children using retinal imaging. Pediatrics 2012, 130, e1227–e1234. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, T.; Okuyama, M.; Miyasaka, M. Characteristics that distinguish abusive from non-abusive head trauma among young children who underwent head computed tomography in Japan. Pediatrics 2008, 122, e841–e847. [Google Scholar] [CrossRef]
- Myhre, M.; Grøgaard, J.; Dyb, G.; Sandvik, L.; Nordhov, M. Traumatic head injury in infants and toddlers. Acta Paediatr. 2007, 96, 1159–1163. [Google Scholar] [CrossRef][Green Version]
- Tung, G.A.; Kumar, M.; Richardson, R.C.; Jenny, C.; Brown, W.D. Comparison of accidental and nonaccidental traumatic head injury in children on non-contrast computed tomography. Pediatrics 2006, 118, 626–633. [Google Scholar] [CrossRef]
- Vinchon, M.; Defoort Dhellemmes, S.; Desurmont, M.; Dhellemmes, P. Accidental and nonaccidental head injuries in infants: A prospective study. J. Neurosurg. 2005, 102, 380–384. [Google Scholar] [CrossRef]
- Keenan, H.T.; Runyan, D.K.; Marshall, S.W.; Nocera, M.A.; Merten, D.F. A Population-Based Comparison of Clinical and Outcome Characteristics of Young Children With Serious Inflicted and Noninflicted Traumatic Brain Injury. Pediatrics 2004, 114, 633–639. [Google Scholar] [CrossRef][Green Version]
- Bechtel, K.; Stoessel, K.; Leventhal, J.M.; Ogle, E.; Teague, B.; Lavietes, S.; Banyas, B.; Allen, K.; Dziura, J.; Duncan, C. Characteristics that distinguish accidental from abusive injury in hospitalized young children with head trauma. Pediatrics 2004, 114, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.; Risdon, R.A.; Krous, H.F. Shaken baby syndrome. BMJ 2004, 328, 720–721. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.W.; Block, R.; Committee on Child Abuse and Neglect; American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics 2009, 123, 1409–1411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parks, S.E.; Annest, J.L.; Hill, H.A.; Karch, D.L. Pediatric Abusive Head Trauma: Recommended Definitions for Public Health Surveillance and Research; National Center for Injury Prevention and Control (U.S.), Division of Violence Prevention: Atlanta, GA, USA, 2012.
- Leetch, A.N.; Woolridge, D. Emergency Department Evaluation of Child Abuse. Emerg. Med. Clin. N. Am. 2013, 31, 853–873. [Google Scholar] [CrossRef]
- Lopes, N.R.; Eisenstein, E.; Williams, L.C. Abusive head trauma in children: A literature review. J Pediatr. 2013, 89, 426–433. [Google Scholar] [CrossRef][Green Version]
- Graupman, P.; Winston, K.R. Nonaccidental head trauma as a cause of childhood death. J. Neurosurg. Pediatr. 2006, 104 (Suppl. S4), 245–250. [Google Scholar] [CrossRef]
- Kesler, H.; Dias, M.S.; Shaffer, M.; Rottmund, C.; Cappos, K.; Thomas, N.J. Demographics of abusive head trauma in the Commonwealth of Pennsylvania. J. Neurosurg. Pediatr. 2008, 1, 351–356. [Google Scholar] [CrossRef]
- Parmar, C.D.; Sinha, A.K.; Hayhurst, C.; May, P.L.; O’Brien, D.F. Epidural hematoma formation following trivial head trauma in a child with osteogenesis imperfecta. J. Neurosurg. Pediatr. 2007, 106 (Suppl. S1), 57–60. [Google Scholar] [CrossRef]
- Duhaime, A.C. Demographics of abusive head trauma. J. Neurosurg. Pediatr. 2008, 1, 349–350. [Google Scholar] [CrossRef]
- Starling, S.P.; Patel, S.; Burke, B.L.; Sirotnak, A.P.; Stronks, S.; Rosquist, P. Analysis of Perpetrator Admissions to Inflicted Traumatic Brain Injury in Children. Arch. Pediatr. Adolesc. Med. 2004, 158, 454–458. [Google Scholar] [CrossRef][Green Version]
- Starling, S.P.; Holden, J.R.; Jenny, C. Abusive Head Trauma: The Relationship of Perpetrators to Their Victims. Pediatrics 1995, 95, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Ma, C.-X.; Carter, R.L.; Ariet, M.; Feaver, E.A.; Resnick, M.B.; Roth, J. Risk factors for infant maltreatment: A population-based study. Child Abus. Negl. 2004, 28, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Wygnanski-Jaffe, T.; Morad, Y.; Levin, A.V. Pathology of retinal hemorrhage in abusive head trauma. Forensic Sci. Med. Pathol. 2009, 5, 291–297. [Google Scholar] [CrossRef]
- Babl, F.E.; Pfeiffer, H.; Kelly, P.; Dalziel, S.R.; Oakley, E.; Borland, M.L.; Kochar, A.; Dalton, S.; Cheek, J.A.; Gilhotra, Y.; et al. Paediatric abusive head trauma in the emergency department: A multicentre prospective cohort study. J. Paediatr. Child Health 2019, 56, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Greeley, C.S. Abusive Head Trauma: A Review of the Evidence Base. Am. J. Roentgenol. 2015, 204, 967–973. [Google Scholar] [CrossRef]
- Geddes, J.F.; Hackshaw, A.K.; Vowles, G.H.; Whitwell, H.L. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 2001, 124, 1290–1298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morad, Y.; Kim, Y.M.; Armstrong, D.C.; Huyer, D.; Mian, M.; Levine, A.V. Correlation between retinal abnormalities and intracranial abnormalities in the shaken baby syndrome. Am. J. Ophthalmol. 2002, 134, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Hymel, K.P.; Karst, W.; Marinello, M.; Herman, B.E.; Frazier, T.N.; Carroll, C.L.; Armijo-Garcia, V.; Musick, M.; Weeks, K.; Haney, S.B.; et al. Pediatric Brain Injury Research Network (PediBIRN) Investigators. Screening for pediatric abusive head trauma: Are three variables enough? Child Abus. Negl. 2022, 125, 105518. [Google Scholar] [CrossRef]
- Kanya Iyer, A.; Lemos, N.P. Are we looking for retinal haemorrhages? Med. Sci. Law 2019, 59, 70–71. [Google Scholar] [CrossRef]
- La Russa, R.; Maiese, A.; Cipolloni, L.; Di Fazio, N.; Delogu, G.; De Matteis, A.; Del Fante, Z.; Manetti, F.; Frati, P.; Fineschi, V. Diagnostic assessment of traumatic brain injury by vacuum extraction in newborns: Overview on forensic perspectives and proposal of operating procedures. Front. Biosci. 2022, 27, 79. [Google Scholar] [CrossRef]
- Squier, W. The “Shaken Baby” syndrome: Pathology and mechanisms. Acta Neuropathol. 2011, 122, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Piteau, S.J.; Ward, M.G.; Barrowman, N.J.; Plint, A.C. Clinical and radio-graphic characteristics associated with abusive and nonabusive head trauma: A systematic review. Pediatrics 2012, 130, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Elinder, G.; Eriksson, A.; Hallberg, B.; Lynøe, N.; Sundgren, P.M.; Rosén, M.; Engström, I.; Erlandsson, B.E. Traumatic shaking: The role of the triad in medical investigations of suspected traumatic shaking. Acta Paediatr. 2018, 107 (Suppl S472), 3–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKeag, H.; Christian, C.W.; Rubin, D.; Daymont, C.; Pollock, A.N.; Wood, J. Subdural hemorrhage in pediatric patients with enlargement of the sub arachnoid spaces. J. Neurosurg. Pediatr. 2013, 11, 438–444. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Chowdhury, V.; Jacobs, M.B.; Moran, K.T.; Martin, F.J.; Coronet, M.T. A systematic review of the diagnostic accuracy of ocular signs in pediatric abusive head trauma. Ophthalmology 2010, 117, 983–992.e17. [Google Scholar] [CrossRef]
- Kivlin, J.D.; Simons, K.B.; Lazoritz, S.; Ruttum, M.S. Shaken baby syndrome. Ophthalmology 2000, 107, 1246–1254. [Google Scholar] [CrossRef]
- Watts, P.; Maguire, S.; Kwok, T.; Talabani, B.; Mann, M.; Wiener, J.; Lawson, Z.; Kemp, A. Newborn retinal hemor-rhages: A systematic review. J. AAPOS 2013, 17, 70–78. [Google Scholar] [CrossRef]
- Levin, A.V. Retinal hemorrhage in abusive head trauma. Pediatrics 2010, 126, 961–970. [Google Scholar] [CrossRef][Green Version]
- Levinson, J.D.; Pasquale, M.A.; Lambert, S.R. Diffuse bilateral retinal hemorrhages in an infant with a coagulopathy and prolonged cardiopulmonary resuscitation. J. AAPOS 2016, 20, 166–168. [Google Scholar] [CrossRef][Green Version]
- Rivera, F. Population-based study of fall injuries in children and adolescents resulting in hospitalization or death. Pediatrics 1993, 92, 61–63. [Google Scholar]
- Chadwick, D.L.; Bertocci, G.; Castillo, E.; Frasier, L.; Guenther, E.; Hansen, K.; Herman, B.; Krous, H.F. Annual Risk of Death Resulting from Short Falls Among Young Children: Less Than 1 in 1 Million. Pediatrics 2008, 121, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, G.; Forbes, B.J. The eye in child abuse: Key points on retinal hemorrhages and abusive head trauma. Pediatr. Radiol. 2014, 44 (Suppl. S4), S571–S577. [Google Scholar] [CrossRef] [PubMed]
- Terson, P.D.A. Hemorrhage in the vitreous body during cerebral hemorrhage. La Clin. Ophthalmol. 1900, 22, 309–312. [Google Scholar]
- Muller, P.J.; Deck, J.H.N. Intraocular and optic nerve sheath hemorrhage in cases of sudden intracranial hypertension. J. Neurosurg. 1974, 41, 160–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koto, T.; Takubo, K.; Ishida, S.; Shinoda, H.; Inoue, M.; Tsubota, K.; Okada, Y.; Ikeda, E. Hypoxia Disrupts the Barrier Function of Neural Blood Vessels through Changes in the Expression of Claudin-5 in Endothelial Cells. Am. J. Pathol. 2007, 170, 1389–1397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Firsching, R.; Muller, C.; Pauli, S.U.; Voellger, B.; Rohl, F.W.; BehrensBaumann, W. Noninvasive assessment of intracranial pressure with venous ophthalmodyna mometry. Clinical article. J. Neurosurg. 2011, 115, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Lantz, P.E.; Carlson, J.; Mott, R. Extensive Hemorrhagic Retinopathy, Perimacular Retinal Fold, Retinoschisis, and Retinal Hemorrhage Progression Associated with a Fatal Spontaneous, Non-Traumatic, Intracranial Hemorrhage in an Infant. (Abstract presented 21 February 2013 at the Am. Acad. Forens. Sci. Annual Meeting, Washington, DC). Available online: http://www.aafs.org/sites/default/files/pdf/ProceedingsWashingtonDC2013.pdf (accessed on 15 January 2023).
- Levin, A.V.; Christian, C.W.; Committee on Child Abuse and Neglect, Section on Ophthalmology. The eye exam ination in the evaluation of child abuse. Pediatrics 2010, 126, 376–380. [Google Scholar] [CrossRef][Green Version]
- Coats, B.; Binenbaum, G.; Peiffer, R.L.; Forbes, B.J.; Margulies, S.S. Ocular hemorrhages in neonatal porcine eyes from single, rapid rotational events. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4792–4797. [Google Scholar]
- Gabaeff, S.C. Exploring the controversy in child abuse pediatrics and false accusations of abuse. Leg. Med. 2016, 18, 90–97. [Google Scholar] [CrossRef][Green Version]
- Abed Alnabi, W.; Tang, G.J.; Eagle, R.C., Jr.; Gulino, S.; Thau, A.; Levin, A.V. Pathology of perimacular folds due to vitreoretinal traction in abusive head trauma. Retina 2019, 39, 2141–2148. [Google Scholar]
- Mills, M. Funduscopic lesions associated with mortality in shaken baby syndrome. J. AAPOS 1998, 2, 67–71. [Google Scholar] [CrossRef]
- Binenbaum, G.; Christian, C.W.; Ichord, R.N.; Ying, G.-S.; Simon, M.A.; Romero, K.; Pollock, A.N.; Forbes, B.J. Retinal hemorrhage and brain injury patterns on diffusion-weighted magnetic resonance imaging in children with head trauma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2013, 17, 603–608. [Google Scholar] [CrossRef][Green Version]
- Reynolds, J.D.; Olitsky, S.E. (Eds.) Pediatric Retina; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Kellogg, N.D. American Academy of Pediatrics Committee on Child Abuse and Neglect. Evaluation of suspected child physical abuse. Pediatrics 2007, 119, 1232–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vinchon, M.; de Foort-Dhellemmes, S.; Desurmont, M.; Delestret, I. Confessed abuse versus witnessed accidents in infants: Comparison of clinical, radiological, and ophthalmological data in corroborated cases. Child’s Nerv. Syst. 2010, 26, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Högberg, G.; Colville-Ebeling, B.; Högberg, U.; Aspelin, P. Circularity bias in abusive head trauma studies could be diminished with a new ranking scale. Egypt. J. Forensic Sci. 2016, 6, 6–10. [Google Scholar] [CrossRef][Green Version]
- Ewing-Cobbs, L.; Prasad, M.; Kramer, L.; Louis, P.T.; Baumgartner, J.; Fletcher, J.M.; Alpert, B. Acute neuroradiological findings in young children with inflicted or noninflicted traumatic brain injury. Child’s Nerv. Syst. 2000, 16, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, G.; Forbes, B.J.; Reghupathi, R.; Judkins, A.; Rorke, L.; Margulies, S.S. An animal model to study retinal hemorrhages in nonimpact brain injury. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2007, 11, 84–85. [Google Scholar] [CrossRef]
- Serbanescu, I.; Brown, S.M.; Ramsay, D.; Levin, A.V. Natural animal shaking: A model for non-accidental head injury in children? Eye 2008, 22, 1–3. [Google Scholar] [CrossRef][Green Version]
- Coats, B.; Binenbaum, G.; Smith, C.; Peiffer, R.L.; Christian, C.W.; Duhaime, A.-C.; Margulies, S.S.; Shuman, M.J.; Hutchins, K.D.; Reynolds, B.B.; et al. Cyclic head rotations produce modest brain injury in infant piglets. J. Neurotrauma 2016. Epub ahead of print. [Google Scholar] [CrossRef][Green Version]
- Glowinski, S.; Majdanik, S.; Glowinska, A.; Majdanik, E. Trauma in a shaken infant? A case study. Aggress. Violent Behav. 2021, 56, 101515. [Google Scholar] [CrossRef]
- Gabaeff, S.C. Challenging the Pathophysiologic Connection between Subdural Hematoma, Retinal Hemorrhage and Shaken Baby Syndrome. WestJEM 21.2 March Issue 2011, 12, 144–158. [Google Scholar]
- Lynøe, N.; Elinder, G.; Hallberg, B.; Rosén, M.; Sundgren, P.; Eriksson, A. Insufficient evidence for ‘shaken baby syndrome’—A systematic review. Acta Paediatr. 2017, 106, 1021–1027. [Google Scholar] [CrossRef][Green Version]
- Strouse, P.J. Shaken baby syndrome is real. Pediatr. Radiol. 2018, 48, 1043–1047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lantz, P.E.; Adams, G.G.W. Postmortem Monocular Indirect Ophthalmoscopy. J. Forensic Sci. 2005, 50, 1450–1452. [Google Scholar] [CrossRef]
- Emerson, M.V.; Jakobs, E.; Green, W.R. Ocular autopsy and histopathologic features of child abuse. Ophthalmology 2007, 114, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Wygnanski-Jaffe, T.; Levin, A.V.; Shafiq, A.; Smith, C.; Enzenauer, R.W.; Elder, J.E.; Morin, J.D.; Stephens, D.; Atenafu, E. Postmortem Orbital Findings in Shaken Baby Syndrome. Am. J. Ophthalmol. 2006, 142, 233–240. [Google Scholar] [CrossRef]
- Gnanaraj, L.; Gilliland, M.G.F.; Yahya, R.R.; Rutka, J.T.; Drake, J.; Dirks, P.; Levin, A.V. Ocular manifestations of crush head injury in children. Eye 2005, 21, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Puanglumyai, S.; Lekawanvijit, S. The importance of optic nerve sheath hemorrhage as a postmortem finding in cases of fatal abusive head trauma: A 13-year study in a tertiary hospital. Forensic Sci. Int. 2017, 276, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Migueis, G.F.J.; Fernandes, F.A.O.; Ptak, M.; Ratajczak, M.; Alves de Sousa, R.J. Detection of bridging veins rupture and subdural haematoma onset using a finite element head model. Clin. Biomech. 2019, 63, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Thomas, N.J.; Gertz, S.J.; Beca, J.; Luther, J.F.; Bell, M.J.; Wisniewski, S.R.; Hartman, A.L.; Tasker, R.C.; Investigators of the Approaches and Decisions in Acute Pediatric Traumatic Brain Injury (ADAPT) Study. Tripartite stratification of the Glasgow Coma Scale in children with severe traumatic brain injury and mortality: An analysis from a multi-center comparative effectiveness study. J. Neurotrauma. 2017, 34, 2222–2229. [Google Scholar] [CrossRef]
- Riffenburgh, R.S. Ocular hemorrhage in autopsies of child abuse victims. Clin. Surg. Opthalmol. 2005, 23, 178–186. [Google Scholar]
- Kodikara, S.; Pollanen, M. Fatal pediatric head injury due to toppled television: Does the injury pattern overlap with abusive head trauma? Leg. Med. 2012, 14, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Budenz, D.L.; Farber, M.G.; Mirchandani, H.G.; Park, H.; Rorke, L.B. Ocular and Optic Nerve Hemorrhages in Abused Infants with Intracranial Injuries. Ophthalmology 1994, 101, 559–565. [Google Scholar] [CrossRef]
- Altinok, D.; Saleem, S.; Zhang, Z.; Markman, L.; Smith, W. MR imaging findings of retinal hemorrhage in a case of nonaccidental trauma. Pediatr. Radiol. 2009, 39, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Fineschi, V.; Padovano, M.; Scopetti, M.; Rossi-Espagnet, M.C.; Giannì, C. Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma. Brain Sci. 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; May, K.; Talbot, J.F.; Parsons, M.A. Incidence, distribution, and duration of birth-related retinal hemorrhages: A prospective study. J. AAPOS 2006, 10, 102–106. [Google Scholar] [CrossRef]
- Binenbaum, G.; Mirza-George, N.; Christian, C.W.; Forbes, B.J. Odds of abuse associated with retinal hemorrhages in children suspected of child abuse. J. AAPOS 2009, 13, 268–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maguire, S.A.; Watts, P.O.; Shaw, A.D.; Holden, S.; Taylor, R.H.; Watkins, W.J.; Mann, M.K.; Tempest, V.; Kemp, A.M. Retinal haemorrhages and related findings in abusive and non-abusive head trauma: A systematic review. Eye 2012, 27, 28–36. [Google Scholar] [CrossRef][Green Version]
- Sturm, V.; Knecht, P.B.; Landau, K.; Menke, M.N. Rare retinal haemorrhages in translational accidental head trauma in children. Eye 2008, 23, 1535–1541. [Google Scholar] [CrossRef][Green Version]
- Kivlin, J.D.; Currie, M.L.; Greenbaum, V.J.; Simons, K.B.; Jentzen, J. Retinal hemorrhages in children following fatal motor vehicle crashes: A case series. Arch. Ophthalmol. 2008, 126, 800–804. [Google Scholar] [CrossRef][Green Version]
- Binenbaum, G.; Rogers, D.L.; Forbes, B.J.; Levin, A.V.; Clark, S.A.; Christian, C.W.; Liu, G.T.; Avery, R. Patterns of Retinal Hemorrhage Associated With Increased Intracranial Pressure in Children. Pediatrics 2013, 132, e430–e434. [Google Scholar] [CrossRef][Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Barth, T.; Altmann, M.; Batzlsperger, C.; Jägle, H.; Helbig, H. Unilaterale Netzhautblutungen bei Säuglingen—2 Fälle von Schütteltrauma? [Unilateral retinal hemorrhage in infants-two cases of shaken baby syndrome?]. Ophthalmologe 2020, 117, 1033–1036. [Google Scholar] [CrossRef]
- Fieß, A.; Dithmar, S.; Kölb-Keerl, R.; Kunze, A.; Riße, M.; Knuf, M.; Bauer, J. Retinale Blutungen und venöse Stase bei einem 10 Monate alten Säugling nach Sturz? [Retinal bleeding and venous stasis in a 10-month-old infant after a fall?]. Ophthalmologe 2016, 113, 694–698. [Google Scholar] [CrossRef]
- Bais, B.; Kubat, B.; Motazedi, E.; Verdijk, R.M. β-Amyloid Precursor Protein and Ubiquitin Immunohistochemistry Aid in the Evaluation of Infant Autopsy Eyes with Abusive Head Trauma. Am. J. Ophthalmol. 2015, 160, 1285–1295.e6. [Google Scholar] [CrossRef] [PubMed]
- Lantz, P.E.; Schoppe, C.H.; Thibault, K.L.; Porter, W.T. Smartphone Image Acquisition During Postmortem Monocular Indirect Ophthalmoscopy. J. Forensic Sci. 2015, 61, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Bais, B.; Karst, W.A.; Kubat, B.; Verdijk, R.M. Persistent Retinal Iron in Abusive Head Trauma. J. Forensic Sci. 2016, 61, 1693–1696. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Barnes, J.K.; Fung, T.H.M.; Elston, J.S.; Patel, C.K. Non-contact ultra-widefield retinal imaging of infants with suspected abusive head trauma. Eye 2017, 31, 353–363. [Google Scholar] [CrossRef][Green Version]
- Oshima, T.; Yoshikawa, H.; Koda, Y.; Ohtani, M.; Tsukamoto, S.; Mimasaka, S. Four intracranial injury cases with peripapillary scleral hemorrhage—Reconsidering the mechanism of hemorrhage. Leg. Med. 2017, 27, 5–9. [Google Scholar] [CrossRef]
- Del Bigio, M.R.; Phillips, S.M. Retroocular and Subdural Hemorrhage or Hemosiderin Deposits in Pediatric Autopsies. J. Neuropathol. Exp. Neurol. 2017, 76, 313–322. [Google Scholar] [CrossRef][Green Version]
- Delteil, C.; Kolopp, M.; Capuani, C.; Humez, S.; Boucekine, M.; Leonetti, G.; Torrents, J.; Tuchtan, L.; Piercecchi, M.-D. Histological dating of subarachnoid hemorrhage and retinal hemorrhage in infants. Forensic Sci. Int. 2019, 303, 109952. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G. Novel in vivo depiction of optic nerves hemorrhages in child abuse: A 3D-SWI pilot study. Neuroradiology 2021, 63, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, R.; Todeschi, J.; Wiedemann-Fode, A.; Stella, I.; Joud, A.; Klein, O. Ophthalmological lesions in shaken baby syndrome: A retrospective analysis of 133 consecutive cases (1992–2018). Neurochirurgie 2022, 68, 367–372. [Google Scholar] [CrossRef]
- Maiese, A.; Iannaccone, F.; Scatena, A.; Del Fante, Z.; Oliva, A.; Frati, P.; Fineschi, V. Pediatric Abusive Head Trauma: A Systematic Review. Diagnostics 2021, 11, 734. [Google Scholar] [CrossRef]
- Oliva, A.; Grassi, S.; Cazzato, F.; Jabbehdari, S.; Mensi, L.; Amorelli, G.; Orazi, L.; Arena, V.; Lepore, D. The role of retinal imaging in the management of abusive head trauma cases. Int. J. Legal Med. 2022, 136, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Bulirsch, L.M.; Loeffler, K.U.; Holz, F.G.; Koinzer, S.; Nadal, J.; Müller, A.M.; Herwig-Carl, M.C. Spatial and temporal immunoreaction of nestin, CD44, collagen IX and GFAP in human retinal Müller cells in the developing fetal eye. Exp. Eye Res. 2022, 217, 108958. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 15 January 2023).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute, 2017; Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 15 January 2023).
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Kahlil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an Umbrella review approach. Int. J. Evid.-Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef][Green Version]
- Christian, C.W.; Levin, A.V.; Council on Child Abuse and Neglect; Section on Ophthalmology; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. The Eye Examination in the Evaluation of Child Abuse. Pediatrics 2018, 142, e20181411. [Google Scholar] [CrossRef][Green Version]
- Saleh, M.; Schoenlaub, S.; Desprez, P.; Bourcier, T.; Gaucher, D.; Astruc, D.; Speeg-Schatz, C. Use of digital camera imaging of eye fundus for telemedicine in children suspected of abusive head injury. Br. J. Ophthalmol. 2009, 93, 424–428. [Google Scholar] [CrossRef]
- Gunda, D.; Cornwell, B.O.; Dahmoush, H.M.; Jazbeh, S.; Alleman, A.M. Pediatric Central Nervous System Imaging of Non-accidental Trauma: Beyond Subdural Hematomas. RadioGraphics 2018, 39, 213–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lambert, S.R.; Johnson, T.E.; Hoyt, C.S. Optic Nerve Sheath and Retinal Hemorrhages Associated With the Shaken Baby Syndrome. Arch. Ophthalmol. 1986, 104, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Luigi Crudele, G.D.; Galante, N.; Fociani, P.; Del Gobbo, A.; Tambuzzi, S.; Gentile, G.; Zoja, R. The forensic application of the Glycophorin A on the Amussat’s sign with a brief review of the literature. J. Forensic Leg. Med. 2021, 82, 102228. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.F. What’s new in the diagnosis of head injury? J. Clin. Pathol. 1997, 50, 271–274. [Google Scholar] [CrossRef][Green Version]
- The Ophthalmology Child Abuse Working Party. Child abuse and the eye. Eye 1999, 13 Pt 1, 3–10. [Google Scholar] [CrossRef]
- Delteil, C.; Humez, S.; Boucekine, M.; Jouvet, A.; Hedouin, V.; Fanton, L.; Leonetti, G.; Tuchtan, L.; Piercecchi, M.-D. Histological dating of subdural hematoma in infants. Int. J. Leg. Med. 2018, 133, 539–546. [Google Scholar] [CrossRef]
- Turillazzi, E.; Karch, S.B.; Neri, M.; Pomara, C.; Riezzo, I.; Fineschi, V. Confocal laser scanning microscopy. Using new technology to answer old questions in forensic investigations. Int. J. Leg. Med. 2007, 122, 173–177. [Google Scholar] [CrossRef]
- Reichard, R.R.; White, C.L.; Hogan, R.N.; Hladik, C.L.; Dolinak, D. Beta-amyloid precursor protein immunohistochemistry in the evaluation of pediatric traumatic optic nerve injury. Ophthalmology 2004, 111, 822–827. [Google Scholar] [CrossRef]

| Retina | |
|---|---|
| RH Type | Main Findings |
| Mild | Few in number, intraretinal, confined to posterior pole |
| Moderate | Numerous, multi-layered, extended over ora serrata |
| Severe | Same aspects of moderate one, but bilateral |
| Other aspects | Macular folds, emovitreus |
| Other ocular structures | |
| Anatomical district | Main findings |
| Cornea | Abrasions, lacerations, cloudings |
| Crystalline lens | Dislocations, damage to suspensory ligaments of lens, ciliar body and muscle |
| Conjunctiva | Subjunctival hemorrhages |
| Optical nerve sheaths | Hemorrhage, most frequently subdural |
| Periorbital adipose tissue and extraocular muscles | Hemorrhage |
| Non-AHT Conditions Related to RH | Incidence | Timing | Risk Factors/Positive Elements | Mechanism | Morphologic Aspect | Exclusion Elements |
|---|---|---|---|---|---|---|
| Birth trauma | Approximately one-third of newborns [38] | First two days of life, 85% of cases heal within 2 weeks [79,80] | Vacuum-assisted delivery [31] | Perinatal hemodynamic changes, ocular compression, prostaglandin release | Often numerous, extended over ora serrata (such as AHT-related) but only intraretinal, with rare retinal folds | RH with numerous, diffuse, extraretinal, duration extended over the first month of life |
| Accidental head injury | Less than 4% according to multiple authors; short falls have an RH incidence close to 0% [36,81,82,83] | Same as AHT | Unambiguous and consistent history given by parents, presence of witnesses, other lesions compatible with referred kind and force of impact | Direct impact, Terson’s Syndrome, rapid acceleration and rotational movements of the head | Often confined to posterior pole, few in number, rarely subretinal. Severe accidents or impacts may determine extended lesions [84] | Absence of other lesions, suspicious behavior and history given by parents, absence of witnesses |
| Raised ICP | Not estimated | Same as AHT | Severe elevation of ICP | Terson’s Syndrome | Superficial, intraretinal, located on or close to the optic disc [84] | Absence of papilledema (present in only 10% cases of AHT), other different patterns |
| Systemic diseases | Variable | Coagulopathy (leukemia, thrombocytopenia, severe anemia, Vitamin K and factors deficiencies, and hemolytic uremic syndrome); raised ICP (glutaric aciduria type 1, meningitis); thrombosis of retinal artery (e.g., endocarditis), damage to the retinal endothelium (e.g., vasculitis) | Related to specific pathology | Deficiency of coagulation mechanisms | Low number and extension | Lack of diagnosis from accurate clinical and laboratory assessments |
| Paper | Year | Country | Category | Aim | Number of Subjects | Age Range of the Studied Population | Methodology | Specific Investigations/Reactants | Statistical Analysis and Validation | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| Bais et al. [89] | 2016 | The Netherlands | Retrospective cross-sectional study | Investigation of new markers as diagnostic tools for discrimination between AHT and non-AHT | 37 deceased infants (21 AHT cases, 16 controls) | 10 to 1041 days | Immunohistochemistry | β-APP, ubiquitin, GFAP | Yes | Need for further experimentation for international validation |
| Lantz et al. [90] | 2016 | USA | Technical note | Description of smartphone still-image capture techniques in PMIO | Not applicable | Not applicable | Employment of smartphone flashlight and camera in addition to traditional PMIO | Not applicable | No | First and only article on the subject |
| Bais et al. [91] | 2016 | The Netherlands | Case report | Description of a long-term hemosiderin persistence in an AHT case | 1 deceased infant, prior victim of AHT | 5 months | Histopathology | Hematoxylin and eosin (H&E); Perl’s iron stain | No | Absence of a large cohort |
| Fieß et al. [88] | 2016 | Germany | Case report | Illustration of the correct process to diagnose AHT from eye examinations | 1 infant | 10 months | Clinical examination, autopsy, histopathology | Ophtalmoscopy and histological confirmation by H&E staining | No | Absence of a large cohort, no statistical validation |
| Yusuf et al. [92] | 2017 | United Kingdom | Case series | Audit the use of non-contact ultra-widefield retinal imaging in infants with suspected abusive head trauma (AHT) | 5 infants | 1 to 15 months | Clinical assessment in living subjects | P200MA Scanning Laser Ophthalmoscope | No | Absence of a large cohort, no statistical validation |
| Puanglumyai et al. [71] | 2017 | Thailand | Case series | Demonstration of ONSH importance over RH in AHT diagnosis | 11 deceased infants, victims of AHT | 1 to 24 months | Autopsy, histopathology | Removal of orbital roof, bilateral exposure and fixation of ONs, H&E staining | No | Absence of a large cohort, no statistical validation |
| Oshima et al. [93] | 2017 | Japan | Case report | Investigating the mechanism of peripapillary scleral hemorrhages | 4 subjects. of whom 1 infant was the victim of AHT | 1 month | Autopsy, histopathology | Microscopic observation of peripapillary scleral hemorrhages | No | Simple description of an encountered case |
| Del Bigio et al. [94] | 2017 | Canada | Retrospective monocentric study | Creating an injury timing interval chart based on microscopic findings | 62 infants, of whom 9 were victims of AHT | 8 to 124 weeks | Autopsy, histopathology | Hematoxylin and eosin (H&E); Perl’s iron stain; Martius scarlet blue | Yes | Absence of complete ocular examination |
| Delteil et al. [95] | 2019 | France | Retrospective multicenter study | Construction of a histological diagnostic tool for RH timing | 83 deceased infants (48 males, 35 females), victims of AHT | 0 to 36 months | Histopathology | Hematoxylin and eosin (H&E); Perl’s iron stain | Yes | - |
| Zuccoli et al. [96] | 2020 | Italy | Retrospective monocentric study | Providing ONSH evidence in survival AHT cases | 13 infant victims of AHT | 1.2 to 20.8 months | MRI | 3D-SWI protocol | Yes | Absence of control subjects; lack of intracranial findings analysis |
| Barth et al. [87] | 2020 | Germany | Case report | Highlighting the possibility of unilateral RHs in confirmed AHT cases | 2 infants | 2 and 2.5 months | Clinical assessment in living subjects | Ophthalmoscopy | No | Anecdotal validity, despite growing evidence in literature of unilateral RHs among AHT victims |
| Cartocci et al. [78] | 2020 | Italy | Review | Broad view on the main MRI findings in the central nervous system in cases of AHT | Not applicable | Not applicable | MRI | Combination of coronal T2-w, T1-w and axial GRE T2-w protocols to detect extra-axial hemorrhages such RHs | No | Lack of large-cohort studies available at the time; variability in techniques across considered studies |
| Maiese et al. [98] | 2021 | Italy | Review | New immunohistochemical technique proposal | Not applicable | Not applicable | Histopathology | Application of Glycophorin A immunoreaction and confocal laser scanning microscopy to RHs | No | Need for large scale validation of the proposed technique |
| Oliva et al. [99] | 2021 | Italy | Retrospective monocentric study | Combination of RETcam and OCT as a diagnostic and prognostic tool in AHT victims | 6 infants | 0.6 to 10 months | Clinical assessment in living subjects | Combination of RETcam and OCT | Yes | Need for further application of the proposed protocol |
| Bulirsch et al. [100] | 2021 | Germany | Prospective cross-sectional study | Investigation of retinal Müller cells immunoreactivity in response to AHT in children | 37 infants, of whom 5 were victims of AHT | 1 to 8 months | Histopathology | Hematoxylin and eosin (H&E); Periodic-acid Schiff (PAS) reaction; Immunoreaction against Nestin, CD44, collagen type IX, GFAP | Yes | First article on the subject, need for a large cohort |
| Moskwa et al. [97] | 2022 | France | Retrospective monocentric study | Strengthening the correlation between specific ophthalmological lesions and AHT | 133 infants | 14 days to 10 months | Clinical assessment in living subjects | Ophthalmoscopy | Yes | Need for a multicentric data collection and analysis of other variables |
| Selection (Star Number) | Comparability (Star Number) | Outcome (Star Number) | |
|---|---|---|---|
| Bais et al. [89] | 3 | 1 | 3 |
| Puanglumyai et al. [71] | 2 | Not applicable † | 2 |
| Del Bigio et al. [94] | 4 | 1 | 2 |
| Delteil et al. [95] | 2 | Not applicable † | 2 |
| Zuccoli et al. [96] | 1 | Not applicable † | 3 |
| Moskwa et al. [97] | 3 | Not applicable † | 2 |
| Oliva et al. [99] | 1 | Not applicable † | 3 |
| Bulirsch et al. [100] | 2 | 1 | 3 |
| Barth et al. [87] | Fieß et al. [88] | Bais et al. [91] | Yusuf et al. [92] | Oshima et al. [93] | |
|---|---|---|---|---|---|
| Were patient’s demographic characteristics clearly described? | Unclear | Yes | Yes | Unclear | Unclear |
| Was the patient’s history clearly described and presented as a timeline? | Unclear | Yes | Yes | Yes | Unclear |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes | Yes | Yes |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes | Yes | Yes |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes | Yes | Yes |
| Was the post-intervention clinical condition clearly described? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Were adverse events (harms) or unanticipated events identified and described? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes |
| Cartocci et al. [78] | Maiese et al. [98] | |
|---|---|---|
| Is the review question clearly and explicitly stated? | Yes | Yes |
| Were the inclusion criteria appropriate for the review question? | Yes | Yes |
| Was the search strategy appropriate? | Unclear | Yes |
| Were the sources and resources used to search for studies adequate? | Yes | Yes |
| Were the criteria for appraising studies appropriate? | Yes | Yes |
| Was critical appraisal conducted by two or more reviewers independently? | No | Yes |
| Were there methods to minimize errors in data extraction? | No | No |
| Were the methods used to combine studies appropriate? | Unclear | Yes |
| Was the likelihood of publication bias assessed? | No | No |
| Were recommendations for policy and/or practice supported by the reported data? | Yes | Yes |
| Were the specific directives for new research appropriate? | Yes | Yes |
| Item | Technique | Morphology/Quantification | Attributed PTI | Notes |
|---|---|---|---|---|
| RBCs | H&E | Intact | First 24 h | Differentiation from autolytic mechanisms by comparison with other structures |
| Lysed | From 2 to 24 days | |||
| Mixed | ||||
| Fibrin/platelets | H&E | Presence/Absence | First 72 h | - |
| Leukocytes | H&E | PMNs and/or lymphocytes | PMNs from 12 h to 17 days (peak within 48–96 h); lymphocytes from 12 h to 23 days (peak within 72–96 h) | - |
| Macrophages | CD68 immunolabeling | 200× magnification field count for semi-quantitative study | From 72 h (minimal PTI observed: 12 h) | Cells per filed proposed: 0–3 |
| Hemoglobin degradation | Perls Prussian blue stain | Detection of siderophages and/or hematoid deposits within affected tissue | From 72 h | Semi-quantitative classification as absent, early, moderate, or abundant |
| Neovascularization | H&E | Absent/present | From the 6th to the 70th day | Grades of presence: capillary proliferation, giant capillaries, arterioles |
| Fibrous organization | H&E | Absent/present | From the 2nd week | Aspects of retinal sclerosis or ON atrophy |
| Estimated PTI | Microscopic Alterations |
|---|---|
| 0–3 days | Organization of fibrin and platelets inside hemorrhage |
| From the 2nd day | Lysis of RBCs |
| From the 6th hour to the 23rd day | Lymphocytes migration inside RH |
| From the 12th hour to the 17th day | Macrophages migration inside RH |
| From the 4th day | Siderophages inside RH; fibrous organization with fibroblasts inside RH |
| From the 4th day to 1 month | Collagen deposition |
| From the 7th day | Neovascularization |
| From the 8th day | Retinal sclerosis |
| Immunoreactants | Function | Results |
|---|---|---|
| Nestin | Marker of stem cell regeneration, intermediate filaments production | Immunoreaction observed in AHT cases, but also in fetal and adult eyes. |
| CD44 | Marker of stem cell regeneration | High specificity for AHT cases. |
| GFAP | Intermediate filaments production, reactive glia cells activation | Immunopositivity found in AHT cases as well as in a premature infant. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fazio, N.; Delogu, G.; Morena, D.; Cipolloni, L.; Scopetti, M.; Mazzilli, S.; Frati, P.; Fineschi, V. New Insights into the Diagnosis and Age Determination of Retinal Hemorrhages from Abusive Head Trauma: A Systematic Review. Diagnostics 2023, 13, 1722. https://doi.org/10.3390/diagnostics13101722
Di Fazio N, Delogu G, Morena D, Cipolloni L, Scopetti M, Mazzilli S, Frati P, Fineschi V. New Insights into the Diagnosis and Age Determination of Retinal Hemorrhages from Abusive Head Trauma: A Systematic Review. Diagnostics. 2023; 13(10):1722. https://doi.org/10.3390/diagnostics13101722
Chicago/Turabian StyleDi Fazio, Nicola, Giuseppe Delogu, Donato Morena, Luigi Cipolloni, Matteo Scopetti, Sara Mazzilli, Paola Frati, and Vittorio Fineschi. 2023. "New Insights into the Diagnosis and Age Determination of Retinal Hemorrhages from Abusive Head Trauma: A Systematic Review" Diagnostics 13, no. 10: 1722. https://doi.org/10.3390/diagnostics13101722
APA StyleDi Fazio, N., Delogu, G., Morena, D., Cipolloni, L., Scopetti, M., Mazzilli, S., Frati, P., & Fineschi, V. (2023). New Insights into the Diagnosis and Age Determination of Retinal Hemorrhages from Abusive Head Trauma: A Systematic Review. Diagnostics, 13(10), 1722. https://doi.org/10.3390/diagnostics13101722





